Abstract
Geographic variation in species interactions can have major effects on distributions. Effects of such variation can be particularly evident for invasive species, in which variation in competitive ability can influence invasive success and impacts. We tested the hypothesis that coexistence or exclusion of the resident mosquito Aedes aegypti results from variation among local populations of the invasive Aedes albopictus in competitive interactions with A. aegypti. We also examined the role of variation in fecundity-size relationship in these competitive interactions. We compared competitive abilities of nine North American populations of A. albopictus, three populations from each of three site types: extinction of A. aegypti following A. albopictus invasion, coexistence following A. albopictus invasion, and A. albopictus allopatric to A. aegypti. Competition among larvae from each A. albopictus population and a single A. aegypti population was tested in laboratory microcosms in a response-surface design. We found interpopulation differences in competitive ability of A. albopictus, but no strong patterns among site types. Extinction sites had steeper average fecundity-size relationships than coexistence sites and allopatric sites, but this did not translate into superior population performance. Certain individual A. albopictus populations had exceptionally large competitive effects on A. aegypti or poor competitive responses to competition from A. aegypti, but competitive effect and response were not correlated. These results suggest that interpopulation variation in the competitive ability of A. albopictus may only partly explain geographic pattern of coexistence with or extinction of A. aegypti. Environmental differences among regions may affect the competitive ability of A. albopictus and influence its invasion success and impact.
Keywords: Competitive asymmetry, Competitive hierarchies, Fecundity-size relationship, Invasive species, Reproductive tactics
INTRODUCTION
Determining the strength, nature, and causes of intraspecific variation remains a important question for population and community ecology (Travis 1996, Keller and Taylor 2008). Geographic heterogeneity in both abiotic conditions and biotic interactions are likely to create a mosaic of local selection that, along with genetic variation derived from founder effects and gene flow, shape the spatial distribution of traits within a species (Keller and Taylor 2008). Geographic variation in species interactions can be a major cause of geographic patterns in the diversity of genotypes and phenotypes (Travis 1996, Gómez-Mestre and Tejedo 2002). Despite these important effects of geographic variation of species interactions, most empirical studies of species interactions have been restricted to documenting the presence of species interactions as important factors for local populations (Travis 1996).
Geographic variation in competitive ability is particularly interesting in the context of biological invasions (Sakai et al. 2001, Keller and Taylor 2008). Differentiation of competitive ability is a prominent hypothesis for how invasive species vary between native and introduced ranges (the Evolution of Increased Competitive Ability Hypothesis – Siemann and Rogers 2001). Less discussed is the hypothesis of local differentiation of competitive ability within the introduced range of a species, either in response to the different biotic environments encountered by a wide-spread invader (Siemann and Rogers 2001, Sakai et al. 2001) or because of the introduction of heterogeneous genotypes with different competitive abilities (Abbott et al. 2007, Keller and Taylor 2008). The outcome of local competition may play an important role in the invasion success of many species into occupied niches (e.g., Byers 2000, Yasuda et al. 2004, Duyck et al. 2006). Impacts of invasive species may vary across their introduced range from limited effects on natives to competitive displacement of natives (Juliano et al. 2004, Abbott et al. 2007). Investigations of the causes of such variation are of basic interest, as they provide an opportunity to understand how intraspecific variation in species interactions arises (Keller and Taylor 2008), and of potential practical importance, as understanding that variation may aid in forecasting where and when invasive species impacts will be most severe (Abbott et al. 2007). Ideal model systems for such investigations involve wide spread, well studied invaders that vary in their degree of contact with and impact on native species, from no contact, through competitive exclusion of natives, to coexistence with natives.
In the mid-1980s the Asian Tiger mosquito, Aedes albopictus, invaded the continental United States from Japan. Since then, it has spread rapidly in the southeastern United States and become one of the most common human-biting mosquitoes in its new range (Lounibos 2002). The spread of A. albopictus has been associated with a decline, sometimes to local extinction, of a closely related species, Aedes aegypti (O'Meara et al. 1995). Aedes aegypti originated in tropical Africa and probably invaded the Americas in the 16th century (Lounibos 2002). Most field (Juliano 1998, Braks et al. 2004) and laboratory (e.g., Barrera 1996, Murrell and Juliano 2008) competition experiments have shown that A. albopictus are often superior in competition for resources with A. aegypti in water-filled containers. These experiments have typically only involved the effects of competition on the immature stages, ignoring effects that may be expressed in resulting adults (e.g., Costanzo et al. 2005, Leisnham et al. 2008). Despite an apparent competitive advantage for North American A. albopictus, A. aegypti remains dominant in some areas in the southern USA, particularly in urban and southern sites in the Florida peninsula (O'Meara et al. 1995).
At least two hypotheses could account for the observation that A. albopictus is typically superior in competition to A. aegypti in experiments involving only the immature stages, yet A. aegypti coexists with A. albopictus at some sites. One hypothesis is that ecological context, including abiotic or biotic environments, differs among sites, with conditions at some sites favoring A. aegypti (Juliano et al. 2002, 2004; Costanzo et al. 2005). For example, Juliano et al. (2002) found that, in Florida, occupancy of containers was greater for A. aegypti but lower for A. albopictus at warm sites with little winter rainfall compared to relatively cool sites with greater winter rain. The second hypothesis, which we address in this paper, is that local populations of these Aedes differ in their competitive characteristics. This hypothesis has received little attention despite prior studies showing geographic variation in other life history traits of A. albopictus, such incidence of diapausing eggs (Lounibos et al. 2003) and larval growth rate (Armbruster and Conn 2006).
