Summary
Light-emitting diodes (LEDs) are becoming more commonly used as light sources for fluorescence microscopy. We describe the adaptation of a commercially available LED flashlight for use as a source for fluorescence excitation. This light source is long-lived, inexpensive, and is effective for excitation in the range of 440–600 nm.
Keywords: fluorescence microscopy, light-emitting diode, LED, flashlight
Introduction
Traditionally, broad-spectrum mercury gas discharge lamps have been used as sources for excitation in fluorescence microscopes. The advantage of this type of excitation is that mercury lamps provide bright, broad-spectrum excitation that is filtered to provide specific wavelength bands (although the "broad spectrum" emission of mercury lamps is not uniformly distributed and is dominated by 7–8 strong peaks between 300 and 900 nm). While these lamps are the standard for microscopic fluorescence applications, they have a number of limitations. First, the wide range of emitted wavelengths includes ultraviolet (UV) light that is highly deleterious to living biological samples. With modern UV-blocking filters most of this excitation is excluded, but there is always a finite leak of UV that can decrease the viability of very sensitive tissues or cells (Hohman, 2007). For example, cell division is sometimes inhibited when irradiating cells with fluorescence excitation derived from mercury discharge lamps (Khodjakov & Rieder, 2006). Second, the lifespan of a mercury discharge lamp is usually only 200–300 hours, and the intensity of these lamps decays progressively during this time (Martin et al. 2005; Hohman, 2007). Third, gas discharge lamps require at least several minutes to reach an operating equilibrium after being turned on and lamp intensity can fluctuate during use. Therefore, once mercury discharge lamps are turned on, they are usually left on for hours to enable fluorescence measurements as needed without delay (Albeanu et al. 2008), thereby shortening lifetime. Fourth, these lamps generate a significant amount of heat and therefore introduce complications when used in a confined space (Martin et al. 2005). Finally, mercury discharge lamps can explode, thereby damaging lenses and/or mirrors within the lamp housing (Martin et al. 2005).
Light emitting diodes (LEDs) show great promise as alternative light sources for fluorescence microscopy (Martin, 2005; Hohman, 2007; Albeanu et al. 2008). Compared to the broad-spectrum emission of mercury discharge lamps (which is then delimited to specific wavelength bands with filters), LEDs traditionally have been designed to provide illumination at very specific wavelength bands from UV to infrared. However, current "white" LEDs have been designed to emit relatively broad excitation that can be filtered to provide specific wavelength bands as with mercury lamps ("white" LEDs tend to have a preponderance of blue emission, as seen in Fig. 1A and Fig. S1). Moreover, no UV emission is generated as a by-product in LEDs that are not specifically designed for UV emission. When turned on, LEDs can achieve full brightness within microseconds with a constant intensity thereafter; upon being turned off, they extinguish immediately without a prolonged glow (Albeanu et al. 2008). Previously the intensity of LED emission was rather dim, but new generation LEDs are much brighter and can additionally have lifetimes as long as 50,000 hours (or more). LEDs are relatively cool and therefore can be used in confined spaces. Not surprisingly, several companies now offer LED-based excitation systems for fluorescence microscopy to capitalize upon these advantageous characteristics of LEDs. However, since these commercially available LED fluorescence sources are designed to be capable of excitation at many different wavelength ranges, they are expensive. We have adapted a commercially available white LED flashlight for use as a source for fluorescence excitation. This light source has the advantages of LED light sources as itemized above and is effective for fluorescence excitation in the most commonly used range of 440–600 nm for microscopy, especially when coupled with CCD detection.
