Abstract
Purpose
To test that prospective delivery of higher thermal dose is associated with longer tumor control duration.
Experimental Design
122 dogs with a heatable soft tissue sarcoma were randomized to receive a low (2–5 CEM43°CT90) or high (20–50 CEM43°CT90) thermal dose in combination with radiotherapy. Most dogs (90%) received 4–6 hyperthermia treatments over 5 weeks.
Results
In the primary analysis, median (95% CI) duration of local control in the low dose group was 1.2 (0.7–2.1) years versus 1.9 (1.4–3.2) years in the high dose group (logrank p=0.28). The probability (95% CI) of tumor control at one year in the low vs. high dose groups was 0.57 (0.43–0.70) vs. 0.74 (0.62–0.86), respectively. Using multivariable procedure, thermal dose group (p=0.023), total duration of heating (p=0.008), tumor volume (p=0.041) and tumor grade (p=0.027) were significantly related to duration of local tumor control. When correcting for volume, grade and duration of heating, dogs in the low dose group were 2.3 times as likely to experience local failure.
Conclusions
Thermal dose is directly related to local control duration in irradiated canine sarcomas. Longer heating being associated with shorter local tumor control was unexpected. However, the effect of thermal dose on tumor control was stronger than for heating duration. The heating duration effect is possibly mediated through deleterious effects on tumor oxygenation. These results are the first to show the value of prospectively controlled thermal dose in achieving local tumor control with thermoradiotherapy, and they establish a paradigm for prescribing thermoradiotherapy and writing a thermal prescription.
Keywords: Hyperthermia, Thermal Dose, Radiation Therapy, Dog, Thermoradiotherapy
Introduction
Evidence supporting the therapeutic benefit of hyperthermia when added to radiation therapy of solid tumors is mounting. There are 10 positive randomized trials wherein radiation combined with hyperthermia resulted in better outcome than radiation therapy alone. Seven of these trials were in humans 1–7 and three were in dogs 8–10. Despite these positive trials, the clinical application of hyperthermia remains somewhat ill-defined because of uncertainties about: 1) thermal dosimetry and 2) the characteristics of the hyperthermia dose-response relationship.
Thermal dosimetry is complicated by temperature heterogeneity within tumors. This heterogeneity results from heterogeneous energy deposition and also from perfusion-related conductive cooling11–13. Additionally, the temperature heterogeneity is temporally dynamic14, and heat effects are time-dependent15. These issues have made development of a thermal dosimetry unit challenging; a review of the history of thermal dosimetry is available 16. Importantly, shortcomings associated with use of descriptors that take only temperature into account have been identified 17, 18 and methods have been developed to convert time-temperature histories into an equivalent number of minutes exposure to 43°C (CEM43°C = cumulative equivalent minutes of exposure at 43°C) 19. The CEM43°C concept has been shown to be valid for many endpoints of tissue damage in pre-clinical studies, including tumor specific endpoints such as tumor control 20.
Descriptors of the lower end of the temperature distribution in tumors have been shown to have the most relevance to tumor response 8, 18, 20, 21. In 1993 the quantification unit CEM43°CT90 was proposed to characterize the low end of the temperature distribution and also to incorporate duration of treatment 22. CEM43°CT90 describes the equivalent time in minutes that the 10th percentile of the measured temperature distribution (the T90 - representative of the low end of the temperature distribution) is equal to 43°C. Since the introduction of the CEM43°CT90 unit, it has been used widely in hyperthermia studies, but not in a prospective manner. Instead, it has been used as a way to describe a hyperthermia treatment after administration. In fact, thermal dose was not a treatment goal in any of the positive hyperthermia trials noted above. Failure to define thermal dose prospectively leads to uncertainty about the hyperthermia dose response relationship. The effect of increasing heat dose or the specifics of heat dose fractionation on tumor and/or normal tissue response is unknown. Understanding this relationship provides an opportunity for optimization of hyperthermia for cancer therapy much the same way as understanding the differences in radiation response between acute and late responding normal tissues allowed results of radiation therapy to be improved 23, 24.
In prior work we determined that a prescribed thermal dose could be delivered accurately 25. This was accomplished by visual inspection of measured temperatures, estimation of the 10th percentile temperature (T90), and adjustment of power and/or treatment time until the prescribed CEM43°CT90 dose was delivered. The ability to prospectively prescribe and deliver a thermal dose provides an opportunity to conduct trials to elucidate some aspects of the hyperthermia dose response relationship. In this paper we describe a randomized trial in canine sarcomas where thermal dose was prospectively defined and delivered with fractionated radiation therapy. Significant associations between: 1) thermal dose and duration of local tumor control, and 2) heating duration and duration of local control were found after adjusting for some important factors related to tumor characteristics (i.e., tumor volume and tumor grade).
Materials and Methods
A randomized Phase III trial of total thermal dose was conducted in pet dogs with spontaneous soft tissue sarcomas 26. Dogs were treated at the College of Veterinary Medicine at North Carolina State and Colorado State Universities. The dogs were identified from canine patients examined in the oncology clinic at these institutions and the cost of treatment was partially subsidized by the supporting grant. To be eligible for entry, the life expectancy of the dog had to be at least 1 year assuming the tumor was not present. The tumor could not be invading bone and had to be in a location where heating was technically possible. Based on physical examination and medical imaging dogs were required to be free of detectable metastasis at the time of treatment. All owners signed an informed consent and the protocols were approved by the Institutional Animal Care and Use Committee at both participating institutions.
Tumor volume was estimated by multiplying the product of three physically-measured orthogonal diameters by π/6; the maximum allowable volume was 400cm3. A biopsy from each tumor was examined to confirm the diagnosis of sarcoma. Tumors were graded as either low-, intermediate- or high grade based number of mitoses per high power field.