At the same time A. albopictus invaded the United States, A. albopictus originating in tropical Asia (Birungi and Munstermann 2002) invaded, established, and spread in Brazil (Lounibos 2002). Field competition experiments showed that A. albopictus from Brazil were also superior in competition for resources with A. aegypti (Braks et al., 2004), producing effects on A. aegypti that were strikingly similar to those observed for North American A. albopictus (Juliano 1998), and suggesting that competitive ability of A. albopictus from different continental regions (North America vs. Brazil) is broadly similar. However, both of these investigations focused only on differences in the responses of immatures to competitive interactions. Only Black et al. (1989) has evaluated interpopulation variation in the competitive ability of A. albopictus, finding differences in survival among populations. No experiments have compared the competitive abilities of different populations of A. albopictus from sites with different histories of interaction with A. aegypti (i.e., no contact with A. aegypti, A. aegypti extinct since A. albopictus arrival, A. aegypti coexists with A. albopictus). Thus, the role of geographic variation in the competitive ability of A. albopictus in determining coexistence with vs. exclusion of A. aegypti in the Americas remains unknown.
Experimental comparisons of competitive abilities are ideally based on competitive effects on and responses of per capita rate of change (r) (Goldberg and Fleetwood 1987). In competition experiments involving mosquitoes, population performance can be estimated by combining demographic data on survivorship, development time, and size (as a fecundity surrogate), into a composite index of population performance (Livdahl and Sugihara 1984, Juliano 1998). However, a problem with the composite index approach arises when investigators wish to compare different populations for competitive ability, but assume no interpopulation variation in the fecundity-size relationship of a species, which is used to estimate reproductive output in demographic models (Livdahl and Sugihara 1984). Such variation is well known for insects (e.g., Hatle et al. 2002) including A. albopictus (Leisnham et al. 2008), and could be an important contributor to geographic variation in competitive ability.
In this paper, we test the hypothesis that variation in the outcome of A. albopictus invasion (i.e., coexistence vs. exclusion) results from variation among local populations of A. albopictus in competitive interactions with A. aegypti. We explicitly evaluate the role of adult reproductive tactics in these competitive outcomes. We compare the competitive abilities of nine North American populations of A. albopictus with known population histories of invasion and coexistence with A. aegypti. As in previous studies examining geographic variation in species interactions (e.g., Black et al. 1989), we compare populations under a single set of environmental conditions and thus reflect underlying genotypic variation.
To measure the absolute magnitude of heterospecific and conspecific competition, we employed a response surface design (Goldberg and Scheiner 2001), in which regression slopes of population performance vs. hetrospecific and conspecific densities quantify per capita competitive effect and response to interspecific and intraspecific competition, respectively (Goldberg and Fleetwood 1987). We predict that A. albopictus from sites where A. aegypti have gone extinct since A. albopictus arrival (extinction) will have greater competitive effect, or better competitive response, than will A. albopictus from sites where A. aegypti coexist with A. albopictus (coexistence), or from sites where A. aegypti has never occurred (allopatric).
MATERIALS AND METHODS
Collection and maintenance of mosquitoes
Aedes albopictus larvae were collected from nine geographic populations in the eastern United Sates (Appendix A), three populations from each of three site types: extinction of A. aegypti following A. albopictus invasion, coexistence of the two species, and A. albopictus allopatric to A. aegypti. At extinction sites (Bartow, FL., Fort Denaud, FL., and Gainesville, FL.), A. albopictus became established in the early 1990s, and its arrival was associated with a decline and extinction of local A. aegypti in 1990 in Gainesville, 1992 in Bartow, and 1993 in Ft. Denaud as recorded by regular site surveys (O' Meara unpublished data, Juliano et al. 2004). At coexistence sites (Daytona, FL., Fort Myers, FL., and Tampa, FL.), A. albopictus has been established since the early 1990s, yet A. aegypti remains present. Among the allopatric sites, A. albopictus has been established in East St. Louis, IL since the mid 1980s (Hawley 1988), in Bloomington, IN since the early 1990s, and in Washington DC since the late 1990s. Allopatric populations are at latitudes beyond the permanent geographic range of A. aegypti (Darsie and Ward 2005), and therefore likely to have had no contact with A. aegypti since their arrival. All A. albopictus populations originated from artificial containers, in urban or suburban areas, where encounters with other Aedes species (e.g., the native treehole Aedes triseriatus) were relatively rare. Collections sometimes yielded Culex spp., but these were present at coexistence, exclusion, and allopatric sites. Aedes aegypti for this experiment were collected as larvae from a single population in Miami, FL, where A. albopictus is locally absent (unpublished data).
Field collected larvae of A. albopictus and A. aegypti were reared to adulthood at 26°C at 16:8 (L:D) h photoperiod and then released into 0.6-m3 cages. Adults were kept at 26°C and 75% RH at 17:7 (L:D) h photoperiod with a graduated dawn-dusk period. Adults had continuous access to 20% sugar solution. Females were regularly fed anesthetized guinea pigs and laid eggs on paper towels in water-filled cups. Individuals from these eggs were used in the experiment.