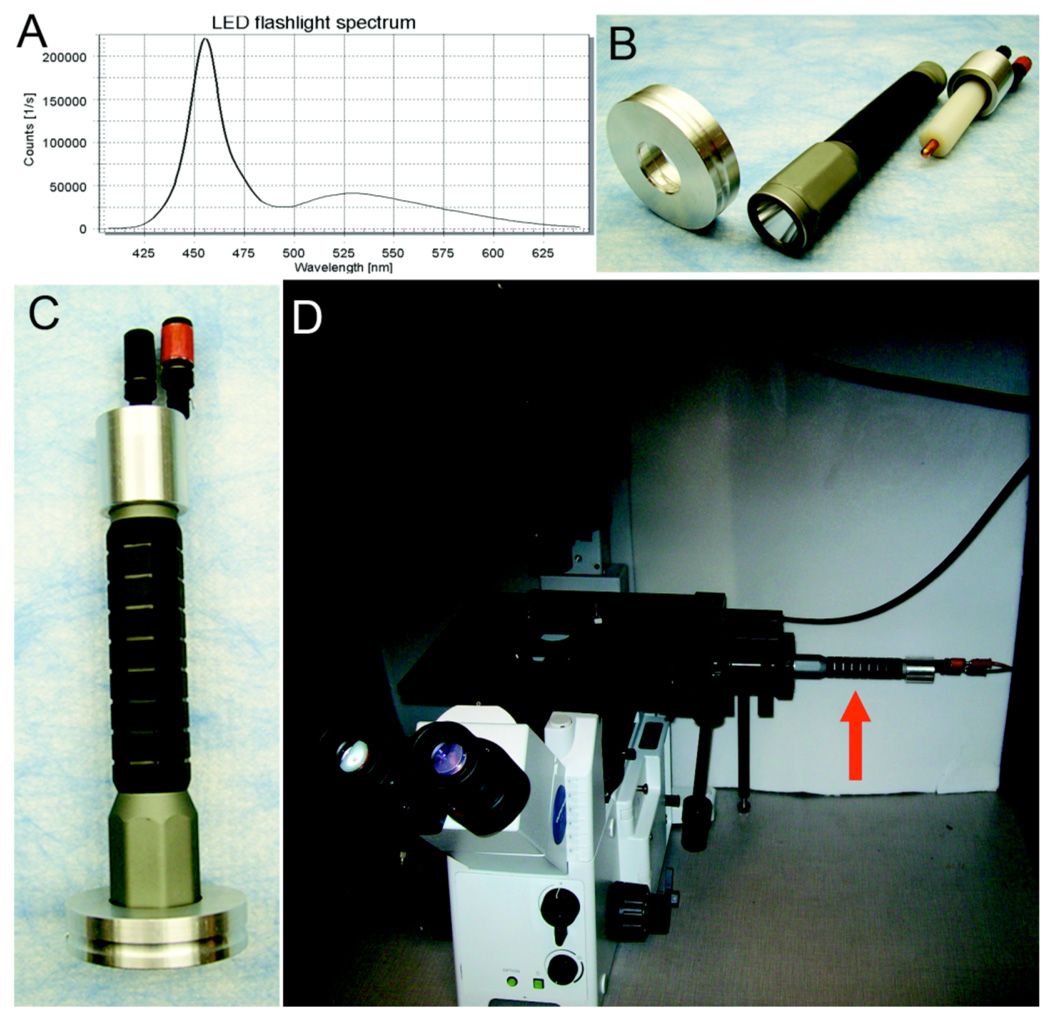
Figure 1.
The LED flashlight as a tool for fluorescence microscopy. A. Emission spectrum from the flashlight from 400 nm to 650 nm. B. Disassembled flashlight excitation source showing the microscope adapter ring on the left, the commercial flashlight in the middle, and the 6 VDC dummy battery adapter on the right. C. The assembled flashlight excitation source. D. The LED flashlight excitation source (red arrow) attached to our Olympus microscope in the light-tight box.
Results and Discussion
We have a CCD-coupled microscope in a light-tight box for luminescence and BRET imaging (Xu et al. 2007) that we wanted to use for fluorescence applications but we needed to keep the box closed throughout imaging measurements; consequently the box could not be opened to change filters, open/close shutters, or focus the microscope. The characteristics of an LED source were attractive for our application, especially the low heat generation and the ability to immediately start and stop fluorescence excitation by simply turning the LED on and off rather than by using a remotely controlled shutter. However, the price of the commercially available LED sources was unappealing. Given that LED flashlights have been used to detect GFP in whole animals (Yang et al. 2005), we investigated whether a simple commercially available white LED flashlight could serve as an excitation source for fluorescence microscopy, thereby reaping the benefits of LED excitation while avoiding the high cost of the excitation sources that have been commercially designed for this purpose. We therefore went to a local camping equipment store and purchased the brightest white single-LED flashlight on the shelf; in our case, this was an Inova Bolt 4.6 watt/6 volt flashlight for a price of approximately USA$ 50. The flashlight’s emission spectrum was measured with a fluorescence spectrophotometer (Quantamaster QM-7/SE, Photon Technology International, Birmingham NJ, USA) and showed strong emission in the 440–480 nm range (Fig. 1A and Fig. S1), which is a suitable excitation range for commonly used fluorescent probes such as: (i) the fluorescent proteins ECFP, EGFP, EYFP; (ii) the fluorescent pH indicator BCECF; (iii) fluorescein and its derivatives (e.g., FITC and various Alexa Fluors); and (iv) nuclear/nucleic acid probes like YO-PRO-1 and YOYO-1. The spectrum of the Inova Bolt flashlight also exhibited a dimmer emission extending into the red and could therefore be used for longer wavelength excitation in conjunction with a CCD detector. The manufacturer estimates the lifetime of the LED in this flashlight to be approximately 50,000 hours.
