Abstract
Background
While process of care is a valuable dimension of quality, process-of-care-based quality indicators (POC-QIs) are ideally associated with meaningful patient outcomes. The relationship between POC-QIs for hospitalized older patients and functional decline, a relevant outcome for older patients, is unknown.
Objective
To assess the relationship between POC-QIs for hospitalized elders and functional decline
Research Design
Observational cohort study.
Subjects
Hospitalized vulnerable elder patients age 65 or older admitted to a general medicine inpatient service from 1 June 2004 to 1 June 2007.
Measures
POC-QIs received by hospitalized patients (measured by ACOVE QIs) and functional decline (increased Activities of Daily Living impairments post discharge).
Results
For 898 vulnerable elder patients, mean adherence to six universally applied quality indicators was 57.8%. After adjustment for factors likely associated with functional decline (comorbidity, vulnerability, baseline functional limitation, number of POC-QIs triggered, length of stay, code status, and interaction between frailty and QI adherence), there was no association between higher quality of care (using the composite score) and increased risk of functional decline. Patients who received a mobility plan were 1.48 (95% CI 1.07-2.05; p=0.017) times more likely to suffer functional decline after discharge. Patients who received an assessment of nutritional status had a lower odds of suffering functional decline after discharge (OR 0.37 (95% CI 0.21-0.64; p<0.001).
Conclusions
Hospitalized vulnerable elders who receive higher quality of care, as measured by ACOVE QIs, are not less likely to suffer decline after discharge.
Keywords: quality measures, hospitalization, functional decline
Introduction
In recent years, much research has been dedicated to identifying relevant and valid measures of quality of healthcare (1). Developing valid measures of quality is important given the increasing focus on measuring and rewarding adherence to quality measures through the increasing use of pay-for-performance and public reporting programs (2). Most of these programs use quality measures that focus on processes of care which can be precisely specified. More importantly, process of care measures do not require the necessary adjusting for disease severity that patient outcome measures, such as mortality, require. In addition to measuring individual care processes, recent attention has focused on the need for composite measures of quality that take into account the multiple care processes indicated in medically complex patients (3). Given the cost of measuring and improving quality of care, adherence to such measures would ideally improve patient outcomes.
To assess quality of care for older patients, the Assessing Care of Vulnerable Elders (ACOVE) quality indicators were developed to offer an objective standard of measuring indicated processes of care (4, 5). These measures were developed using the modified Delphi method and are a series of IF/THEN statements that focus on clinical care processes that should occur for eligible patients. Research has demonstrated that higher quality care, as measured by a composite score of adherence to ACOVE quality indicators, is associated with improved three-year survival in community-dwelling vulnerable older persons (6). While the process-outcome relationship has been examined in community-dwelling older adults, it is not known whether adherence to these measures is associated with improved outcomes for hospitalized older patients, who are at increased risk for functional decline after discharge (7). While the etiology of functional decline in hospitalized patients is multifactorial, several factors that are implicated, such as immobility, poor nutrition, inadequate pain control and delirium, are care processes addressed by ACOVE quality indicators. For example, it is reasonable to hypothesize that to prevent functional decline after discharge, it is important to perform a functional status assessment to target interventions such as physical therapy or rehabilitation. Likewise, failure to assess pain, nutrition, or ordering a mobility plan could all result in functional impairment due to failure to treat pain, order necessary nutritional supplementation, or ensure that patients receive physical therapy. For these reasons, it is possible that adherence to ACOVE process of care measures could be associated with reductions in functional decline in hospitalized older patients, a relevant and important outcome for these patients (8). Therefore, the aim of this study is to assess the relationship between process of care for hospitalized vulnerable elders, as measured by ACOVE process of care quality indicators (POC-QIs), and functional decline after discharge.
Methods
Study Design
Patients were identified using the infrastructure of a pre-existing prospective cohort study of general medicine inpatients at the University of Chicago Medical Center. Each day, trained research assistants approached new adult patients on the inpatient general medicine service to participate in “the Hospitalist Project,” an ongoing, large study of quality of care and resource allocation for hospitalized patients (9). Patients are approached within 48 hours after admission by as research assistant. If a patient is unavailable due to test or procedure, or too sick to participate, the research assistant returns within 24 hours until the patient is interviewed, refuses, or is discharged (without having provided consent). Using this protocol, over 75% of patients who consent are interviewed before hospital day 2 with over 99% by hospital day 4. To assess patients ability to consent to the study and participate in the interview, an abridged form of the Mini-Mental State Exam (MMSE) is administered first (10). A score of 17 or below required the involvement of a proxy during the informed consent process and subsequent interview. Patients over age 65 were administered the Vulnerable Elders Survey (VES-13), a 13-item validated survey that identifies those older patients at risk of functional decline or death, using items based on age, self-rated health and physical function. Those individuals with a VES-13 score of 3 or above were identified as “Vulnerable Elders” (VEs), and identify the subset of patients for whom ACOVE Quality Indicators were developed (11, 12) and were included in this study. Patients who were under age 65 or were over age 65 but not identified as “vulnerable” (VES-13 score <3) were excluded from this study. Patients with a less than one day length of stay were excluded since quality of inpatient care does not likely impact functional outcomes after discharge within such a short period of time. Lastly, patients who died in the hospital, were discharged to hospice, or were initially admitted to an ICU were also excluded since the selected ACOVE quality indicators may not apply in these near death cases. The Institutional Review Board at the University of Chicago approved this study.
