Abstract
Mmp-20 and Klk4 are the two key enamel proteases. Can both enzymes process amelogenin to generate the major cleavage products that accumulate during the secretory stage of amelogenesis? We isolated Mmp-20 and Klk4 from developing pig teeth and used them to digest the tyrosine-rich amelogenin polypeptide (TRAP), the leucine-rich amelogenin protein (LRAP), and 5 fluorescence peptides. We characterized the digestion products by LC-MSMS, SDS-PAGE, and C18 RP-HPLC monitored with fluorescence and UV detectors. Mmp-20 cleaves amelogenin sequences after Pro162, Ser148, His62, Ala63, and Trp45. These cleavages generate all of the major cleavage products that accumulate in porcine secretory-stage enamel: the 23-kDa, 20-kDa, 13-kDa, 11-kDa, and 6-kDa (TRAP) amelogenins. Mmp-20 cleaves LRAP after Pro45 and Pro40, producing the two LRAP products previously identified in tooth extracts. Among these key cleavage sites, Klk4 was able to cleave only after His62. We propose that Mmp-20 alone processes amelogenin during the secretory stage.
Keywords: TRAP, LRAP, enamel, tooth, fluorescent peptides
Introduction
Because of the large size of their developing teeth and their availability from slaughterhouses, pigs have been the predominant model for the study of amelogenin, the most abundant enamel protein (Fincham et al., 1999). Porcine amelogenins and their cleavage products have been isolated and extensively characterized by protein sequencing (Fukae et al., 1979, 1980; Fukae and Shimizu, 1983; Yamakoshi et al., 1989, 1994, 2003). In the pig, there are separate amelogenin genes on the X and Y chromosomes, but these genes secrete proteins having identical amino acid sequences (Ikawa et al., 2005). As in other mammals, the complicated assortment of amelogenins in porcine secretory-stage enamel is due to alternative splicing (Hu et al., 1996) and to proteolysis (Bartlett and Simmer, 1999).
The major porcine amelogenin (P173; Met1-Asp173) has 173 amino acids and an apparent molecular weight of 25 kDa. Most of the secreted P173 is cleaved after Ser148 to generate the 20-kDa amelogenin (P148; Met1-Ser148), which in turn is cleaved after Trp45 to yield the tyrosine-rich amelogenin polypeptide (TRAP; P45; Met1-Trp45) and the 13-kDa amelogenin (Leu46-Ser148) (Fincham et al., 1981). The TRAP cleavage site is conserved in humans and other mammals (Fincham et al., 1989; Fincham and Moradian-Oldak, 1993). Point mutations in AMELX that alter the human amelogenin amino acid sequence near Trp45 cause amelogenesis imperfecta (AI) and also alter the susceptibility of the protein to proteolysis (Li et al., 2001, 2003; Tanimoto et al., 2008).
Another important amelogenin isoform is the leucine-rich amelogenin protein (LRAP) (Fincham et al., 1981; Fincham and Moradian-Oldak, 1993). Cloning of the LRAP mRNA was the first evidence of amelogenin alternative splicing (Gibson et al., 1991). The LRAP mRNA is about 10% as abundant as the message for the major amelogenin isoform (Yuan et al., 1996). In the pig, LRAP (P56) is cleaved after Pro45 or Pro40, and these cleavage products accumulate in the matrix (Fincham and Moradian-Oldak, 1993).
The only known extracellular proteases in developing enamel are Mmp-20 (Bartlett et al., 1996) and kallikrein 4 (Klk4) (Simmer et al., 1998), and both of these enzymes are critical for proper dental enamel formation (Hart et al., 2004; Kim et al., 2005). Enamel proteases degrade enamel proteins, which facilitates their removal from the matrix, allowing for thicker individual crystallites and a higher degree of mineralization (Smith, 1998; Lu et al., 2008). Enamel proteases may also play a role in processing enamel proteins to generate products that serve separate functions than the parent proteins. In this study, we tested the ability of Mmp-20 and Klk4 to catalyze the cleavages that generate the P173 and LRAP cleavage products that accumulate in secretory-stage enamel.