Dogs were randomized, if deemed “heatable”, to receive either a low (2–5 CEM43°CT90, target of 2) or high (20–50 CEM43°CT90, target of 50) cumulative thermal dose as summed over 5–10 treatments over 5 weeks. Heatability is defined below. Hyperthermia treatments were constrained to last between 12 and 120 minutes, depending on the rate of thermal dose acquisition. Based on evidence that 5 CEM43°CT90 is necessary for improvement in tumor response compared to radiation alone 21, 22, doses in this study were chosen to be above and below that value. A large difference between total doses was selected to avoid dose overlap between groups. An important distinction of this study was the exclusion of non-heatable tumors. Some tumors cannot be heated because of perfusion-related conductive cooling. If administration of the higher thermal dose was not possible, based on the rate of thermal dose acquisition during the first treatment session, the dog was not randomized. All tumors had to be capable of being heated to the high thermal dose group because heatability was determined prior to randomization.
Tumors were imaged using computed tomography (CT) and thermometry catheters were placed according to RTOG guidelines 27, 28. Hyperthermia was induced using scanning spiral or annular array microwave applicators operating between 140–433 mHz. Deionized water was used as the coupling medium. Surface cooling prevented skin temperature from exceeding 43°C. Upper temperature limits of 43°C and 48°C were placed on normal tissue and tumor, respectively. Heating and thermometry equipment at each institution was identical, having been compiled and tested by one of the authors (TVS). Training sessions had been held previously to assure that hyperthermia operators were consistent in methodology.
Temperatures were recorded along the path of the catheters by use of an automated translation device29 or by manual pullback. These temperatures were examined visually and the T90 estimated by identifying the temperature representing the 10th percentile. Continuous monitoring of T90 was used to gauge whether sufficient thermal dose was being delivered to meet the definition of heatability. Using a thermal isoeffect formula, a table of T90 vs. CEM43°CT90/hr was constructed and used to determine if the T90 was adequate to reach a minimum of 2 CEM43CT90 during the first treatment. A T90 of 40.1°C is required to deliver 2.0 CEM43°CT90 in 120 min; this rate of dose acquisition is necessary to meet the minimum thermal dose in the high dose group (20 CEM43°CT90) using 10 treatments of 120 minutes duration each. If the T90 was less than 40.1°C, the position of the applicator was adjusted. If this failed to result in a T90 of 40.1°C the tumor was deemed unheatable and the animal was not randomized. Determination of heatability was usually possible in the first 15 minutes following power application. Animals with unheatable tumors were transferred to other therapy protocols and were not considered further in this trial.
If a T90 of 40.1°C was possible, the Duke University Protocol office was telephoned during the hyperthermia procedure and the patient randomized to either the high or low dose group. Randomization was stratified by tumor volume (1–100 cm3 vs. 101–400 cm3), tumor grade (low and intermediate vs. high) and institution (CSU vs. NCSU). Once the patient was randomized the length of each hyperthermia fraction was adjusted so that approximately 20% of the desired total dose was given at each hyperthermia fraction, or until 120 minutes elapsed. If 20% of the total dose could not be given in one 2-hour treatment an additional treatment was given later that week after at least 48 hours. This strategy aimed to deliver the thermal dose in five hyperthermia treatment sessions, but allowed up to 10 sessions without a protocol violation.
Dogs were under general anesthesia for radiation and hyperthermia. Anesthesia was induced using controlled mask inhalation of isoflurane in 100% oxygen. Induction was followed by orotracheal intubation and anesthesia maintenance using isoflurane and an inspired fractional oxygen content of 1.0. All tumors were irradiated to a total dose of 56.25 Gy given in 25 daily fractions of 2.25 Gy; fractions were given Monday through Friday. Cobalt photons were used at North Carolina State University and 6MV x-rays at Colorado State University. Tumors were typically treated using parallel opposed beams with dose computed manually except for more complex anatomic locations where CT based planning was used. Hyperthermia treatments were given after the daily radiation fraction. After treatment was completed, dogs were re-evaluated at 1,2,3,5,7,9 and 12 months and then at 3-month intervals. Re-evaluation consisted of physical examination, tumor measurement, radiographs of the thorax, and assessment of regional lymph nodes. Tumor volume was measured at each follow-up as previously described. Local failure was defined as an increase in tumor volume >125% of the smallest tumor volume measured on at least two consecutive examinations. Acute thermal burn and deep tissue or third degree burns were assessed as measures of hyperthermia complications.
The main endpoint of interest was duration of local tumor control. This is defined as the time from date of the first hyperthermia treatment until local failure. Based on our prior work we estimated the median duration of local tumor control in the low dose group to be 400 days 30, 31. We wanted 80% power to detect an increase in median duration of local control to 2 years (82.5% increase) in the high dose group with a one-sided alpha of 0.05. This required 44 evaluable dogs per treatment group. To allow for possible patient dropouts and withdrawals it was planned to randomize 96 dogs. Four interim analyses were planned.
Data were entered into a database at Duke University Medical Center. Regular assessments of data entry compliance were conducted and any missing data immediately rectified. Data were prepared for analysis by the database programmer. The statistical analysis was done using the intent-to-treat approach (i.e., as randomized). The time-to-event type of endpoints, including duration of local tumor control (the main endpoint of this trial) and secondary endpoints such as event-free survival, local failure-free survival, metastasis-free survival and overall survival, were analyzed using both the Kaplan-Meier method and Cox proportional hazards regression models. The regression approach was used to allow for adjustments for some potentially important prognostic factors 32. Both point estimates such as median time to event and hazard ratio (HR) and their 95% confidence intervals (CIs) were reported. Event-free survival was defined as the time from date of the first hyperthermia treatment until local tumor recurrence, metastasis, amputation or death. Local failure-free survival was defined as the time from date of the first hyperthermia treatment until local tumor recurrence or death. Metastasis-free survival was defined as the time from date of the first hyperthermia treatment until metastasis or death while the overall survival was the time from date of the first hyperthermia treatment until death from any cause.
Observed complication rates by the thermal dose group and the corresponding odds ratio (OR) were reported. The comparison between the two randomized groups to address the second objective (that is, hyperthermia dose can be achieved safely) was done using the Fisher's exact test.