The Inova flashlight is normally powered by two 3 V lithium batteries with an estimated lifetime of 2.5 hours, but as shown in Fig. 1B, we replaced the batteries with a dummy battery component that allowed us to connect the battery terminals to an inexpensive power supply operating at 6 V (we used a Tekpower DC Variable Power Supply # HY152A, which cost USA$ 40 from Amazon.com). The illumination of the LED is therefore controllable remotely from outside our light-tight box by turning on and off the power supply. A simple adaptor was also machined on a lathe from aluminum to connect the flashlight to our microscope's port for the fluorescence lamp housing (Fig. 1B,C); however, the flashlight could also function effectively as an excitation source when simply held in place by a ring-stand and a clamp. Therefore, the mercury lamp (with its mirror and collector lens) was removed and replaced with the LED flashlight. There is no other difference in the optical train between the mercury lamp vs. LED flashlight sources. The reflector within the flashlight acts to focus the beam in the same way as the mirror/lens within a mercury lamp housing. With the mercury lamp housing on the Olympus IX71 inverted microscope replaced with our flashlight, the system appears as in Fig. 1D (a color CCD camera [an Olympus DP72] is coupled to the bottom port on the microscope and is therefore out of view).
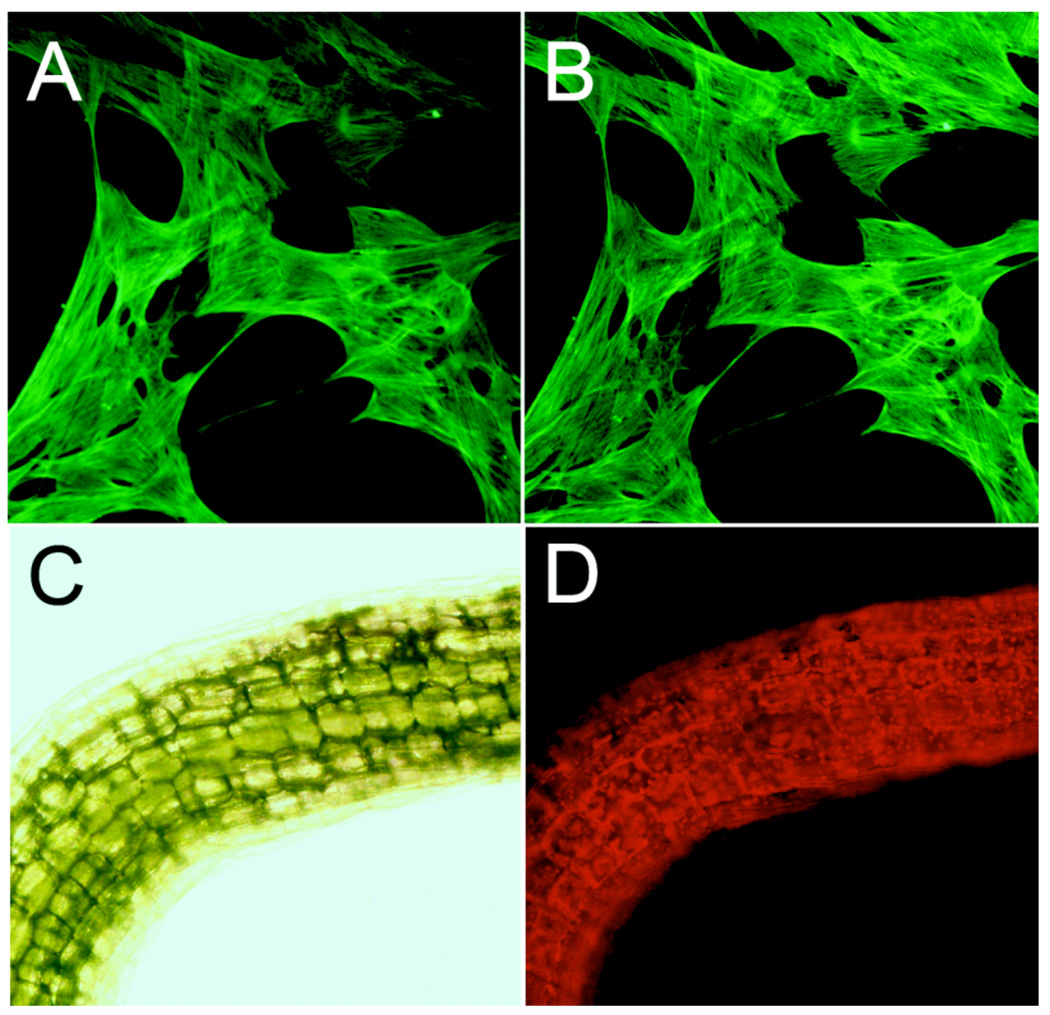
This white LED flashlight source is less intense than a mercury source, but the fluorescence images shown in Figure 2 were easily visible by eye. For excitation at 470/30 nm ("30" = bandpass width at half peak height), the mercury source was 2X more intense than the LED source and at 500/20 nm, it was 4X more intense (Supplemental Table 1). However, in conjunction with a CCD detector, comparable images were achieved using the LED flashlight source with a camera exposure of about 4–5 times the duration of that required by a 100 Watt mercury lamp. For example, the fluorescence images of Alexa Fluor 488-labeled actin filaments in raccoon uterine cells excited by the 100 W mercury lamp and captured with a 1 second exposure (Fig. 2A) are equivalent to those collected by a 5 second exposure with the LED flashlight source (Fig. 2B). As another example, we also used our LED source to excite the red autofluorescence of chlorophyll in the stem of a plant seedling (Figs. 2C & 2D).
Figure 2.
Images from the LED-excitation system. A. Fluorescence of actin filaments in raccoon uterine cells labeled with phalloidin conjugated to Alexa Fluor 488 excited by the 100 W mercury arc lamp with a 1 s exposure. B. The same fluorescent object as in panel A except excited by the LED flashlight with a 5 s exposure. C. A bright field image of the stem from an Arabidopsis seedling. D. The same object as in panel C examined for chlorophyll autofluorescence excited by the LED