Data Collection
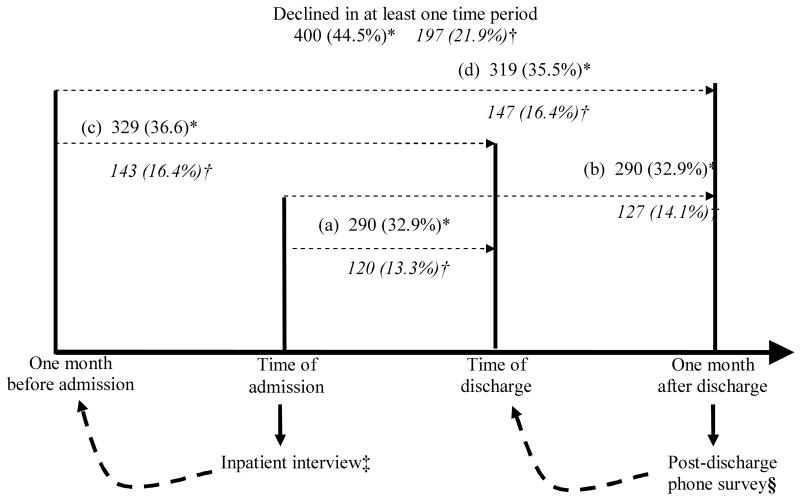
During the inpatient interview, patients were asked to report their functional status using questions of Activities of Daily Living (ADLs) for both the time of admission (present) and for one month prior to admission (retrospective report) (13-15). For each Activity of Daily Living (feeding, transferring, bathing, toileting, dressing, and continence), patients reported whether they were able to complete the activity unassisted or whether help was needed. By convention, an ADL impairment was identified if a patient needed assistance to complete the activity. During a follow-up telephone interview 30 days after discharge, patients were asked to report their functional limitation using the same ADL questions referring to both the time of discharge (retrospective report) and for one month after discharge (present). This protocol has been validated in earlier studies of hospitalized patients by Covinsky and others, and yields patient reports of functional status for four distinct points in time: 1 month prior to admission, upon admission to the hospital, upon discharge from the hospital, and 30 days after discharge (15). (See Appendix Figure 1, http://links.lww.com/A1255.)
Appendix Figure 1.
Patients Reporting Functional Decline Over Time Periods (n = 898)
(a) Admission to discharge; (b) Admission to one month after discharge; (c) One month before admission to discharge; (d) One month before admission to one month after discharge
*Patients experiencing functional decline [emergence of deficits in 1 or more Activities of Daily Living (ADLs)] in given time period, n (%)
†Patients experiencing catastrophic functional decline are in italics (emergence of deficits in 3 or more ADLs) in given time period, n (%)
‡At inpatient interview, patients were asked to retrospectively recall their functional status one month prior to admission
§At post-discharge phone survey, patients were asked to retrospectively recall their functional status at the time of discharge from the hospital
The medical charts of enrolled patients were reviewed by trained research assistants using a computer-based chart abstraction tool designed to reliably determine adherence to 16 ACOVE POC-QIs, as previously reported (16). Six of these quality indicators (formal assessment of cognitive status within 24 hours, assessment of functional status, efforts to improve mobility, discharge planning, documentation of nutritional status and assessment of pain within 24 hours) applied to all hospitalized vulnerable elders, or were “universally applicable.” The remaining ten were condition-specific, and were only applicable if a patient had a condition (e.g. delirium, pressure ulcer, etc.) that triggered the quality indicator. Chart reviewers underwent a monitored training with 25 charts, concluding with a review session to identify discrepancies and correct errors within the abstraction process.
Data Analysis
To measure quality of care, percent adherence to quality indicators across all patients was calculated. In addition, ACOVE composite quality scores were calculated for each patient as the percent of indicators met out of those that each patient was eligible for or had triggered (3, 7).