We also looked at the relationships between other hyperthermia treatment variables and the outcomes to the treatment to aid in our understanding of the dose response curve. The possibility of a different dose response relationship within each of the two thermal dose groups was examined through Cox proportional hazards models with interaction terms between the hyperthermia treatment variables and the thermal dose group indicator being included. A subset analysis of only the high thermal dose patients was explored as well.
All tests were two-sided unless otherwise noted. A p-value of 0.05 or smaller was considered statistically significant. No adjustment was made for multiple testing.
Results
A total of 134 dogs were accrued between July of 1996 and January of 2003. Seventy-five dogs were accrued from North Carolina State University and 59 from Colorado State University. Interim analyses were conducted after 65 and 83 dogs had been accrued and there was no evidence from data that supported stopping the trial prematurely at that time. Another interim analysis was conducted after 112 dogs had been accrued. At this time there appeared to be a disproportionate number of early deaths in the high dose group that were not apparently related to either the tumor or treatment. These deaths were due to intercurrent disease, e.g. pancreatitis, heart failure, second primary neoplasm. Since these deaths could decrease the power to assess treatment effect on local tumor control, it was decided to continue accrual and to weight the randomization 2:1 to the high dose group. Another interim analysis was conducted in December, 2002 and it was decided afterwards to stop the trial as the randomization goal had been met. Of the 134 dogs, 12 had unheatable tumors leaving 122 dogs for randomization. Sixty-four dogs were randomized to the high dose group and 58 to the low dose group.
Baseline characteristics were very similar between thermal dose groups (Table 1), except that there was a slight unbalance in tumor volume. Although it was unexpected, it could happen since the randomization was stratified on the categorized tumor volume (1–100 cm3 vs. 101–400 cm3), not the value of tumor volume itself. This could have some implications on statistical analyses of the trial endpoints, as an adjustment for tumor volume might be necessary to remove such unbalance between the two thermal dose arms. Overall median follow-up (95% confidence interval) was 3.0 (2.5–4.1) years while some dogs were followed for more than six years. Eighty-seven (71%) of the dogs received 5 hyperthermia treatments; 110 (90%) received between 4–6 treatments (Table 2). Note that there were 8 dogs (4 in each thermal dose group) that did not finish all planned hyperthermia treatments due to various reasons.
Table 1.
Baseline characteristics of dogs in low vs. high thermal dose group.
| Variable | Low Dose Group | High Dose Group |
|---|---|---|
| Age (yr) (mean ± SD) | 9.7±3.0 | 9.9±2.5 |
| Dogs Entered, By Institution1 | ||
| CSU | 26 | 30 |
| NCSU | 32 | 34 |
| Tumor Volume (cm3) [median (IQR2)] | 22 (12–99) | 39 (1212–79) |
| Histologic Type [n, (%)] | ||
| Sarcoma, NOS3 | 11 (19.0%) | 10 (15.6%) |
| Fibrosarcoma | 9 (15.5%) | 10 (15.6%) |
| Myxosarcoma | 4 (6.9%) | 4 (6.3%) |
| Hemangiopericytoma | 15 (25.9%) | 20 (31.3%) |
| Neurofibrosarcoma | 17 (29.3%) | 20 (31.3%) |
| Other | 2 (3.4%) | 0 |
| Tumor Site [n, (%)] | ||
| Head or oral | 2 (3.4%) | 0 |
| Trunk | 12 (20.7%) | 9 (14.1%) |
| Extremity | 44 (75.9%) | 55 (85.9%) |
| Tumor Grade [n, (%)] | ||
| Low | 37 (63.8%) | 36 (56.3%) |
| Intermediate | 14 (24.2%) | 18 (28.1%) |
| High | 7 (12.1%) | 10 (15.6%) |
| Gender [n, (%)] | ||
| Male | 3 (5.2%) | 6 (9.4%) |
| Male, neutered | 25 (43.1%) | 29 (45.3%) |
| Female | 2 (3.5%) | 1 (1.6%) |
| Female, neutered | 28 (48.3%) | 28 (43.8%) |
CSU=Colorado State University; NCSU=North Carolina State University
IQR = (1st quartile–3rd quartile).
NOS = not otherwise specified.
Table 2.
Number of heat treatments received by each dog, by treatment group and overall.
| Number of Heat Treatments | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Low Dose (N=58) | 3 | 0 | 1 | 7 | 47 | 0 |
| High Dose (N=64) | 0 | 4 | 4 | 10 | 40 | 6 |
| Overall (N=122) | 3 (2.5%) | 4 (3.3%) | 5 (4.1%) | 17 (13.9%) | 87 (71.3%) | 6 (4.9%) |
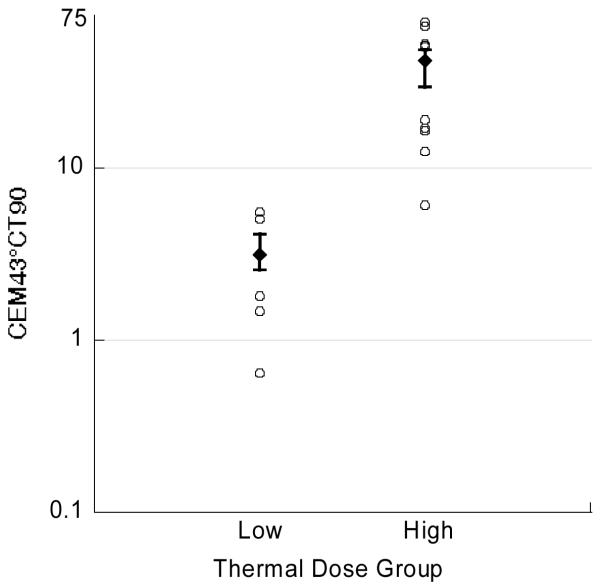
The median (interquartile range or IQR) number of temperature points was 21 (16–27). There was excellent temperature discrimination between thermal dose groups (Figure 1). The mean (standard deviation or SD) CEM43°CT90 values for the low versus high dose groups were 3.3 (1.0) and 38.6 (12.3) minutes, respectively (Table 3). There were 15 dogs with total CEM43°CT90 values outside the range specified in the protocol, 5 in the low dose group and 10 in the high dose group (Figure 1). The magnitude of this discrepancy was considered to be of unlikely significance and these dogs were analyzed as randomized (intention to treat).