Experiment 1: Competition
The experiment had a replicated, blocked design with within-block replication of each site type. Egg availability prevented us from replicating each population in every block. For each block, eggs of both species were hatched synchronously in a solution of 0.44 g nutrient broth per 1 L deionized (DI) water. Within 24 h, larvae were rinsed and transferred into the experiment. The experiment consisted of the following initial combinations of larvae (A. albopictus: A. aegypti): 10:0, 20:0, 40:0, 10:10, 20:20, 10:30, 30:10, 0:10, 0:20, and 0:40 to create a response surface design (Goldberg and Scheiner 2001). Each combination was replicated three times for each of the nine A. albopictus populations, yielding 189 experiment units with A. albopictus, 189 experimental units with A. aegypti, and 270 total experimental units (400-ml cups containing 350 ml DI water and provisioned with 0.70 g of dried senescent live oak (Quercus virginiana) leaves). Cups were set up four days prior to the addition of larvae to allow microbial communities to establish. On days 14, 28, 42, and 56 after the start of each replicate, 0.70 g of additional dried live oak was added to each cup to avoid complete resource depletion and to mimic the natural condition of continuing resource inputs to containers. One cup containing high density A. albopictus (40:0) was lost.
The experiment was housed in an environmental chamber at 28 °C and 14:10 (L:D) h photoperiod to approximate summer climate and photoperiod conditions at all sites. Treatments were randomly assigned cups and cup position was shuffled daily. Each day we collected pupae into individual vials and held them until adult emergence. Adults were killed by drying (24 h, 50°C) and females were weighed and their wing lengths were measured. Dry mass and wing length of adult females were highly correlated (A. albopictus:, r2 = 0.760, n = 1127, P < 0.0001; A. aegypti: r2 = 0.744, n = 766, P < 0.0001), and wing length was used as a measure of female size to estimate fecundity of A. albopictus and A. aegypti (see below).
For each cup, proportion survivorship to adulthood (both sexes), mean female dry mass, and mean female wing length were recorded. Daily eclosion of females and their wing lengths were used to calculate λ’, a composite index of population finite rate of increase based on r’, which estimates the realized per capita rate of population change (dN/N dt = r, the exponential growth rate) for each replicate cohort (Livdahl and Sugihara 1984; details in Appendix B).
Experiment 2: Fecundity – size relationships
We used a regression equation relating female wing length to fecundity for A. aegypti: f(wx) = 0.5 [− 8.616 + 2.50(wx 3)] (r2 = 0.875, n = 206, P < 0.001, Briegel 1990), where wx is female wing length. To test differences in the relationship between fecundity and body size in A. albopictus, A. albopictus larvae from each colony were reared to adulthood in the laboratory. As adults eclosed they were placed in 20-L nylon screen cages and within 5–10 days were fed to repletion from an anaesthetized mouse, then isolated in 600 ml containers with a 40 ml cup of water lined with paper towel for oviposition. Eggs were counted and the mean dry mass determined for 10 randomly chosen eggs from each female. Eggs were weighed in groups of 5–10, to 0.1 µg. After oviposition, all females were killed, dissected, and numbers of mature eggs (stages 4 and 5, Detinova 1962) in their ovaries counted. Fecundity was calculated by adding laid and unlaid mature eggs. Wings of all females were removed and measured. A total 318 females (24–43 for each population) entered the experiment. Killing and dissecting females after the first gonotrophic cycle is consistent with most prior studies that have examined the fecundity of A. albopictus (e.g., Armbruster and Hutchinson 2002). Data on the parity of wild A. albopictus females suggest that the average female matures but one batch of eggs (Hawley 1988).
Data analyses
Competition
For each species, linear models with effects of densities of A. albopictus and A. aegypti (continuous variables), population (class variable), and block (class variable) were tested with λ’ and its main demographic components (survivorship and mean female mass) as dependent variables. Collectively we refer to these dependent variables as “population performance”. An effect of competition was detected as a significant slope for a species’ performance versus heterospecific or conspecific density. If population affects the outcome of competition, we expect an interaction between density and population on λ’, survivorship, and mean female mass. Aedes albopictus populations with stronger effects on A. aegypti will yield steeper slopes of A. aegypti population performance vs. A. albopictus density. Populations of A. albopictus with better competitive responses to A. aegypti will yield shallower slopes of A. albopictus population performance vs. A. aegypti density. Interactions between heterospecific and conspecific densities were tested but not significant, and thus they were removed from models. Association of competitive effect and response among populations was tested by estimating Spearman rank correlation between slopes, with a strong negative correlation expected if strong competitive effect and response are associated across populations.
Populations selected for this study were not a random sample of all possible populations of each site type. Therefore, in all analyses, population was treated as a fixed effect and statistical inferences extend only to the populations selected. This approach follows that of previous studies on geographic variation of life history traits (e.g. Reznick et al. 2001, Leisnham et al. 2008). For all analyses, we tested for significant differences among populations using pairwise contrasts (Scheiner 2001), with sequential Bonferroni correction for all possible comparisons (33) within each analysis. We tested for site type effects using the a priori contrast comparing mean values of λ’, survivorship, and mean female mass among extinction vs. coexistence vs. allopatric sites.
We arcsine-square root transformed proportion surviving, and log transformed both λ’ + 1 and mass to meet assumptions of normality and homogeneity of variances. All analyses were done using SAS (SAS Institute Inc. 2003) using experiment-wise α = 0.05.