Decline in functional status was defined as the emergence of any new ADL impairments when compared to the baseline period prior to admission (15, 17-22). Changes in ADL limitation for each individual patient were calculated for four time periods: (1) one month prior to admission to one month after discharge; (2) one month before admission to time of discharge; (3) time of admission to one month after discharge; (4) time of admission to time of discharge. These changes were recorded as binary variables for each time period, with 1 indicating the emergence of new ADL impairments and 0 indicating no new ADL impairments. Because emergence of isolated ADL impairments may be temporary, the emergence of 3 or more new ADL limitations, also named “catastrophic functional decline,” also was constructed as a binary variable for each of the four time periods (23).
Multivariable logistic regression was performed to assess the effect of quality score, as measured by adherence to ACOVE POC-QIs, on functional decline in each of the four time periods. In addition, for the 6 quality indicators that were universally applicable to all patients, multivariable logistic regression was performed to test the effect of adherence to individual quality indicators on functional decline for the time period one month before admission to one month after discharge, which is the time period most sensitive to acute hospitalization. All logistic regression models controlled for observable characteristics that may also affect functional decline such as: Charlson comorbidity score (24), VES-13 score or level of vulnerability, number of baseline ADL limitations, log of the length of stay, code status and the number of quality indicators triggered for each patient. Charlson comorbidity score, a marker of comorbidity burden routinely used in risk adjustment, was constructed using principal and secondary diagnosis from administrative data (25). Higher VES-13 scores indicate increased risk of functional decline (12), and number of baseline ADL limitations was included because a patient with fewer limitations has more potential for loss of ADL independence. Patients who are sicker likely experience longer lengths of stay and are at greater risk of functional decline after discharge. In addition, patients whose code status is less than “full” may also be sicker and more likely to decline. Patients with more geriatric conditions would trigger more indicators, and also be possibly more prone to functional decline (3, 26). In addition, because indicated care processes may have differing effects across increasing levels of patient vulnerability, an interaction term between VES-13 score and quality score or quality indicator adherence was included. In addition to these covariates, models were also adjusted for routine demographic characteristics including age, race, and gender. All models were clustered by attending to control for individual physician practice. Predicted probabilities of functional decline for patients receiving and not receiving a specific quality indicator were estimated from models. All analyses were repeated using catastrophic functional decline as the outcome variable. Statistical analyses were conducted using Stata 9.0 with p < 0.05 (Stata Corporation, College Station, Texas).
To assess for response bias in our sample, we compared routine demographic characteristics and quality indicator adherence for patients who received follow-up to those that eligible patients that were lost to follow-up. In addition, to test for the possibility of confounding due to patient illness, we examined the association between illness covariates (Charlson score, VES-13 score, number of baseline ADL limitations, length of stay, code status and the number of quality indicators triggered) and adherence to quality indicators.