Figure 1.
Median thermal dose in each thermal dose group. Error bars represent the 25th and 75th percentile. There were 5 dogs in the low dose group (3 lower and 2 higher) and 10 dogs in the high dose group (5 lower and 5 higher) whose thermal dose fell outside the prescribed range. These dogs are represented by the open circles. Note log scale on ordinate.
Table 3.
Mean thermal dose parameters for the low and high dose groups.
| Variable | Low Dose Group Mean (SD) n=58 | High Dose Group Mean (SD) n=63 | p-value (t-test) |
|---|---|---|---|
| Overall Mean T10 (°C) | 44.2 (1.6) | 46.0 (1.4) | <0.0001 |
| Overall Mean T50 (°C) | 41.7 (1.0) | 43.2 (1.0) | <0.0001 |
| Overall Mean T90 (°C) | 39.6 (0.9) | 40.5 (0.8) | <0.0001 |
| Total CEM43°CT10 (min) | 1140 (2103) | 8294 (21719) | 0.0116 |
| Total CEM43°CT50 (min) | 98 (138) | 918 (1354) | <0.0001 |
| Total CEM43°CT90 (min) | 3.3 (1.0) | 38.6 (12.3) | <0.0001 |
| Duration of 1st HT (min) | 26.2 (14.8) | 68.3 (32.9) | <0.0001 |
| Total Duration of all HTs (min) | 118 (59) | 315 (146) | <0.0001 |
Absolute temperatures in the low dose group (T10, T50, and T90) were lower than in the high dose group (Table 3). Based on Spearman correlation, there was a significant relationship between mean T90 and mean T50 in the low (Spearman correlation coefficient (SCC)=0.33; p=0.011) and high (SCC=0.40; p=0.001) dose groups. However, there was not a significant relationship between T90 and T10 in either the low (SCC=−0.05; p=0.72) or high (SCC=0.05; p=0.69) dose groups. An analysis using the Pearson correlation coefficient yielded similar results (data not shown).
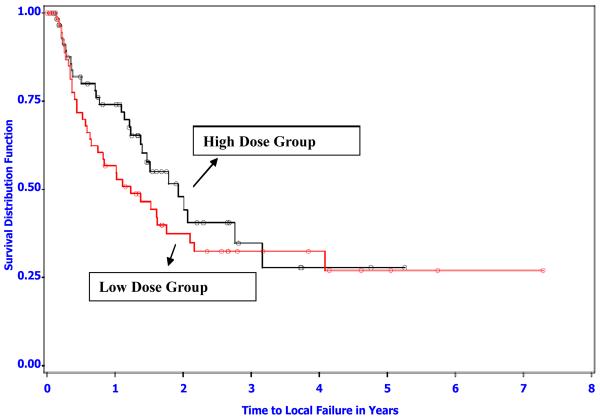
Sixty of the 122 dogs (49.2%) experienced local tumor recurrence as the first event (n=56) or as the first event in combination with another event (n=4) (Table 4). The median (95% confidence interval) time to local tumor recurrence in the low dose group was 1.2 (0.7–2.1) years versus 1.9 (1.4–3.2) years in the high dose group (log-rank p=0.28) (Figure 2). Focusing specifically at the one year follow-up time, the probability of being tumor free (95% confidence interval) was 0.57 (0.43–0.70) in the low dose group versus 0.74 (0.62–0.86) in the high dose group. The probability for either dose group is not contained in the 95% confidence interval for the other group; the time to local failure appeared to have reached the biggest difference between the two thermal dose groups around one year after entering the study (see Figure 2).
Table 4.
Enumeration of first events by treatment group and overall.
| Dose Group | N | No Event | Local Recurrence | Metastasis | Death | Local + Metastasis | Local + Death | Metastasis + Death |
|---|---|---|---|---|---|---|---|---|
| Low | 58 | 8 | 32 | 3 | 10 | 1 | 1 | 3 |
| High | 64 | 11 | 24 | 3 | 24 | 2 | 0 | 0 |
| Overall | 122 | 19 | 56 | 6 | 34 | 3 | 1 | 3 |
Figure 2.
Kaplan-Meier survival distribution function estimates of time to local failure for the low and high thermal dose groups (logrank p-value=0.28). There were 35 and 28 local occurrences in the low and high dose group, respectively. Open symbols represent censored observations (death due to any cause with no local recurrence (15 and 24 for low and high), living patients with no local occurrence (7 and 8 for low and high), and amputations including tumor site (1 and 4 for low and high)).
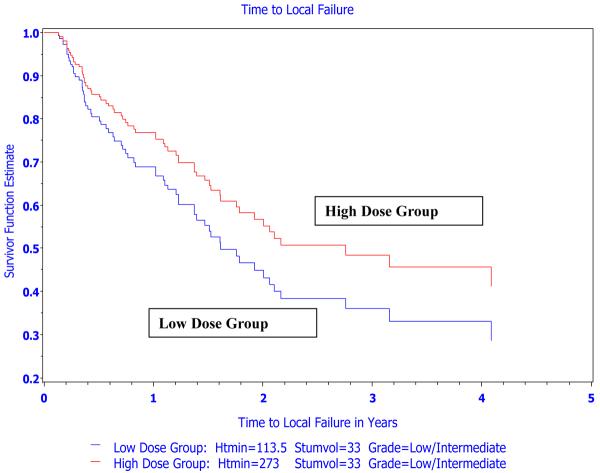
The Kaplan-Meier analysis of local tumor control did not take other potentially influential variables into consideration. Thus, univariate Cox proportional hazards regression including the following variables entered one at a time was performed: thermal dose parameters (T10, T50, T90, CEM43°CT10, CEM43°CT50, and CEM43°CT90), tumor site, histologic tumor type, tumor volume, tumor grade, year entered, and institution. Following this univariate analysis, the most predictive variables from each related category were entered into a model containing total heat duration and thermal dose group. A backward elimination approach was followed to obtain the best parsimonious model where all included variables had a p-value ≤ 0.05. Based on backward elimination, 4 variables were identified that were significantly related to duration of local tumor control: thermal dose group, total duration of heating, tumor volume and tumor grade (Table 5). A stepwise proportional hazards regression was also performed which resulted in this same parsimonious model. For heating duration and tumor volume, the hazard ratio refers to incremental changes of 30 min and 30 cm3, respectively (i.e., unit change = 30). Therefore, after correcting for heating duration, tumor volume and tumor grade, there was a statistically significant association between thermal dose group and duration of local tumor control (Figure 3; hazard ratio=2.3 for low vs. high thermal dose groups, 95% CI: 1.1–4.6, p-value=0.023). This finding supports the hypothesis that thermal dose group, quantified using CEM43°CT90, had a biologic relationship to local tumor control when hyperthermia is combined with radiation.