Materials & Methods
All experimental procedures involving the use of animals were reviewed and approved by the University of Michigan Institutional Animal Care and Use Committee.
Preparation of Soft and Hard Enamel Samples
Tooth germs of permanent molars were surgically extracted from the maxillae and mandibles of six-month-old pigs at the Michigan State University Meat Laboratory (East Lansing, MI, USA). The enamel organ epithelia and dental pulp tissue were removed by means of tissue forceps. The soft, cheese-like enamel was separated from the crown with a spatula. We obtained hard enamel samples by scraping the remaining hard, chalky enamel.
Separation of Soft Enamel and Hard Enamel Extracts
We sequentially extracted soft or hard enamel scrapings to obtain neutral and alkaline extracts. The neutral extract (supernatant) was obtained by homogenization of the enamel samples in Sörensen buffer (made by mixing Na2HPO4 and KH2PO4 to achieve a final phosphate concentration of 50 mM and a pH of 7.4), followed by centrifugation. The pellet was re-suspended in carbonate buffer (made by mixing NaHCO3 and Na2CO3 to a final concentration of 50 mM and a pH of 10.8), homogenized, and then centrifuged. The supernatant was designated as alkaline extract.
Purification of Porcine Klk4
The hard enamel-neutral extract was raised to 40% saturation by the addition of ammonium sulfate, then centrifuged. The supernatant was raised to 65% saturation and centrifuged. The 40-65% saturation pellet was re-suspended in 0.5 M acetic acid. Acid-insoluble material was pelleted by centrifugation. The acid-soluble supernatant was applied to a C18 RP-HPLC column (Discovery® C18, 1.0 cm x 25 cm, Sigma-Aldrich/Supelco, Bellefonte, PA, USA) equilibrated with 0.05% trifluoroacetic acid (TFA) and eluted with a linear gradient (20-80%) of acetonitrile containing 0.05% TFA at a flow rate of 1.0 mL/min. The fraction containing Klk4 was buffer-exchanged with 50 mM Tris-HCl (pH 7.4) with the use of a regenerated cellulose membrane (Ultracel; NMWL: 3000; Millipore, Billerica, MA, USA).
Purification of Porcine Mmp-20
The soft enamel-alkaline extract was fractionated by ion exchange chromatography in a Q-SepharoseTM Fast Flow column (1.6 cm x 20 cm, GE Healthcare Biosciences, Little Chalfont, UK) equilibrated with 50 mM Tris-HCl/6 M urea (pH 7.4) and eluted with a linear NaCl gradient (0-0.5 M). Mmp-20 eluted in the fourth peak was de-salted and concentrated by ultrafiltration (Ultracel; Millipore Corporation) and lyophilized. The lyophilized sample was dissolved with 20 mM sodium citrate/6 M urea (pH 7.4) and applied to a Heparin SepharoseTM 6 Fast Flow column (1.6 cm x 20 cm, GE Health-care) equilibrated with the same buffer and eluted with a step NaCl gradient (0.2, 0.5, and 2 M at a flow rate of 0.2 mL/min). Mmp-20 eluted in the second peak, which was buffer-exchanged with 50 mM Tris-HCl (pH 7.4) with the use of a regenerated cellulose membrane (Ultracel; NMWL: 3000; Millipore).
Isolation of LRAP and TRAP and Recombinant Pig Amelogenin (rP172)
LRAP was commercially synthesized (YenZym, Burlingame, CA, USA). TRAP (in the soft enamel, alkaline extract) was fractionated by ion exchange chromatography (2nd peak) and then by size exclusion chromatography on a Superdex G200 column (1.6 cm x 100 cm, GE Healthcare) equilibrated with 50 mM Tris-HCl/4 M guanidine (pH 7.4). TRAP (again in the 2nd peak) was de-salted and concentrated with an ultrafiltration membrane and lyophilized. The LRAP and TRAP samples were further purified by C18 RP-HPLC. The expression and isolation of rP172 have been described previously (Ryu et al., 1999).