Results
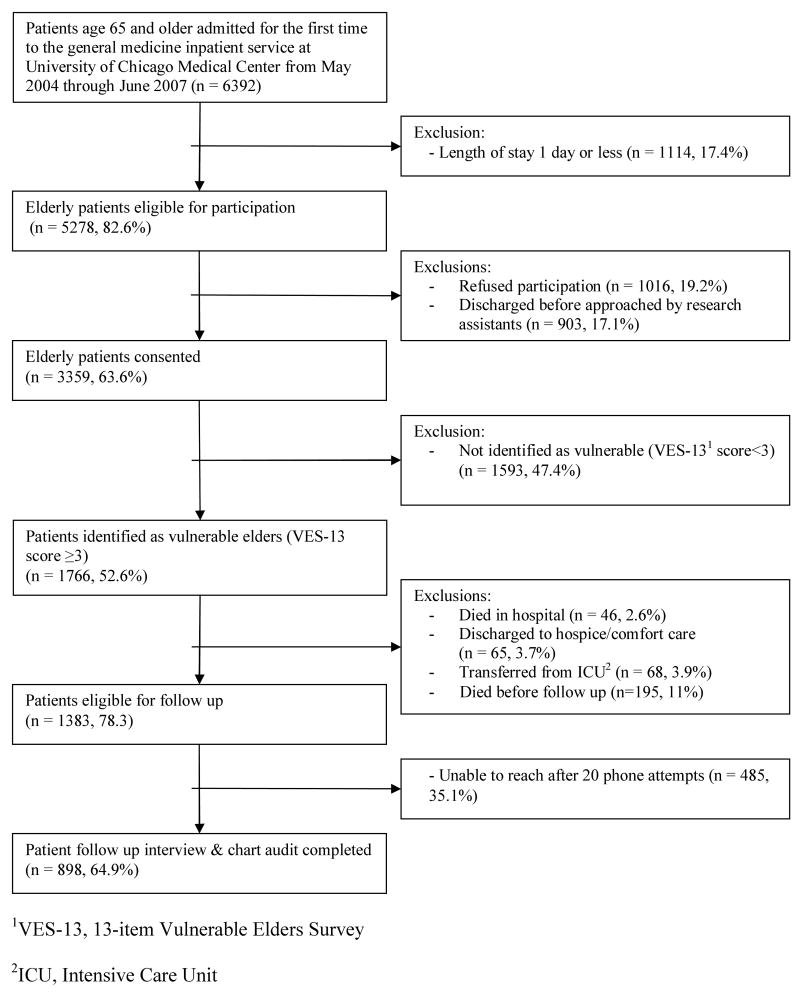
From May 2004 through June 2007, 6392 elderly patients were first-time admittees to the general medicine inpatient service at the University of Chicago Medical Center (Figure 1). Of the 5278 (82.6%) who stayed in the hospital more than 1 day, 3359 (63.6%) consented to participate in the study. 1766 (52.6%) of the consenting patients were identified as vulnerable elders through administration of the VES-13, of whom 46 (2.6%) died in the hospital, 65 (3.7%) were discharged to hospice or comfort care, 68 (3.9%) were transferred from an ICU. Another 195 (11%) of patients died before the one month follow-up interview. Of the remaining 1383 patients eligible for follow-up, 898 (64.9%) of patients completed the telephone follow-up interview.
Figure 1.
Patient recruitment to and exclusion from study.
Patient characteristics are described in Table 1. The 898 patients with complete functional status data were predominantly African American and female with a mean age of 79.3 years (SD 8.2). The mean VES-13 score was 5.6 (SD 2.0), with mean length of hospital stay 5.2 (SD 4.5) days. At the time of admission, 161 (17.9%) patients had impaired cognitive status and 565 (62.9%) of patients reported impairments in at least one Activity of Daily Living (ADL). Patients who successfully completed follow-up were more likely to be be African-American (71% vs. 63%, p=0.012), with a shorter length of stay (5.3 days vs. 6.7 days, p=0.007), lower VES score (5.6 vs. 5.9, p=0.005), trigger fewer quality indicators (7.9 indicators vs. 8.4 indicators, p<0.001, but have a higher Charlson score (1.9 vs. 1.7, p=0.03). There was no difference in adherence to quality indicators or composite quality score between those patients that completed follow-up and those that did not.
Table 1.
Demographics of Study Sample (n = 898)
| Demographic | Value |
|---|---|
| Age, mean (SD) [years] | 79.3 (8.2) |
| Female, n (%) | 633 (70.5) |
| Black, n (%) | 667 (74.3) |
| Hispanic, n (%) | 25 (3.1) |
| Low income (< $15,000/year), n (%) | 199 (22.2) |
| Education: | |
| High school or less, n (%) | 537 (59.8) |
| Some college or more, n (%) | 261 (29.1) |
| Do not know, n (%) | 100 (11.1) |
| VES-13 score, mean (SD) [range 3 – 11] | 5.6 (2.0) |
| Length of hospital stay, mean (SD) | 5.2 (4.5) |
| Any ADL disability, n (%)* | 565 (62.9) |
| Impaired cognitive status, n (%)† | 161 (17.9) |
| Average Charlson Score, mean (SD) | 1.9 (1.6) |
At time of admission to hospital
Measured as MMSE score < 17 signifying need for a proxy
For those ACOVE POC-QIs that were universally applicable, or not triggered by a specific condition (formal assessment of cognitive status within 24 hours, assessment of functional status, efforts to improve mobility, discharge planning, documentation of nutritional status and assessment of pain within 24 hours), percent adherence ranged from 6.3% to 97.5%, with a mean adherence of 57.9%. (Table 2) The average number of POC-QIs triggered per patient was 8.0 (SD 1.8). For the 417 patients that triggered between 5 to 7 indicators, mean quality score was 53.1 (50.8 - 55.4). For the 387 patients that triggered between 8 to 10 indicators, mean quality score was 64.4 (63.0 -65.9). 94 patients triggered more than 10 indicators and had a mean quality score of 62.4 (60.0-64.7). There was a positive relationship between number of quality indicators triggered and overall quality score (r=0.33, P<0.001).
Table 2.