Table 5.
Variables significantly related to duration of local tumor control in a final parsimonious Cox multivariable proportional hazards regression model following backward elimination. The hazard ratio, its 95% confidence interval (CI) for group comparison or a meaningful increment in a continuous predictor, and p-value for testing hazard ratio=1 are reported.
| Predictor | Unit Change | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|---|
| Thermal Dose Group | Low vs. High | 2.28 | 1.12–4.64 | 0.023 |
| Total Duration of Heating (min; continuous) | 30 | 1.09 | 1.02–1.17 | 0.008 |
| Tumor volume (cm3; continuous) | 30 | 1.09 | 1.00–1.19 | 0.041 |
| Tumor Grade | High vs. Low/Intermediate | 2.19 | 1.09–4.37 | 0.027 |
Figure 3.
Estimated survival distribution functions of time to local failure for a “typical” dog in the low and high thermal dose groups from the Cox proportional hazards model. There is a significant association between thermal dose group and time to local failure after controlling for total duration of heating, tumor volume and tumor grade (hazard ratio of low vs. high=2.28 (95% CI: 1.12–4.64); p-value=0.023). Duration of heating and tumor volume values used in the estimation of survival fuctions were median values for the respective group and overall, respectively. Htmin=total duration of heat treatment; median duration of heating in the thermal dose group was used in the plot. Stumvol=median tumor volume over all dogs in trial.
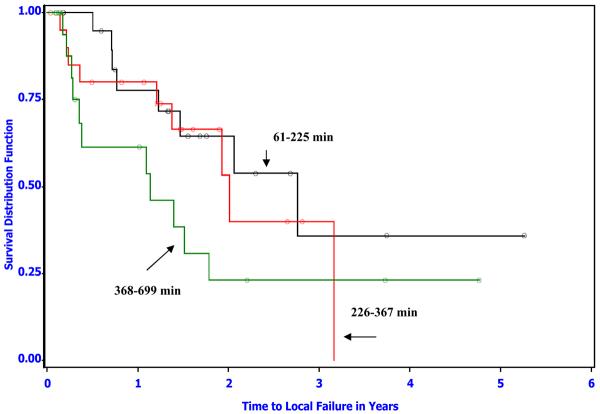
Tumor grade, tumor volume and overall duration of hyperthermia were also related to duration of local tumor control. The finding of an effect of tumor volume and grade were expected as these have been identified previously as significant predictors of outcome in canine sarcomas 8, 20, 31, 33. The mean total duration of heating (SD) in the low versus high dose groups were 118 (59) minutes versus 315 (146) minutes respectively. Finding an association between total duration of heating and duration of local tumor control was unexpected, especially considering the direction of the association was opposite to what might be expected intuitively (Table 5; hazard ratio=1.1 for a 30-minute increase in the total duration of heating, 95% CI: 1.02–1.17, p-value=0.008). In other words, increasing duration of hyperthermia within each thermal dose group was associated with decreasing duration of local tumor control (Figure 4). However, based on the estimated hazard ratios, the strength of this association was not as great as for thermal dose group (Table 5). That is, there was a significant overall hyperthermia thermal dose effect in spite of the negative effect of the total duration of heating.
Figure 4.
Kaplan-Meier survival distribution function estimates of time to local failure for dogs in the high thermal dose group. Total heating duration was divided into thirds for the analysis (n=21 dogs each curve). The longest heating duration is associated with shorter duration of tumor control. The number of local recurrences was 8, 9, and 11 while the median time to local failure was 2.8, 2.0 and 1.1 years for the three heating duration groups in increasing order, respectively.
The endpoints of event-free survival, overall survival, local failure-free survival and metastasis free survival were also examined. We did not find an association between thermal dose group and any of these endpoints. However, the association between these endpoints and overall duration of heating, tumor volume and tumor grade remained significant (data not shown).
With regard to toxicity, 19 (30%) of dogs in the high dose group experienced some form of acute toxicity compared to 8 (14%) of dogs in the low dose group (odds ratio=2.6; Fisher exact p=0.05). None of these acute toxicities were serious. The incidence of deep tissue burn or third-degree burn was not significantly different between the high and low thermal dose groups, 9 (14%) vs. 4 (7.0%) dogs respectively (odds ratio=2.2; Fisher exact p=0.25). Some of the deep burns were serious and required daily bandage change and/or surgical debridment.
Discussion
This is the first prospective hyperthermia clinical trial where a significant association between prescribed thermal dose, quantified as CEM43°CT90, and duration of local tumor control has been identified. When correcting for tumor volume, tumor grade and overall duration of heating, dogs in the low dose group were 2.3 times as likely to experience local failure as dogs in the high dose group. The results of this trial are relevant to future hyperthermia trials. In most trials conducted to date, thermal dose has not been defined. Energy has been applied with the goal of “heating” the tumor and little to no attention has been given prospectively to administering a specified thermal dose or thermal prescription. Our results are important in that they provide additional support for the value of hyperthermia in combination with radiation for some tumors, but the results also make it important to consider thermal dose a priori rather than a posteriori.