Digestion of LRAP and TRAP by Mmp-20 or Klk4
LRAP or TRAP (100 µg) was incubated in 200-µL reactions with 21 µg of Mmp-20 or 1.25 µg of Klk4 extract for 1, 2, 4, 8, 12, and 24 hrs at 37°C in 50 mM Tris-HCl/5 mM CaCl2 (pH 7.4) and analyzed by SDS-PAGE. The 24-hour digests were analyzed by LC-MS/MS.
Digestion of FRET-Amelogenin Peptide by Mmp-20 or Klk4
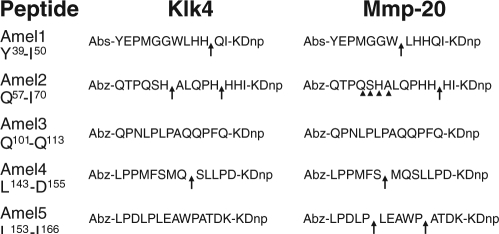
Five porcine amelogenin fluorescence resonance energy transfer (FRET) peptides were synthesized and purified by JPT Peptide Technologies (Berlin, Germany). Each was labeled with 2-aminobenzoyl (Abz) and 2,4-dinitrophenyl (Dnp): Amel1 (Tyr39-Ile50) Abz-YEPMGGWLHHQI-KDnp; Amel2 (Gln57-Ile70) Abz-QTPQSHALQPHHHI-KDnp; Amel3 (Gln101-Gln113) Abz-QPNLPLPAQQPFQ-KDnp; Amel4 (Leu143-Asp155) Abz-LPPMFSMQSLLPD-KDnp; and Amel5 (Leu153-Lys166) Abz-LPDLPLEAWPATDK-KDnp. Each FRET peptide (100 µg) was incubated in 200-µL reactions with 21 µg of Mmp-20 or 1.25 µg of Klk4 extract in 50 mM Tris-HCl/10 mM CaCl2 (pH 7.4) at 37°C for 24 or 48 hrs, and aliquots were analyzed by LC-MS/MS or fractionated by RP-HPLC and monitored with UV (230 nm) and fluorescence detectors (excitation λ 320 nm/emission λ 420 nm).
SDS-PAGE, Western Blotting, and Zymography
SDS-PAGE was performed with Novex® 18% or 4-20% Tris-Glycine Gel (Invitrogen, Carlsbad, CA, USA). The gels were stained with SimplyBlueTM SafeStain (Invitrogen), and apparent molecular weights estimated by comparison with SeeBlue® Plus2 PreStained Standards (Invitrogen). Duplicate SDS-PAGE gels were transblotted onto nitrocellulose membranes (HybondTM-P; 0.45 µm, GE Healthcare) and immunostained with a polyclonal antibody against recombinant pig Klk4 (Hu et al., 2000) or an anti-peptide antibody specific for the Mmp-20 hemopexin-like domain (Fukae et al., 1998). Blocking with 5% non-fat milk was followed by overnight incubation with a 1:10,000 dilution of antibody in Tween Tris-buffered saline (TTBS) containing 5% milk. The membranes were washed 3x in TTBS and incubated with anti-rabbit IgG secondary antibody (GE Healthcare) at a dilution of 1:10,000 for 1 hr. Immunostaining was performed by chemiluminescent detection (for Mmp-20) with the ECL Plus Western Blotting Detection System (GE Healthcare) or by colorimetric detection (for Klk4) with diaminobenzidine (DAB) (Sigma, St. Louis, MO, USA). Zymograms were performed on SDS polyacrylamide gels containing 10% gelatin or 12% casein. Reactions were performed in 50 mM Tris buffer/10 mM CaCl2 (pH 7.4) at 37°C for 24 hrs.
Mass Spectrometry (LC-MSMS)
LC-MSMS was performed by Nextgen Sciences (Ann Arbor, MI, USA) on purified LRAP and TRAP, their digests, and on the digests of the 5 FRET peptides after 24 or 48 hrs.