Adherence to Universally Applied ACOVE Quality Indicators (n = 898)
| ACOVE Quality Indicators | Number triggered | Number Met | % Adherence |
|---|---|---|---|
| IF a vulnerable elder is admitted to the hospital for any acute or chronic illness or any surgical procedure, THEN the evaluation should include, within 24 hours, cognitive status. | 898 | 39 | 4.3% |
| IF a vulnerable elder is admitted to a hospital or is new to a physician practice, THEN multidimensional assessment of cognitive ability and assessment of functional status should be documented. | 898 | 377 | 41.9% |
| IF a vulnerable elder is found to have problems with gait, strength (e.g., <= 4 out of 5 on manual muscle testing, or the need to use his or her arms to rise from a chair), or endurance (e.g., dyspnea on mild exertion), THEN an exercise program should be offered. | 853 | 564 | 66.1% |
| IF a vulnerable elder is admitted to the hospital, THEN the discharge planning should begin within 48 hours. | 898 | 776 | 86.4% |
| IF a vulnerable elder is hospitalized, THEN his or her nutritional status should be documented during the hospitalization by evaluation of oral intake or serum biochemical testing (e.g., albumin, prealbumin, or cholesterol). | 898 | 842 | 93.8% |
| ALL vulnerable elders should be screened for chronic pain during the initial evaluation period. | 898 | 849 | 54.5% |
| Total | 5343 | 3087 | 57.8% |
400 (44.5%) patients reported functional decline in any of the four time periods. For the time period from admission to discharge, 290 (32.9%) patients reported functional decline. The same fraction of patients reported decline from admission to one month after discharge. When using one month before admission as the baseline, slightly greater numbers of patients reported functional decline [319 (35.5%) from one month before admission to one month after discharge; 329 (36.6%) from one month before admission to discharge]. Patient reports of catastrophic functional decline followed similar patterns during the four time periods, with 197 (21.9%) patients reporting catastrophic functional decline in any of the four time periods. 120 (13.3%) reported catastrophic decline from admission to discharge, and 127 (14.1%) reported catastrophic decline from admission to one month after discharge. The number of patients reporting catastrophic decline was similar [143 (15.9%)] for both one month before admission to discharge and 147 (16.4%) one month before admission to one month after discharge. (Appendix Figure 1, http://links.lww.com/A1255)
In multivariate logistic regression testing the effect of overall quality score on functional decline, there was no observable relationship in any of the four time periods. Examining the relationship between covariates [VES-13 score, baseline ADL limitations, number of QIs triggered, Charlson index (measure of comorbidity burden), and interaction between VES-13 score and quality score] confirmed the importance of controlling for these factors in examining the relationship between quality and outcomes. As predicted, patients that were more frail (higher VES-13 score), with more comorbidities (higher Charlson index), more geriatric conditions (triggered more quality indicators), but with preserved physical function (fewer ADL limitations at baseline) were significantly more likely to suffer functional decline between one month before admission and one month after discharge. These relationships were consistent for all four time periods of functional decline (Table 3).
Table 3.
Relationship between Overall Quality Score and Functional Decline (n = 898)
| Model | Functional Decline‡ | Catastrophic Functional Decline§ | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) |
P value | Odds Ratio (95% CI) |
P value | |
| Association between Quality Score* and Functional Decline during each Time Period† | ||||
| (a) admit to discharge | 1.00 (0.99-1.01) | 0.73 | 0.99 (0.98-1.01) | 0.38 |
| (b) admit to one month after discharge | 1.00 (0.99-1.01) | 0.59 | 0.99 (0.98-1.00) | 0.23 |
| (c) one month before admit to discharge | 1.00 (0.99-1.01) | 0.60 | 1.00 (0.98-1.01) | 0.57 |
| (d) one month before admit to one month after discharge | 0.99 (0.99-1.00) | 0.18 | 1.00 (0.99-1.01) | 0.86 |
| Association between Disease Severity and Covariates | ||||
| Average VES-13 score | 1.49 (1.18-1.88) | <0.001 | 1. 41 (1.00-1.97) | 0.05 |
| Baseline ADL limitations | 0.71 (0.66-0.77) | <0.001 | 0.63 (0.57-0.70) | <0.001 |
| Charlson score | 1.13 (1.04-1.24) | 0.006 | 1.08 (0.96-1.22) | 0.18 |
| Number of QIs triggered | 1.27 (1.16-1.38) | <0.001 | 1.32 (1.21-1.45) | 0.001 |
| QI adherence & VES-13 score interaction | 1.00 (0.99- 1.00) | 0.13 | 1.00 (0.99- 1.00) | 0.25 |
| Log Length of Stay | 1.35 (1.04-1.75) | 0.02 | 1.53 (1.16-2.00) | 0.002 |
| DNR/DNI Status | 1.10 (0.61-1.99) | 0.74 | 1.00 (0.99-1.01) | 0.86 |
Quality score calculated as total number of quality indicators met divided by total number of quality indicators triggered multiplied by 100