Importantly, only tumors where the prescribed thermal dose could be delivered were included in the trial. This is different from nearly all other hyperthermia trials wherein nonheatable tumors have been included. Inclusion of nonheatable tumors in a hyperthermia trial will dilute the power to detect a beneficial hyperthermia effect. Also, the finding of a thermal dose effect in this study suggests that heatability should be determined in all clinical hyperthermia settings. It is of questionable value to invest the cost and effort of administering clinical hyperthermia, from both hospital and patient perspectives, to patients with tumors where a prospectively determined thermal dose cannot be delivered.
We did not find a thermal dose group effect on local tumor control in a Kaplan-Meier analysis (Figure 2), although the difference in local tumor control at 1 year between thermal dose groups is notable. However, a thermal dose effect was identified in a multivariable proportional hazards regression analysis where the effect of other variables on local tumor control could be considered. In that analysis, thermal dose group was significantly associated with duration of local tumor control, along with grade, volume and total duration of heating. We note that there are some limitations associated with the time to local tumor control endpoint due to the competing risks issue. Competing risks were introduced because dogs who died or whose tumor site was amputated before local failure were censored for the endpoint of duration of local tumor control. When this type of censoring is relatively heavy, as in this study (16 dogs in the low and 28 in the high dose group for a total of 44 dogs or 36%), and could be related to the endpoint in question and censor subjects in the two groups in a non-independent way, the standard survival data analysis methodology would no longer produce an unbiased and/or efficient estimation and inference results. However, time to local control is an endpoint routinely used in radiation related cancer clinical trials. Thus, other endpoints, such as overall survival, local failure-free survival, and event-free survival may be preferable and were also evaluated. Though we did not find an association between thermal dose group and those endpoints this does not diminish the significance of the results of the local control analysis.
In some settings the potential complications associated with placement of thermometry catheters has been argued to outweigh their value 34. In that study it is important to note the thermometry catheters were not removed between hyperthermia fractions. These complications, however, may prompt some to avoid the use of invasive thermometry completely. With the results of this trial, and another in humans with superficial tumors conducted at Duke University Medical Center where thermal dose was applied prospectively and a positive association with local tumor control was found 35, there is no rationale for failing to measure temperature during clinical hyperthermia when it is combined with radiation. Temperature measurement can be used to guide delivery of a prescribed dose and also to avoid administration of hyperthermia to patients with unheatable tumors. The development of magnetic resonance based thermometry will eliminate the invasiveness of measuring intratumoral temperatures during hyperthermia but until noninvasive MR thermometry is ready for clinical use the role of invasive thermometry cannot be dismissed36, 37.
We acknowledge that as a result of variation in spatial resolution of intratumoral temperature there may be some uncertainty in quantifying thermal dose based on invasive measurements. In this trial, temperature was measured at a relatively large number of points and this would tend to reduce such uncertainty. However, until non-invasive thermometry becomes a clinical reality it will not be possible to quantify the complete temperature distribution accurately. Spatial distribution of temperatures could have an impact on the biologic and physiologic effects of hyperthermia. The fact that a relationship between CEM43°CT90 and local control duration was found using invasive thermometry suggests this temperature descriptor may be even more robust in the setting of noninvasive thermometry.
The difference in absolute temperature (T10, T50, and T90) between thermal dose groups (Table 3) was not planned. This difference likely resulted from more conservative heating in the low dose group to avoid giving the entire prescribed dose in 1 or 2 hyperthermia treatments. These temperature differences are not complicating factors regarding interpretation of the results of this trial. The finding of an inverse relationship between overall duration of heating and time to local control, and other survival-related endpoints, was also unexpected. Based on relative risk values (Table 4), thermal dose group had more influence on duration of local tumor control than did total duration of heating. However, the finding of an effect of total duration of heating independent of thermal dose group (Fig 4) is provocative and suggests that physiologic alterations created by heating, e.g. perfusion, oxygen consumption, interstitial pressure, may be influential in determining tumor response following thermoradiotherapy combinations.
The effect of heating on tumor oxygenation is of particular interest. In some human trials where oxygen has been measured before and after tumor heating, increases in oxygenation have been documented 38, 39. But, a decrease in tumor oxygenation at higher temperatures has also been documented 40, 41. Thus it is likely there is a point of diminishing return for the beneficial oxygenation effects of hyperthermia at higher temperatures. For example, consider only dogs in the high dose group (low dose dogs would be similar). Dogs in the high dose group received a median thermal dose of 41.7 CEM43°CT90. The time required to deliver this dose varied considerably; 25th and 75th percentile total heating times were 203 and 381 minutes, respectively – nearly a factor of 2 difference. Longer heating time would be required when it is difficult to achieve a high T90 (due to regions of high perfusion for example). As noted above, based on Spearman and Pearson correlation, there was not a significant positive relationship between T90 and T10 within thermal dose groups. This means that dogs with a lower T90 (as might occur due to high perfusion) could have very high T10 values. Because of the lower T90, heating time in these dogs would be prolonged subjecting the tumor to the potentially deleterious effects of the higher temperatures, such as a decrease in oxygenation.
There are other data to support this hypothesis. In a previously published canine trial, high intratumoral temperature ranges (low T90, high T10 for example) were associated with a complete response rate of 18%. When the temperature range was smaller, the complete response rate was 87.5% 20. Though the canine Phase III trial described herein was not designed to assess the effect of heating duration, its identification is compelling evidence in support of investigating the physiologic consequences of how a therapeutic thermal dose is fractionated. The problem with available data on hyperthermia effects on oxygenation is that they are only snapshots in time. What is badly needed is a more complete understanding of the temporal effects of hyperthermia not only on oxygenation but on parameters such as interstitial fluid pressure 42, and gene and cytokine expression 43, 44 throughout the course of treatment.
A prescribed cumulative CEM43°CT90 may be achieved by adjustment of T90, the number of hyperthermia fractions and/or the duration of each hyperthermia fraction. Results from this trial suggest that how these parameters are adjusted to reach a cumulative thermal dose will influence the biologic effect of that thermal dose. Based on prior findings of decreased tumor oxygenation at higher temperatures 39–41 and our finding of an inverse association between total duration of heating and local control duration, adjustment of fractionation of the hyperthermia dose may be extremely important with regard to optimization of the hyperthermia effect.