Results
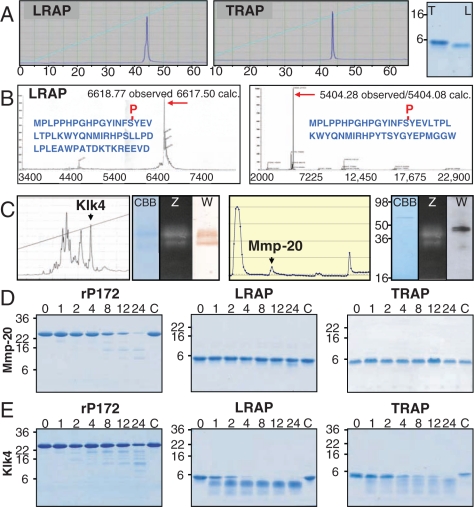
TRAP from developing pig teeth and synthetic LRAP were purified until they displayed a single chromatographic peak on a C18 RP-HPLC column and a single CBB-stained band on SDS-PAGE (Fig. 1A). LC-MSMS analysis confirmed the identities of the proteins and that both were phosphorylated (Fig. 1B). Klk4 and Mmp-20 were isolated from the hard and soft enamel, respectively. Klk4 was evident as two bands migrating above 30 kDa on SDS-PAGE, gelatin zymograms, and on Western blots (Fig. 1C) (Tanabe, 1984). We purified Mmp-20 taking advantage of its heparin-binding affinity. Intact Mmp-20 was evident on casein zymograms and on Western blots as a doublet at 45 and 41 kDa. An Mmp-20 catalytic domain was also evident on the zymograms (Fig. 1C). Both enzymes were able to process recombinant pig amelogenin into multiple cleavage products by 24 hrs, but the patterns of cleavage by Mmp-20 and Klk4 differed (Figs. 1D, 1E). Mmp-20 processed LRAP into slightly smaller products, but did not process TRAP (Fig. 1D). Klk4 degraded both LRAP and TRAP (Fig. 1E).
Figure 1.
Purification and characterization of porcine LRAP, TRAP, Klk4, and Mmp-20. (A) The leucine-rich amelogenin protein (LRAP) and the tyrosine-rich amelogenin peptide (TRAP) were purified until they represented single chromatographic peaks on a C18 RP-HPLC column and a single band on SDS-PAGE. The numbers under the chromatograms indicate time (in min). (B) Mass spectrometry of LRAP gave a value of 6618.77 Da (calculated mass, 6617.50), and TRAP gave a value of 5404.28 (calculated mass, 5404.08), which closely matched the calculated masses of the single phosphosphorylated proteins. (C) The final purification of Klk4 was on a C18 column, where Klk4 was collected from a well-defined chromatographic peak and appeared as a doublet above 30 kDa on SDS-PAGE stained with Coomassie Brilliant Blue (CBB), on a gelatin zymogram (Z), and on a Western blot (W). The final purification of Mmp-20 was on a heparin affinity column. Mmp-20 could not be detected by SDS-PAGE, but was evident on a casein zymogram (Z) and on a Western blot stained with an anti-peptide antibody raised against its C-terminal hemopexin-like domain. The Mmp-20 catalytic domain appears as lower bands on the zymogram, but is not detected by the antibody raised against the hemopexin-like domain.(D) Standard aliquots of Mmp-20 and (E) Klk4 were used to digest recombinant pig amelogenin (rP172), LRAP, and TRAP for 0, 1, 2, 4, 8, 12, and 24 hrs. The protein without enzyme was run as a control (C). Both enzymes digested rP172, but produced different patterns of digestion products. Mmp-20 showed little activity against LRAP, but was able to produce a slightly smaller product by 24 hrs. Klk4 degraded LRAP by 8 hrs and generated multiple products. Mmp-20 showed no appreciable activity against TRAP, while Klk4 degraded TRAP.