Fecundity-size
MANCOVA was used to test for differences between populations (predictor variable) in the relationships of wing length (predictor variable) with fecundity and egg size (dependent variables), using F statistics derived from Pillai’s Trace (SAS Institute Inc. 2003). We interpret contributions of dependent variables to significant MANCOVA effects using Standardized Canonical Coefficients (SCCs; Scheiner 2001). Population was a class variable and wing length a continuous variable. Interaction of wing length with population was also included. Thirty seven females either did not lay eggs before dying or their eggs were damaged, and thus their eggs were not weighed and they were excluded from the MANCOVA. Although we tested population differences in the relationship of wing length with fecundity and egg size using data from all populations, we conducted separate univariate linear regressions of fecundity on wing length for each population to estimate f(wx) for calculating λ’ for each population.
RESULTS
Experiment 1: Competition
Estimated finite rate of increase λ’
The origin of A. albopictus influences its competitive effect on A. aegypti (F8, 159=2.42, P=0.0170) and response to competition from A. aegypti (F8, 158=3.28, P=0.0017) (Fig. 1, Appendix C). Bartow had the strongest negative effect of A. albopictus density on A. aegypti λ’ (i.e., steepest negative slope) (Fig. 1, Appendix C), and there was a difference between Bartow and Bloomington (P < 0.0001). Ft. Denaud had the strongest negative effect of A. aegypti density on A. albopictus λ’, (Fig. 1, Appendix C), and was different to all other populations (P < 0.0002). Aedes albopictus (conspecific) density negatively affected A. albopictus λ’ (F1, 158=4.10, P=0.0447) similarly for all populations (F8, 158=0.04, P=1.0000). Competitive effect and response slopes were uncorrelated (rs = 0.0167, P = 0.948), indicating inconsistent ranking of populations of A. albopictus in competitive effect and response (Appendix C). Separate linear regressions of fecundity on wing length for use in estimating λ’ for each population yielded r2 values from 0.336 to 0.763.
Fig. 1.
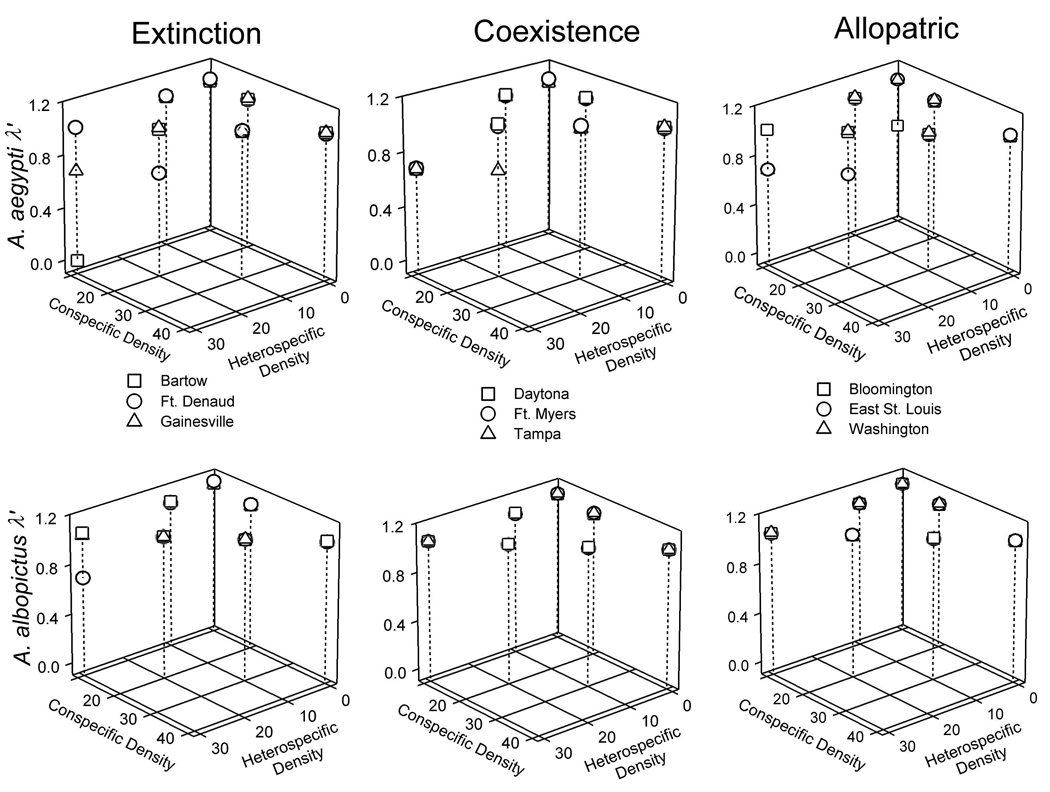
Observed heterospecific and conspecific effects of larval densities on backtransformed λ’ of A. aegypti and A. albopictus. Panels are grouped by species (rows) and origin of A. albopictus populations where A. aegypti has gone extinct, coexists, or has never been (allopatric) (columns). Predicted slopes are presented in Fig C1 in Appendix C.
Survivorship
Heterospecific (A. aegypti) density negatively affected A. albopictus survivorship (Fig. 2), with a steeper slope for Ft. Denaud compared to Ft. Myers and for extinction sites compared to coexistence sites (F8, 158=2.23, P=0.0280; Appendix C). Conspecific density negatively affected A. albopictus survivorship (F1, 158=149.65, P<0.0001) similarly for all populations (F8, 158=1.25, P<0.2746). Heterospecific (F1, 159=324.95, P<0.0001) and conspecific (F1, 159=195.36, P<0.0001) densities negatively affected A. aegypti survivorship similarly for all populations (F1, 159=1.20, P=0.3025 and F1, 159=0.81, P=0.5918; Appendix C).