With regard to toxicity from hyperthermia, 13 of 122 (11%) dogs in this trial experienced serious thermal toxicity. Serious toxicity was more common to the high dose group (9 vs. 4) but the difference was not statistically significant (p=0.25), as this trial was not designed to test such a small toxicity rate difference. Nevertheless, it is logical to assume that using higher temperatures will be associated with a greater probability of complications. This relationship between temperature and complications is another reason to investigate other fractionation schemes.
In summary, the main result of the phase III study was improved local control at 1 year between high and low thermal dose but no overall difference in local control with time based on the primary analysis. However, upon multivariable regression analysis there was improved local control in animals receiving high thermal dose vs. low thermal dose. Finally, local control was diminished for animals receiving protracted heating times. These data add additional support to the clinical use of hyperthermia in conjunction with radiation therapy. Importantly, they justify continued use of invasive thermometry until non-invasive thermometry becomes available. Further, they point to the need to improve our knowledge of the physiologic effects of hyperthermia treatment with different fractionation schemes.
Acknowledgments
Supported by Grant PO1CA42745 from the Department of Health and Human Services, National Institutes of Health
References
- 1.Datta N, Bose A, Keppor H, Gupta S. Head and neck cancers: results of thermoradiotherapy versus radiotherapy. Int J Hyperthermia. 1990;6:479–486. doi: 10.3109/02656739009140944. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard J, Gonzalez Gonzalez D, Hushof M, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet. 1995;345:540–543. doi: 10.1016/s0140-6736(95)90463-8. [DOI] [PubMed] [Google Scholar]
- 3.Sneed P, Stauffer P, McDermott M, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/- hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:287–295. doi: 10.1016/s0360-3016(97)00731-1. [DOI] [PubMed] [Google Scholar]
- 4.Sugimachi K, Kuwano H, Ide H, Toge T, Saku M, Oshiumi Y. Chemotherapy combined with or without hyperthermia for patients with oesophageal carcinoma: a prospective randomized trial. Int J Hyperthermia. 1994;10:485–493. doi: 10.3109/02656739409009352. [DOI] [PubMed] [Google Scholar]
- 5.Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys. 1994;28:163–169. doi: 10.1016/0360-3016(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 6.van der Zee J, Gonzalez Gonzalez D, van Rhoon G, van Dijk J, van Putten W, Hart A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355:1119–1125. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 7.Vernon C, Hand J, Field S, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys. 1996;35:731–744. doi: 10.1016/0360-3016(96)00154-x. [DOI] [PubMed] [Google Scholar]
- 8.Dewhirst M, Sim D, Sapareto S, Connor W. Importance of minimum tumor temperature in determining early and long-term responses of spontaneous canine and feline tumors to heat and radiation. Cancer Res. 1984;44:43–50. [PubMed] [Google Scholar]
- 9.McChesney Gillette S, Dewhirst M, Gillette E, et al. Response of canine soft tissue sarcomas to radiation or radiation plus hyperthermia: a randomized phase II study. Int J Hyperthermia. 1992;8:309–320. doi: 10.3109/02656739209021786. [DOI] [PubMed] [Google Scholar]
- 10.Gillette E, McChesney S, Dewhirst M, Scott R. Response of canine oral carcinomas to heat and radiation. Int J Radiat Oncol Biol Phys. 1987;13:1861–1867. doi: 10.1016/0360-3016(87)90353-1. [DOI] [PubMed] [Google Scholar]
- 11.Gelvich E, Maxokhin V. Resonance effects in applicator water boluses and their influence on SAR distribution patterns. Int J Hyperthermia. 2000;16:113–128. doi: 10.1080/026567300285321. [DOI] [PubMed] [Google Scholar]
- 12.Weinbaum S, Jiji M. A new simplified bioheat equation for the effect of blood flow on local average tissue temperature. J Biomech Eng. 1985;107:131–139. doi: 10.1115/1.3138533. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Roemer R. The effects of large blood vessels on temperature distritbutions during simulated hyperthermia. J Biomech Eng. 1992;114:473–481. doi: 10.1115/1.2894097. [DOI] [PubMed] [Google Scholar]
- 14.Acker J, Dewhirst M, Honore G, Samulski T, Tucker J, Oleson J. Blood perfusion measurements in human tumours: evaluation of laser Doppler methods. Int J Hyperthermia. 1990;6:287–304. doi: 10.3109/02656739009141139. [DOI] [PubMed] [Google Scholar]
- 15.Dewey W, Hopwood L, Sapareto S, Gerweck L. Cellular responses to combinations of hyperthermia and radiation. Radiology. 1977;123:463–474. doi: 10.1148/123.2.463. [DOI] [PubMed] [Google Scholar]
- 16.Hand J, Machin D, Vernon C, Whaley J. Analysis of thermal parameters obtained during Phase III trials of hyperthermia as an adjunct to radiotherapy in the treatment of breast carcinoma. Int J Hyperthermia. 1997;13:343–364. doi: 10.3109/02656739709046538. [DOI] [PubMed] [Google Scholar]
- 17.Leopold K, Dewhirst M, Samulski T, et al. Relationships among tumor temperature, treatment time, and histopathological outcome using preoperative hyperthermia with radiation in soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1992;22:989–998. doi: 10.1016/0360-3016(92)90798-m. [DOI] [PubMed] [Google Scholar]