flashlight source (5 s exposure). For panels A&B, the microscopic objective was 20X, and the fluorescence filters were EX 470/30; EM 520/40 (Chroma ZsGreen filter set #42002); for panels C&D, the microscopic objective was 10X; for panel D, the fluorescence filters were EX 500/20; EM 520LP (Chroma YFP filter set #410290). See Supplementary Material for more information about the spectrum and intensity of the LED source we used as well as information on the fluorescence filter sets.
Therefore, for fluorescence microscopy using excitation in the most commonly used range of wavelengths, this flashlight system allows an effective way to reap the benefits of LED excitation. The total cost of this flashlight and power supply (~$ 90 total) was less than the cost of a single replacement lamp for a mercury discharge fluorescence source and has a predicted lifespan of ~300 X longer. This technology provides a way to economically add LED excitation capability to an existing mercury-discharge fluorescence microscope. It may also be an excellent single-source solution for schools with limited resources to set up laboratory exercises involving fluorescence microscopy or research laboratories that wish to economize in the current challenging era of limited research budgets.
Supplementary Material
Acknowledgements
We thank Jeff Jones and Mark Boyd (Olympus America Inc.) for the loan of the DP72 camera, and Bruce and Roger Williams for machining the adapter and dummy battery. This project was supported by a grant from the NIMH (MH080035).
References
- Albeanu DF, Soucy E, Sato TF, Meister M, Murthy VN. LED arrays as cost effective and efficient light sources for widefield microscopy. PLoS One. 2008;3:e2146. doi: 10.1371/journal.pone.0002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman B. LED light source: major advance in fluorescent microscopy. Biomed.Instrum. Technol. 2007;41:461–464. doi: 10.2345/0899-8205(2007)41[461:LLSMAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. Imaging the division process in living tissue culture cells. Methods. 2006;38:2–16. doi: 10.1016/j.ymeth.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Agostini HT, Hansen LL. Light emitting diode microscope illumination for green fluorescent protein or fluorescein isothiocyanate epifluorescence. BioTechniques. 2005;38:204–206. doi: 10.2144/05382BM06. [DOI] [PubMed] [Google Scholar]
- Xu X, Soutto M, Xie Q, Servick S, Subramanian C, von Arnim AG, Johnson CH. Imaging Protein Interactions with BRET in Plant and Mammalian Cells and Tissues. Proc. Natl. Acad. Sci. USA. 2007;104:10264–10269. doi: 10.1073/pnas.0701987104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Luiken G, Baranov E, Hoffman RM. Facile whole-body imaging of internal fluorescent tumors in mice with an LED flashlight. BioTechniques. 2005;39:170–172. doi: 10.2144/05392BM02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.