Fig. 2.
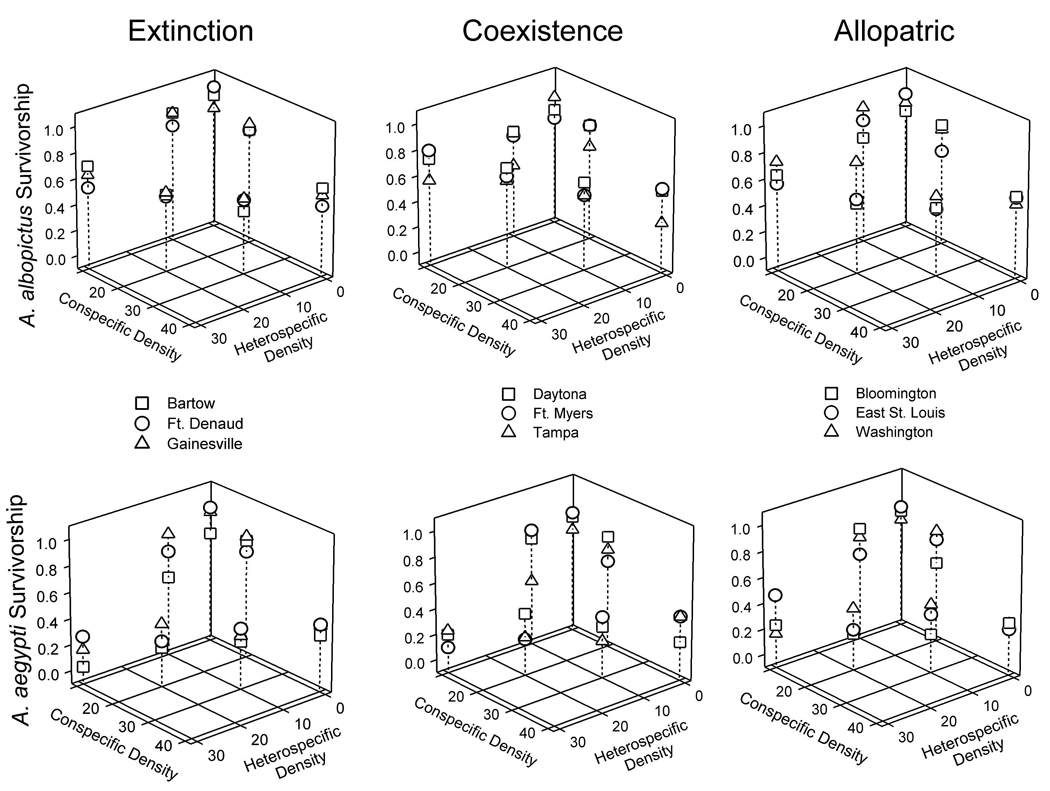
Observed heterospecific and conspecific effects of larval densities on backtransformed survival of A. aegypti and A. albopictus. Panels are grouped by species (rows) and origin of A. albopictus populations where A. aegypti has gone extinct, coexists, or has never been (allopatric) (columns). Predicted slopes are presented in Fig C3 in Appendix C.
Adult female size
Heterospecific and conspecific densities negatively affected A. albopictus mass (F1, 157=32.28, P<0.0001 and F1, 157=4.72, P=0.0314) and A. aegypti mass (F1, 146=18.95, P<0.0001 and F1, 157=14.50, P=0.0002) (Appendix C). Density effects on body size were consistent among populations (Appendix C).
Experiment 2: Fecundity - size relationship
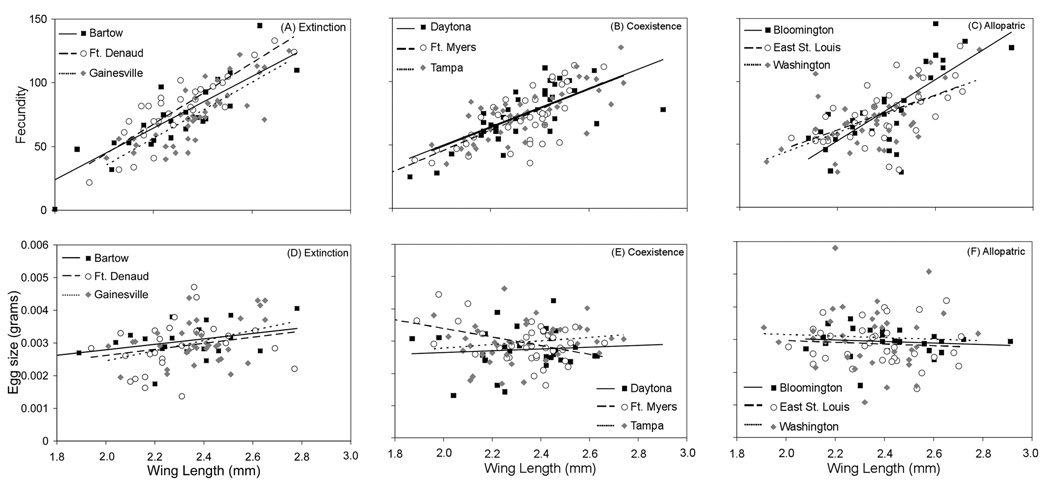
Wing length positively affected fecundity of A. albopictus in all populations, and showed steeper slopes in extinction sites than coexistence and allopatric sites (Table 1, Fig. 3). Wing length had a small positive effect on egg size of A. albopictus from extinction sites, but not in coexistence and allopatric populations (Fig. 3). Differences between extinction sites and coexistence sites and between extinction sites and allopatric sites were approximately equally attributable to differences in both fecundity and egg size (see SCCs in Table 1). All multivariate pairwise contrasts between individual populations for the wing*population interaction were not significant after Bonferroni correction.