- 18.Leopold K, Dewhirst M, Samulski T, et al. Cumulative minutes with T90 greater than Tempindex is predictive of response to hyperthermia and radiation. Int J Radiat Oncol Biol Phys. 1993;25:841–847. doi: 10.1016/0360-3016(93)90314-l. [DOI] [PubMed] [Google Scholar]
- 19.Sapareto S, Dewey W. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 20.Dewhirst M, Sim D. The utility of thermal dose as a predictor of tumor and normal tissue response to combined radiation and hyperthermia. Cancer Res. 1984;44:4772–4780. [PubMed] [Google Scholar]
- 21.Sneed P, Gutin P, Stauffer P, et al. Thermoradiotherapy of recurrent malignant brain tumors. Int J Radiat Oncol Biol Phys. 1992;23:853–861. doi: 10.1016/0360-3016(92)90659-6. [DOI] [PubMed] [Google Scholar]
- 22.Oleson J, Samulski T, Leopold K, et al. Sensitivity of hyperthermia trial outcomes to temperatures and time: implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys. 1993;15:289–297. doi: 10.1016/0360-3016(93)90351-u. [DOI] [PubMed] [Google Scholar]
- 23.Fu K, Pajak T, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 24.Withers H, HD TJ, Peters L. A new isoeffect curve for change in dose per fraction. Radiother Oncol. 1983;1:187–191. doi: 10.1016/s0167-8140(83)80021-8. [DOI] [PubMed] [Google Scholar]
- 25.Thrall D, Rosner G, Azuma C, et al. Using units of CEM 43C T90, local hyperthermia thermal dose can be delivered as prescribed. Int J Hyperthermia. 2000;16:415–428. doi: 10.1080/026567300416712. [DOI] [PubMed] [Google Scholar]
- 26.Page R, Thrall D. Soft Tissue Sarcomas and Hemangiosarcomas. In: Ettinger S, Feldman E, editors. Textbook of Veterinary Internal Medicine. 5 ed. W.B. Saunders Co.; Philadelphia: 2000. pp. 529–535. [Google Scholar]
- 27.Sapozink M, Corry P, Kapp D, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia for deep-seated malignancy. Int J Radiat Oncol Biol Phys. 1991;20:1109–1115. doi: 10.1016/0360-3016(91)90212-m. [DOI] [PubMed] [Google Scholar]
- 28.Dewhirst M, Phillips T, Samulski T, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia. Int J Radiat Oncol Biol Phys. 1990;18:1249–1259. doi: 10.1016/0360-3016(90)90466-w. [DOI] [PubMed] [Google Scholar]
- 29.Engler M, Dewhirst M, Winget J, Oleson J. Automated temperature scanning for hyperthermia treatment monitoring. Int J Radiat Oncol Biol Phys. 1987;13:1377–1382. doi: 10.1016/0360-3016(87)90233-1. [DOI] [PubMed] [Google Scholar]
- 30.McChesney S, Gillette E, Dewhirst M, Withrow S. Influence of WR 2721 on radiation response of canine soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1986;12:1957–1963. doi: 10.1016/0360-3016(86)90132-x. [DOI] [PubMed] [Google Scholar]
- 31.Thrall D, Prescott D, Samulski T, et al. Radiation plus local hyperthermia versus radiation plus the combination of local and whole body hyperthermia in canine sarcomas. Int J Radiat Oncol Biol Phys. 1996;34:1087–1096. doi: 10.1016/0360-3016(95)02260-0. [DOI] [PubMed] [Google Scholar]
- 32.Cox D, Oakes . Analysis of Survival Data. Chapman and Hall; London: 1984. [Google Scholar]
- 33.Kuntz C, Dernell W, Powers B, Devitt C, Straw R, Withrow S. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986–1996) J Am Vet Med Assoc. 1997;211:1147–1151. [PubMed] [Google Scholar]
- 34.van der Zee J, Peer-Valstar J, Rietveld P, de Graaf-Strukowska L, van Rhoon G. Practical limitations of interstitial thermometry during deep hyperthermia. Int J Radiat Oncol Biol Phys. 1998;40(I):1015–1017. doi: 10.1016/s0360-3016(98)00008-x. [DOI] [PubMed] [Google Scholar]
- 35.Jones E, Dewhirst M, Vujaskovic Z, et al. Hyperthermia improves the complete response rate for superficial tumors treated with radiation: results of a prospective randomized trial testing the thermal dose parameter CEM43T90. Int J Radiat Oncol Biol Phys. 2003;57:s253–s254. [Google Scholar]
- 36.Samulski T, MacFall J, Zhang Y, Grant W, Charles C. Non-invasive thermometry using magnetic resonance diffusion imaging: potential for application in hyperthermic oncology. Int J Hyperthermia. 1992;8:819–829. doi: 10.3109/02656739209005029. [DOI] [PubMed] [Google Scholar]
- 37.MacFall J, Prescott D, Charles H, Samulski T. 1H MRI phase thermometry in vivo in canine brain, muscle, and tumor tissue. Med Phys. 1996;23:1775–1782. doi: 10.1118/1.597760. [DOI] [PubMed] [Google Scholar]
- 38.Brizel D, Scully S, Harrelson J, et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res. 1996;56:5347–5350. [PubMed] [Google Scholar]
- 39.Jones E, Prosnitz L, Dewhirst M, et al. Thermochemoradiotherapy Improves Oxygenation in Locally Advanced Breast Cancer. Clin Cancer Res. 2004;10:4287–4293. doi: 10.1158/1078-0432.CCR-04-0133. [DOI] [PubMed] [Google Scholar]
- 40.Vujaskovic Z, Song C. Physiological mechanisms underlying heat-induced radiosensitization. Int J Hyperthermia. 2004;20:163–174. doi: 10.1080/02656730310001619514. [DOI] [PubMed] [Google Scholar]
- 41.Vujaskovic Z, Poulson J, Gaskin A, et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys. 2000;46:179–185. doi: 10.1016/s0360-3016(99)00362-4. [DOI] [PubMed] [Google Scholar]
- 42.Milosevic M, Fyles A, Wong R, et al. Interstitial fluid pressure in cervix cancer: within-tumor heterogeneity and relationship to oxygen tension. Cancer (Phila) 1998;82:2418–2426. doi: 10.1002/(sici)1097-0142(19980615)82:12<2418::aid-cncr16>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 43.Le Q, Denko N, Giaccia A. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- 44.Stein I, Neeman M, Shweiki D, Itin A, Keshet E. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulaton with other ischemia-induced genes. Molec Cell Biol. 1995;15:5363–5368. doi: 10.1128/mcb.15.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]