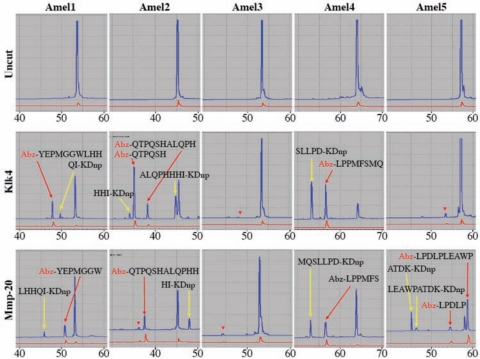
Single aliquots of Klk4 and Mmp-20 did not exhibit detectable chromatographic peaks at 230 nm (Appendix Fig. 2) and were used to digest 5 fluorescent peptides covering all of the major amelogenin (P173) cleavage sites that were previously identified by characterization of amelogenin cleavage products isolated from porcine secretory-stage enamel (Fukae and Shimizu, 1983; Yamakoshi et al., 1994, 2003). After 24 hrs, the digestions were characterized by LC-MSMS and by RP-HPLC monitored by UV and fluorescence detectors (Figs. 2, 3). Mmp-20 cleaved amelogenin sequences after Trp45 (in Amel1) and Ser148 (in Amel4). In vivo, these cleavages generate the most abundant amelogenin cleavage products in porcine secretory-stage enamel: the 20-kDa (Met1-Ser148), TRAP, and the 13-kDa (Leu46-Ser148) amelogenins. Klk4 was not able to catalyze either of these cleavages. Mmp-20 was also able to catalyze all of the cleavages that generate the less-abundant amelogenin cleavage products in secretory-stage enamel. Mmp-20 cleaved the Amel5 sequence after Pro162 that in vivo generates the 23-kDa (Met1-Pro162) amelogenin and cleaved the Amel2 sequence (in the 48-hour incubation) after His62 and Ala63 that in vivo yields “extended TRAP” (Met1-His62 and Met1-Ala63) and the 11-kDa (Ala63-Ser148 and Leu64-Ser148) amelogenin. Klk4 was not able to catalyze the cleavage of amelogenin sequences after Pro162 or Ala63, but was able to catalyze the cleavage after His62. However, cleavage after both His62 and Ala63 is used to generate the 11-kDa amelogenin in vivo (Appendix Fig. 1).
Figure 2.
Digestion of fluorescent peptides Amel1 (Tyr39-Ile50), Amel2 (Gln57-Ile70), Amel3 (Gln101-Gln113), Amel4 (Leu143-Asp155), and Amel5 (Leu153-Lys166). The numbers under the chromatograms indicate time (in min). The first row shows the UV (blue) and fluorescent (red) chromatograms for each uncut peptide. The second and third rows show each peptide after a 24-hour digestion with a standard aliquot of Klk4 or Mmp-20, respectively. Red arrows point to peaks containing a fluorescent (Abz-containing) cleavage product. Yellow arrows point to cleavage products that do not fluoresce (lack the Abz). Arrowheads point to minor fluorescent (Abz-containing) cleavage products that were not characterized in these 24-hour digests, but became more prominent in 48-hour digests (data not shown). Column 1 (Amel1) shows that Mmp-20 catalyzed the TRAP cleavage (after Trp45), but Klk4 did not. Column 2 (Amel2) shows that Klk4 can cleave after His62. Mmp-20 cleaved Amel2 after His68 near the end of the peptide. This cleavage has not been detected in enamel extracts (in vivo) and might be an artifact of the use of a peptide substrate. Mmp-20 generated multiple cleavages of Amel2 after 48 hrs (Appendix). Neither enzyme showed much activity for Amel3, which contained minor cleavage sites previously shown to be susceptible to cleavage by recombinant Mmp-20 (Ryu et al., 1999). Column 4 (Amel4) shows that Mmp-20, but not Klk4, catalyzes the cleavage after Ser148 that generates the 20-kDa amelogenin. Column 5 (Amel5) shows that Mmp-20, but not Klk4, catalyzes the cleavage after Pro162 that generates the 23-kDa amelogenin.
Figure 3.
Sites cleaved by Klk4 and Mmp-20 in the 5 fluorescent peptides. Arrows indicate sites cleaved after 24 hrs. Arrowheads indicate sites in Amel2 cleaved by Mmp-20 in a 48-hour digest (after Gln60, Ser61, His62, and Ala63). The His62 and Ala63 sites are used in a minor processing pathway that generates extended TRAP and the 11-kDa amelogenin (Appendix).