Table 1.
Least squares MANCOVA on fecundity and mean egg size of A. albopictus in response to the independent variables of Population and Wing length.
| MANCOVA | Standardized Canonical Coefficients (SCCs) |
||||
|---|---|---|---|---|---|
| Source of variation | Pillai’s Trace (F) | df | P | Fecundity | Egg size |
| Population | 1.76 | 16, 526 | 0.0341 | 1.08 | 0.73 |
| Wing length | 163.34 | 2, 262 | <0.0001 | 1.47 | 0.13 |
| Population×Wing Length | 1.89 | 16, 526 | 0.0194 | 1.17 | 0.67 |
| Extinction vs. Allopatric | 4.44 | 2, 262 | 0.0127 | 0.71 | 0.92 |
| Extinction vs. Coexistence | 4.50 | 2, 262 | 0.0120 | 0.81 | 0.88 |
| Allopatric vs. Coexistence | 0.03 | 2, 262 | 0.9703 | 1.20 | −0.53 |
Multivariate pairwise contrasts among site types for the slopes are only shown for the population x wing length interaction for brevity (sequential Bonferroni correction, 3 comparisons). See text for the results of pairwise contrasts between populations. Significant effects and pairwise comparisons are indicated in bold. Only first canonical variates are shown; all second canonical variates are nonsignificant.
Fig. 3.
Relationships of wing length with fecundity (A–C) and egg size (D–F) in A. albopictus from populations originating from sites where A. aegypti has gone extinct, coexists, or never been (allopatric).
DISCUSSION
There are inherent interpopulation differences in competitive effect and response of A. albopictus. We predicted that A. albopictus from sites where A. aegypti has gone extinct (extinction) would have stronger competitive effects on or better competitive responses to A.aegypti, compared to A. albopictus from sites where A. aegypti persists (coexistence), or is absent (allopatric). However, our data yielded no strong patterns among extinction, coexistence, or allopatric sites. Instead, certain populations stood out as having large competitive effects (Bartow) or poor competitive responses (Ft. Denaud). As both of these populations are from extinction sites, interpopulation variability in competitive ability is unlikely to explain variation in invasion success or impact of A. albopictus among these sites.
Most A. albopictus populations, except Ft. Denaud, yielded greater λ’ than A. aegypti at high combined densities. This result is consistent with previous field (Juliano 1998, Braks et al. 2004) and laboratory (Barrera 1996, Murrell and Juliano 2008) experiments using natural leaf detritus as the nutrient base, and with competitive superiority of A. albopictus over A. aegypti under these conditions. Competition affected female size in the same way for all A. albopictus populations, and all A. albopictus populations had the same effect on A. aegypti size. This suggests that differential effects of A. albopictus population on λ ’ are mostly the result of effects on survivorship. Nevertheless, we observed slightly different conclusions for λ’ and survivorship of both species, reaffirming the importance of estimating population rate of increase in competition studies (Livdahl and Sugihara 1984).
Although extinction sites had steeper average fecundity-size relationships than did coexistence sites and allopatric sites, this did not translate into generally superior population performance. To test whether interpopulation differences in fecundity-size relationships (f(wx)) were important determinants of overall competitive interactions, we also calculated λ’ using one f(wx) from a regression pooling data from all populations. Although λ’ values changed, statistical conclusions about competitive advantage and interpopulation differences in competitive effects and responses were unchanged. Thus, as observed by Juliano (1998), patterns in competitive interactions for these populations are not strongly related to fecundity-size relationships.
Consistent with prior work on A. albopictus reproduction (e.g., Leisnham et al. 2008), fecundity and egg size were positively correlated, yielding no evidence of a trade-off between fecundity and investment per offspring across populations. Although interpopulation differences in reproduction were primarily attributable to fecundity, differences in egg size were not trivial. Aedes albopictus from extinction sites had a strong relationship between wing length and egg size compared to coexistence sites (Fig. 1). Egg size does not enter into calculations of λ’ but it may be related to fitness of A. albopictus. In A. aegypti and other insects, females hatching from large eggs grow faster, attain greater adult size, and lay more and larger eggs than females hatching from small eggs (Steinwascher 1984, Azevedo et al. 1997, Fox and Czesak 2000). Interspecific comparisons among Aedes mosquitoes also show egg survival time is correlated with egg volume at both high and low humidity (Sota and Mogi 1992). Thus, larger eggs may yield superior fitness in A. albopictus, and this advantage for large females may contribute to greater population-level competitive ability of A. albopictus at certain sites, and contribute to displacement of A. aegypti.