Results derived from multivariate logistic regression that tests the effect of quality score on functional decline and adjusts for covariates listed [average VES-13 Score, Charlson Score, interaction between quality score and VES-13 score, number of baseline ADL limitations, DNR/DNI status, log length of stay, number of Quality Indicators triggered, demographic characteristics (age, race, gender), and clustered for attending subject]
Functional Decline defined as the emergence of one or more new deficits in Activities of Daily Living (ADLs) in given time period
Catastrophic Functional Decline defined as the emergence of deficits in 3 or more ADLs in a given time period
P < 0.05
To examine the effect of individual quality indicators, analysis was focused on functional decline during the time period from one month before admission to one month after discharge, the time period which is likely most affected by in-hospital processes. In this analysis, patients who had a documented effort to improve mobility (i.e. order for physical therapy) were more likely to experience functional decline (OR 1.5, 95% CI 1.1-2.0, p=0.017) and catastrophic functional decline (OR 1.9, 95% CI 1.2-3.1, p=0.010). In contrast, patients who received documentation of nutritional status were less likely to experience functional decline (OR 0.4, 95% CI 0.2-0.6, p<0.001) and catastrophic functional decline (OR 0.3, 95% CI 0.1-0.6, p=0.001). Of note, these findings were consistent across all time periods.
Because there is the possibility of confounding due to patient illness (sicker patients that are more likely to experience functional decline may be more or less likely to receive certain care processes), the association between illness covariates (Charlson score, VES-13 score, number of baseline ADL limitations, length of stay, code status and the number of quality indicators triggered) and adherence to quality indicators was also examined. Patients that received above median quality score were more likely to have a longer length of stay (5.6 days vs. 4.9 days, p=0.009) and trigger more quality indicators (8.2 indicators vs. 7.7 indicators, p<0.001). With respect to individual quality indicators, patients who had a higher VE score (OR 1.1 95% CI 1.0- 1.2; p=0.018) who triggered more quality indicators (OR 1.35, 95% CI 1.2-1.5, p<0.001), and had a longer length of stay (OR 2.2, 95% CI 1.7-2.9, p<0.001) had a significantly greater likelihood of having a mobility plan. Patients who triggered more quality indicators were 22.7 (95% CI 6.7 -76.8, p<0.001) times more likely to have a nutritional status assessment.
Discussion
These results demonstrate that increased quality of care, as measured by the composite score of adherence to ACOVE process of care measures, is not significantly associated with reduced risk of functional decline in hospitalized vulnerable elder patients. Interestingly, those patients with a documented effort to improve mobility were more likely to experience functional decline after discharge. In addition, patients who receive an assessment of nutritional status were less likely to experience functional decline.
In attempting to understand these findings, it is important to consider the possibility of confounding due to patient illness. For example, patients who are more frail, have more geriatric conditions and therefore trigger more quality indicators, and had a longer length of stay were more likely to receive a plan for mobility. This raises the possibility that clinicians were more likely to document of an attempt to improve mobility (i.e. a physical therapy order) for sicker patients that were more likely to also decline. In the case of nutritional status assessment, this explanation does not appear to be responsible. Although patients who trigger more quality indicators are more likely to receive a nutritional status assessment, patients who receive nutritional status assessments were less likely to experience functional decline after discharge. Future research will be needed to understand this finding. It is unlikely that the simple documentation of nutritional status is protective against functional decline. A more plausible explanation may be that receiving an assessment of nutritional status is a proxy for also receiving related therapies that may guard against functional decline, such as nutritional supplementation. However, it is also that an unmeasured confounder is responsible for this finding.
While attempts were made to adjust for a wide variety of pertinent covariates, it is likely that relevant confounding variables were omitted. This study highlights the difficulty of assessing process-outcome causal relationships in observational study designs. Although we considered an instrumental variable approach (28), we were unable to identify a potential variable that would relate to whether patients would receive certain quality indicators. Future research should therefore include more aggressive measurement and controlling of covariates, in particular focusing on possible selection effects. Randomized controlled trials that compare usual care to “high quality care” are likely needed to rigorously evaluate the effect of quality of care.