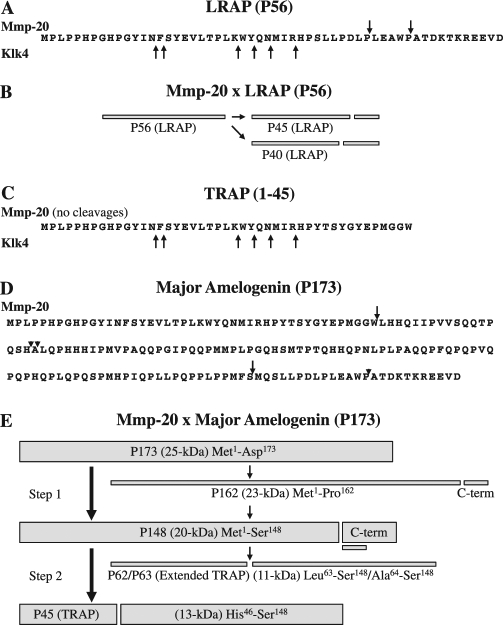
The cleavage of fluorescent peptides and characterization of the sites cleaved by mass spectrometry clearly demonstrated that Mmp-20 is capable of cleaving amelogenin sequences at all of the sites that are cleaved during the secretory stage to generate the main cleavage products of the major amelogenin isoform (P173). Because of its smaller size (56 amino acids), LRAP was synthesized in its entirety without fluorescent tags, digested with Mmp-20 and Klk4, and the sites cleaved were identified by LC-MSMS analysis. Mmp-20 cleaved LRAP after Pro45 and Pro40, whereas Klk4 did not cleave at these sites, but instead degraded LRAP by cleaving at other sites (Figs. 4A, 4B). Finally, Mmp-20 showed little to no activity against TRAP, whereas KLK4 degraded TRAP (Fig. 4C).
Figure 4.
Summary of the digestion of porcine LRAP, TRAP, and the major amelogenin isoform (P173) by Mmp-20 and Klk4. (A) The porcine LRAP sequence. Downward pointing arrows mark the two processing sites (after Pro40 and Pro45) catalyzed by Mmp-20. Upward pointing arrows are degradation sites catalyzed by Klk4, as determined by LC-MSMS analysis of a 24-hour digest of LRAP. (B) Diagram showing the processing of LRAP by Mmp-20. (C) The porcine TRAP sequence. Mmp-20 has very low catalytic activity against TRAP. Upward pointing arrows are degradation sites catalyzed by Klk4, as determined by LC-MSMS analysis of a 24-hour digest of TRAP. (D) Porcine 173-amino-acid major amelogenin (P173) sequence. Downward arrows show the major Mmp-20 processing sites; arrowheads show the minor Mmp-20 processing sites. (E) Diagram showing the major and minor processing sites that generate the most abundant amelogenin cleavage products in porcine secretory-stage enamel. The secreted protein (P173) is usually processed in two steps. The initial cleavage is after Ser148 and generates P148, the most abundant amelogenin cleavage product in the matrix. The second cleavage is after Trp45 and generates TRAP and the 13-kDa amelogenins. A less-used processing pathway involves an initial cleavage after Pro162, generating the 23-kDa amelogenin, which is cleaved again to generate P148. P148 is also cleaved in a less-often-used alternative pathway that generates extended TRAP and the 11-kDa amelogenin.