Our experiment is one of the first to show interpopulation divergence in competitive effect and response for an insect. Competitive effect is usually associated with ability to harvest and deplete scarce resources (Tilman 1982). Harvesting efficiency can contribute to competitive response as well, but response is also affected by physiological efficiency and flexibility, such as reduced metabolic demands when resources are scarce or plasticity of size and time to maturity that may enable a species to maintain positive dN/dt despite competition. Such phenotypic plasticity will not necessarily alter competitive effect (Tilman 1982). In our experiment, there were significant interactions involving population only for interspecific competition and the magnitudes of competitive effect and response were uncorrelated among A. albopictus populations. Competitive effect and response are at least partially independent for plants and invertebrates (e.g., Goldberg and Fleetwood 1987, Joshi and Thompson 1995, Byers 2000). Thus, independent evolution of enhanced effect or response among populations of a wide ranging species, such as A. albopictus, seems possible.
Competition between A. albopictus and A. aegypti is widely assumed to occur via resource depletion, and manipulating resources levels can alter the impact of competition (Juliano 1998, Braks et al. 2004). However, both species may be affected by interference competition produced by water-borne substances (Moore and Fisher 1969, Dye 1984, Broadie and Bradshaw 1991). Competition between invertebrates can involve multiple mechanisms (Byers 2000). Intrapopulation differences in the mechanisms of competition between A. albopictus and A. aegypti merits further investigation as it may explain geographic variation in heterospecific but not conspecific effects between these species in this study.
Our sites were grouped according to the presence/absence of A. aegypti recorded during annual and multi-annual surveys. But site types also differ in environmental conditions. Coexistence sites are warmer, more urban, and seasonally dryer compared to extinction sites (O'Meara et al. 1995, Juliano et al. 2002). Allopatric sites differ considerably in climate compared to coexistence and extinction sites because of latitude (Leisnham et al. 2008). Despite these differences between site types, we observe relatively little consistent difference in competitive ability of A. albopictus populations under the single set of environmental conditions in this study.
Our results suggest that coexistence of A. aegypti with A. albopictus may be mainly determined by the phenotypic responses of both species to environmental conditions, which differ among sites, with conditions at some sites favoring A. aegypti (Juliano et al. 2002, 2004; Costanzo et al. 2005). Aedes albopictus eggs are more sensitive to desiccation that A. aegypti eggs (Costanzo et al. 2005, Juliano et al. 2002), and local coexistence of these species may be possible because warm, dry climates favor A. aegypti and alleviate effects of competition from A. albopictus via differential mortality of A. albopictus eggs (Juliano et al. 2002).
We must also look to other ecological or genetic factors, especially those particular to specific populations, to understand the divergent evolution in competitive ability of A. albopictus. These may include intrapopulation variation of selection on A. albopictus due to local differences in the larval and terrestrial environment, intrapopulation variation of selection on A. albopictus due to temporal changes in the presence/absence of A. aegypti, and non-adaptive variation due to founder effects, genetic drift, and inbreeding. Additionally, geographic differentiation in the competitive ability of A. aegypti may also affect the spread of A. albopictus in North America.
ACKNOWLEDGEMENTS
We thank D. O’Donnell, P. Armbruster, M. Tseng, C. Janiec, L. Sala, E. Murrell, L. Kling, L. Towler, and C. Salmon for field collections, experiment maintenance, or data collection. This research was funded by NIAID grant R01-AI-44793.
LITERATURE CITED
- Abbott KL, Greaves SNJ, Ritchie PA, Lester PJ. Behaviourally and genetically distinct populations of an invasive ant provide insight into invasion history and impacts on a tropical ant community. Biological Invasions. 2007;9:453–463. [Google Scholar]
- Armbruster P, Hutchinson RA. Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae) Journal of Medical Entomology. 2002;39:699–704. doi: 10.1603/0022-2585-39.4.699. [DOI] [PubMed] [Google Scholar]
- Armbruster P, Conn JE. Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae) Annals of the Entomological Society of America. 2006;99:1234–1243. [Google Scholar]
- Azevedo RBR, French V, Partridge L. Life-history consequences of egg size in Drosophila melanogaster. The American Naturalist. 1997;150:250–282. doi: 10.1086/286065. [DOI] [PubMed] [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecological Entomology. 1996;21:117–127. [Google Scholar]
- Birungi J, Munstermann LE. Genetic structure of Aedes albopictus (Diptera: Culicidae) populations based on mitochondrial ND5 sequences: Evidence for an independent invasion into Brazil and United States. Annals of the Entomological Society of America. 2002;95:125–132. [Google Scholar]
- Black WC, Karamjit SR, Turco BJ, Arroyo DC. Laboratory study of competition between United States strains of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 1989;26:260–271. doi: 10.1093/jmedent/26.4.260. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Lourenço-de-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes in Brazil. Annals of the Entomological Society of America. 2004;97:130–139. [Google Scholar]
- Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. Journal of Insect Physiology. 1990;36:165. [Google Scholar]
- Broadie KS, Bradshaw WE. Mechanisms of interference competition in the western treehole mosquito, Aedes sierrensis. Ecological Entomology. 1991;16:145–154. [Google Scholar]
- Byers JE. Competition between two estuarine snails: Implications for invasions of exotic species. Ecology. 2000;81:1225–1239. [Google Scholar]