This study also has a number of limitations. First, it was conducted at a single academic medical institution and may not be generalizable. We measured quality using a small subset of quality indicators and observed patients for only 1 month after discharge. Adherence to certain quality indicators may associated with positive outcomes other than functional decline that were not measured in this study (i.e. cognitive screening could be associated with a reduction in delirium). In addition, although we examined the contribution of early discharge planning and physical therapy initiation in hospital, we did not examine immediate post hospitalization and rehabilitation settings which may be better predictor of functional decline for hospitalized seniors. Missing data biases could have emerged due to individuals lost to follow-up or death since patients who received follow-up appeared less sick than those that did not. Likewise, it is difficult to account for selective differences in difficulty of triggering different QIs in this analysis. In addition, chart documentation has been shown to underestimate the care given to patients, so it is possible that patients received higher quality care than was documented in their charts (29). It is also possible that our definition of functional decline (the emergence of a deficit in a single Activity of Daily Living) is subject to natural fluctuation; however measures of catastrophic functional decline were included to account for this possibility. Finally, as discussed earlier, the observational design of this study could not assess causality, and was only able to establish an association between quality of care and functional decline.
In conclusion, hospitalized vulnerable older patients who receive higher quality of care, as measured by ACOVE quality indicators, are not less likely to suffer from functional decline. To accurately assess the relationship between process of care and patient outcomes for hospitalized older patients, future research should use approaches that are able to address the challenges posed by selective application of care processes.
Table 4.
Individual Quality Indicator Adherence and Functional Decline One Month Before Admission to One Month After Discharge* (n = 898)
| Quality Indicator | Functional Decline‡ | Catastrophic Functional Decline§ | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | |
| Cognitive Status Assessment | 1.38 (0.70-2.73) | 0.35 | 0.60 (0.25-1.43) | 0.25 |
| Functional Status | 1.01 (0.75-1.36) | 0.94 | 1.37 (0.97-1.93) | 0.07 |
| Mobility Plan | 1.48 (1.07-2.05) | 0.017 ‖ | 1.89 (1.16-3.08) | 0.01 ‖ |
| Discharge Planning | 0.76 (0.50-1.15) | 0.19 | 0.61 (0.37-1.00) | 0.05 ‖ |
| Nutritional Status | 0.37 (0.21-0.64) | <0.001 ‖ | 0.28 (0.13-0.60) | <0.001 ‖ |
| Pain Assessment | 0.90 (0.68-1.19) | 0.46 | 1.03 (0.67-1.59) | 0.90 |
Results derived from multivariate logistic regression that tests the effect of QI adherence on functional decline and adjusts for average VES-13 Score, Charlson Score, interaction between quality indicator adherence and VES-13 score, number of baseline ADL limitations, DNR/DNI status, log length of stay, number of Quality Indicators triggered, demographic characteristics (age, race, gender), and clustered for attending subject
Functional Decline defined as the emergence of one or more new deficits in Activities of Daily Living (ADLs) in time period one month before admission to one month after discharge
Catastrophic Functional Decline defined as the emergence of deficits in 3 or more ADLs in a given time period
P < 0.05
Acknowledgments
This study was funded by the Hartford Health Outcomes Geriatrics Research Scholars Award, National Institute for General Medical Sciences, Donald W. Reynolds Foundation, the University of Chicago's John A. Hartford Foundation Center of Excellence in Geriatrics, and Pritzker Summer Research Program. The funding sources had no role in this study.
References
- 1.Bradley EH, Herrin J, Elbel B, et al. Hospital quality for acute myocardial infarction: correlation among process measures and relationship with short-term mortality. JAMA. 2006;296(1):72–8. doi: 10.1001/jama.296.1.72. [DOI] [PubMed] [Google Scholar]
- 2.Rowe JW. Pay-for-performance and accountability: related themes in improving health care. Ann Intern Med. 2006;145(9):695–9. doi: 10.7326/0003-4819-145-9-200611070-00013. [DOI] [PubMed] [Google Scholar]
- 3.Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med. 2007;356(24):2496–504. doi: 10.1056/NEJMsa066253. [DOI] [PubMed] [Google Scholar]
- 4.Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med. 2001;135(8 Pt 2):642–6. doi: 10.7326/0003-4819-135-8_part_2-200110161-00002. [DOI] [PubMed] [Google Scholar]
- 5.Shekelle PG, MacLean CH, Morton SC, et al. Acove quality indicators. Ann Intern Med. 2001;135(8 Pt 2):653–67. doi: 10.7326/0003-4819-135-8_part_2-200110161-00004. [DOI] [PubMed] [Google Scholar]