- Costanzo KS, Kesavaraju B, Juliano SA. Condition specific competition in container mosquitoes: the role of non-competing life-history stages. Ecology. 2005;86:3289–3295. doi: 10.1890/05-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsie RF, Ward RA. Identification and geographical distribution of the mosquitos of North America. Gainesville, North of Mexico: University Press of Florida; 2005. [Google Scholar]
- Detinova TS. Age-grouping Methods in Diptera of Medical Importance. Geneva, Switzerland: World Health Organization; 1962. [PubMed] [Google Scholar]
- Duyck P, David P, Junod G, Brunel C, Dupont R, Quilici S. Importance of competition mechanisms in successive invasions by polyphagous tephritids in La Réunion. Ecology. 2006;87:1770–1780. doi: 10.1890/0012-9658(2006)87[1770:iocmis]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dye C. Competition amongst larval Aedes aegypti: the role of interference. Ecological Entomology. 1984;9:355–357. [Google Scholar]
- Fox CW, Czesak ME. Evolutionary ecology of progeny size in arthropods. Annual Review of Entomology. 2000;45:341–369. doi: 10.1146/annurev.ento.45.1.341. [DOI] [PubMed] [Google Scholar]
- Goldberg DE, Fleetwood L. Competitive effect and response in four annual plants. Journal of Ecology. 1987;75:1131–1143. [Google Scholar]
- Goldberg DE, Scheiner SM. ANOVA and ANCOVA: field competition experiments. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. New York: Oxford University Press; 2001. pp. 77–98. [Google Scholar]
- Gómez-Mestre I, Tejedo M. Geographic variation in asymmetric competition: a case study with two larval anuran species. Ecology. 2002;83:2102–2111. [Google Scholar]
- Hatle JD, Crowley MC, Andrews AL, Juliano SA. Geographic variation of reproductive tactics in lubber grasshoppers. Oecologia. 2002;132:517–523. doi: 10.1007/s00442-002-0994-5. [DOI] [PubMed] [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. Journal of American Mosquito Control Association. 1988;4 Supplement:1–40. [PubMed] [Google Scholar]
- Joshi A, Thompson JN. Alternative routes to the evolution of competitive ability in two competing species. Evolution. 1995;49:616–625. doi: 10.1111/j.1558-5646.1995.tb02298.x. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition. Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Lounibos LP, O'Meara GF. A field test for competitive effects of Aedes albopictus on A. aegypti in South Florida: differences between sites of coexistence and exclusion. Oecologia. 2004;194:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, O'Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130:458–469. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SR, Taylor DR. History, chance and adaptation during biological invasion: separating stochastic phenotypic evolution from response to selection. Ecology Letters. 2008;11:852–866. doi: 10.1111/j.1461-0248.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- Leisnham PT, Sala LM, Juliano SA. Geographic variation in adult survival and reproduction of the mosquito Aedes albopictus. Journal of Medical Entomology. 2008;45:210–221. doi: 10.1603/0022-2585(2008)45[210:gviasa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livdahl TP, Sugihara G. Non-linear interactions of populations and the importance of estimating per capita rates of change. Journal of Animal Ecology. 1984;53:573–580. [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annual Review of Entomology. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Escher RL, Lourenço-de-Oliveira R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae) Annals of the Entomological Society of America. 2003;96:512–518. [Google Scholar]
- Moore CG, Fisher BR. Competition in mosquitoes. Density and species ratio effects on growth, mortality, fecundity, and production of growth retardant. Annals of Entomological Society of America. 1969;62:1325–1331. doi: 10.1093/aesa/62.6.1325. [DOI] [PubMed] [Google Scholar]
- Murrell EG, Juliano SA. The role of detritus type in interspecific competition and population distributions of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) Journal of Medical Entomology. 2008;45:375–383. doi: 10.1603/0022-2585(2008)45[375:dtatoo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Meara GF, Evans LF, Jr, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. Journal of Medical Entomology. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Reznick D, Butler MJ, IV, Rodd H. Life history evolution in guppies. VII. The comparative ecology of high- and low-predation environments. The American Naturalist. 2001;157:12–26. doi: 10.1086/318627. [DOI] [PubMed] [Google Scholar]
- Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O'Neil P, Parker IM, Thompson JN, Weller SG. The population biology of invasive species. Annual Revue of Ecology and Systematics. 2001;32:305–332. [Google Scholar]
- SAS Institute Inc. SAS User's Guide: Statistics. Version 9.1. Cary, NC: SAS Institute Inc.; 2003. [Google Scholar]
- Scheiner SM. MANOVA: multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. Oxford: Oxford University Press; 2001. pp. 99–115. [Google Scholar]
- Siemann E, Rogers WE. Genetic differences in growth of an invasive tree species. Ecology Letters. 2001;4:514–518. [Google Scholar]
- Sota T, Mogi M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia. 1992;90:353–358. doi: 10.1007/BF00317691. [DOI] [PubMed] [Google Scholar]
- Steinwascher K. Egg Size Variation in Aedes aegypti: Relationship to Body Size and Other Variables. American Midland Naturalist. 1984;112:76. [Google Scholar]
- Tilman D. Resource competition and community structure. Princeton, New Jersey, USA: Princeton University Press; 1982. [PubMed] [Google Scholar]
- Travis JT. The significance of geographical variation in species interactions. American Naturalist. 1996;148:51–58. [Google Scholar]
- Yasuda H, Evans EW, Kajita Y, Urakawa K, Takizawa T. Asymetric larval interactions between introduced and indigenous ladybirds in North America. Oecologia. 2004;141:722–731. doi: 10.1007/s00442-004-1680-6. [DOI] [PubMed] [Google Scholar]