- 6.Higashi T, Shekelle PG, Adams JL, et al. Quality of care is associated with survival in vulnerable older patients. Ann Intern Med. 2005;143(4):274–81. doi: 10.7326/0003-4819-143-4-200508160-00008. [DOI] [PubMed] [Google Scholar]
- 7.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–23. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 8.Covinsky KE, Justice AC, Rosenthal GE, et al. Measuring prognosis and case mix in hospitalized elders. The importance of functional status. J Gen Intern Med. 1997;12(4):203–8. doi: 10.1046/j.1525-1497.1997.012004203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meltzer D, Manning WG, Morrison J, et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–74. doi: 10.7326/0003-4819-137-11-200212030-00007. [DOI] [PubMed] [Google Scholar]
- 10.Roccaforte WH, Burke WJ, Bayer BL, et al. Validation of a telephone version of the mini-mental state examination. J Am Geriatr Soc. 1992;40(7):697–702. doi: 10.1111/j.1532-5415.1992.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 11.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49(12):1691–9. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 12.Min LC, Elliott MN, Wenger NS, et al. Higher vulnerable elders survey scores predict death and functional decline in vulnerable older people. J Am Geriatr Soc. 2006;54(3):507–11. doi: 10.1111/j.1532-5415.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 13.Katz S, Ford A, Moskowitz R, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;(185):914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 14.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. [PubMed] [Google Scholar]
- 15.Covinsky KE, Palmer RM, Counsell SR, et al. Functional status before hospitalization in acutely ill older adults: validity and clinical importance of retrospective reports. J Am Geriatr Soc. 2000;48(2):164–9. doi: 10.1111/j.1532-5415.2000.tb03907.x. [DOI] [PubMed] [Google Scholar]
- 16.Arora VM, Johnson M, Olson J, et al. Using assessing care of vulnerable elders quality indicators to measure quality of hospital care for vulnerable elders. J Am Geriatr Soc. 2007;55(11):1705–11. doi: 10.1111/j.1532-5415.2007.01444.x. [DOI] [PubMed] [Google Scholar]
- 17.Covinsky KE, Fortinsky RH, Palmer RM, et al. Relation between symptoms of depression and health status outcomes in acutely ill hospitalized older persons. Ann Intern Med. 1997;126(6):417–25. doi: 10.7326/0003-4819-126-6-199703150-00001. [DOI] [PubMed] [Google Scholar]
- 18.Covinsky KE, Justice AC, Rosenthal GE, et al. Measuring prognosis and case mix in hospitalized elders. The importance of functional status. J Gen Intern Med. 1997;12(4):203–8. doi: 10.1046/j.1525-1497.1997.012004203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covinsky KE, Kahana E, Chin MH, et al. Depressive symptoms and 3-year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–9. doi: 10.7326/0003-4819-130-7-199904060-00004. [DOI] [PubMed] [Google Scholar]
- 20.Covinsky KE, King JT, Jr, Quinn LM, et al. Do acute care for elders units increase hospital costs? A cost analysis using the hospital perspective. J Am Geriatr Soc. 1997;45(6):729–34. doi: 10.1111/j.1532-5415.1997.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 21.Covinsky KE, Martin GE, Beyth RJ, et al. The relationship between clinical assessments of nutritional status and adverse outcomes in older hospitalized medical patients. J Am Geriatr Soc. 1999;47(5):532–8. doi: 10.1111/j.1532-5415.1999.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 22.Covinsky KE, Rosenthal GE, Chren MM, et al. The relation between health status changes and patient satisfaction in older hospitalized medical patients. J Gen Intern Med. 1998;13(4):223–9. doi: 10.1046/j.1525-1497.1998.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayis S, Gooberman-Hill R, Bowling A, et al. Predicting catastrophic decline in mobility among older people. Age Ageing. 2006;35(4):382–7. doi: 10.1093/ageing/afl004. [DOI] [PubMed] [Google Scholar]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 25.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-CM-9 administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 26.Min LC, Wenger NS, Fung C, et al. Multimorbidity is associated with better quality of care among vulnerable elders. Med Care. 2007;45(6):480–8. doi: 10.1097/MLR.0b013e318030fff9. [DOI] [PubMed] [Google Scholar]
- 27.Efron B, Tibshirani R. Bootstrap Methods for Standard Errors, Confidence Intervals, and Other Measures of Statistical Accuracy. Statistical Science. 1986;1:54–77. [Google Scholar]
- 28.Kahn KL, Tisnado DM, Adams JL, Liu H, Chen WP, Hu FA, Mangione CM, Hays RD, Damberg CL. Does ambulatory process of care predict health-related quality of life outcomes for patients with chronic disease? Health Serv Res. 2007;42:63–83. doi: 10.1111/j.1475-6773.2006.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luck J, Peabody JW, Dresselhaus TR, et al. How well does chart abstraction measure quality? A prospective comparison of standardized patients with the medical record. Am J Med. 2000;108(8):642–9. doi: 10.1016/s0002-9343(00)00363-6. [DOI] [PubMed] [Google Scholar]