Discussion
In situ hybridization studies have demonstrated that Mmp-20 is expressed early in enamel formation, while Klk4 is not expressed until the transition stage (Bègue-Kirn et al., 1998; Hu et al., 2000, 2002; Simmer et al., 2004). Digestion of recombinant amelogenin (rP172; Pro2-Asp173) with recombinant Mmp-20 yielded all of the major porcine amelogenin cleavage products that accumulate in secretory-stage enamel (Ryu et al., 1999), while digestion of rP172 by Klk4 produced an entirely different set of cleavages (Ryu et al., 2002). Here we demonstrated, through the digestion of fluorescent peptides, that Mmp-20 is able to digest amelogenin sequences at the exact sites that yield all of the major amelogenin cleavage products: the 23-kDa (Met1-Pro162), 20-kDa (Met1-Ser148), 13-kDa (Leu46-Ser148), 11-kDa (Ala63-Ser148/Leu64-Ser148) extended TRAP (Met1-His62/Met1-Ala63), and TRAP (Met1-Trp45). Klk4 cannot cleave amelogenin sequences at any of the sites that generate the major amelogenin cleavage products (except after His62), but freely cleaves at many other sites. Only Mmp-20 processes LRAP after Pro40 and Pro45, and Mmp-20 does not degrade LRAP further. Bovine Mmp-20 also catalyzes the Pro45 cleavage of LRAP (Li et al., 1999). Klk4, in contrast, degrades both TRAP and LRAP. Besides cleaving at a host of other sites, Klk4 efficiently cleaves phosphorylated TRAP and LRAP after Asn14 and Phe15, near the phosphorylated Ser16.
Our findings support the conclusion that, in developing pig teeth, Mmp-20 is the only significant proteolytic activity in the enamel extracellular space during the secretory stage. It processes amelogenins into a group of cleavage products that accumulate and are only slowly degraded further by Mmp-20. The tyrosine- and leucine-rich amelogenin peptides accumulate because Mmp-20 has little activity to degrade them. Klk4 cannot be active during the secretory stage, since it would prevent their accumulation. The widening of secretory-stage enamel crystals with depth (Daculsi and Kerebel, 1978) is likely due to the slow degradation of amelogenins by Mmp-20. The importance of the processing function of Mmp-20 is suggested by the loss of prism structure and failure of the enamel layer to achieve full thickness in the Mmp20 null mice, while its degradative function is evident by the retention of enamel proteins and the tendency of the enamel to chip off in these mice (Caterina et al., 2002). Klk4, in contrast, does not contribute to the processing of amelogenin, but is dedicated to its degradation during the transition and maturation stages.
Supplementary Material
Acknowledgments
We thank the Michigan State University Meat Laboratory for fresh developing molars.
Footnotes
This investigation was supported by USPHS Research Grants DE015846 and DE016276 from the NIDCR/NIH.
All authors declare that there are no conflicting interests.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Bartlett JD, Simmer JP. (1999). Proteinases in developing dental enamel. Crit Rev Oral Biol Med 10:425-441 [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Simmer JP, Xue J, Margolis HC, Moreno EC. (1996). Molecular cloning and mRNA tissue distribution of a novel matrix metalloproteinase isolated from porcine enamel organ. Gene 183:123-128 [DOI] [PubMed] [Google Scholar]
- Bègue-Kirn C, Krebsbach PH, Bartlett JD, Butler WT. (1998). Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin: tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur J Oral Sci 106:963-970 [DOI] [PubMed] [Google Scholar]
- Caterina JJ, Skobe Z, Shi J, Ding Y, Simmer JP, Birkedal-Hansen H, et al. (2002). Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem 277:49598-49604 [DOI] [PubMed] [Google Scholar]
- Daculsi G, Kerebel B. (1978). High-resolution electron microscope study of human enamel crystallites: size, shape, and growth. J Ultrastruct Res 65:163-172 [DOI] [PubMed] [Google Scholar]
- Fincham AG, Moradian-Oldak J. (1993). Amelogenin post-translational modifications: carboxy-terminal processing and the phosphorylation of bovine and porcine “TRAP” and “LRAP” amelogenins. Biochem Biophys Res Commun 197:248-255 [DOI] [PubMed] [Google Scholar]
- Fincham AG, Belcourt AB, Termine JD, Butler WT, Cothran WC. (1981). Dental enamel matrix: sequences of two amelogenin polypeptides. Biosci Rep 1:771-778 [DOI] [PubMed] [Google Scholar]
- Fincham AG, Hu YY, Pavlova Z, Slavkin HC, Snead ML. (1989). Human amelogenins: sequences of “TRAP” molecules. Calcif Tissue Int 45:243-250 [DOI] [PubMed] [Google Scholar]
- Fincham AG, Moradian-Oldak J, Simmer JP. (1999). The structural biology of the developing dental enamel matrix. J Struct Biol 126:270-299 [DOI] [PubMed] [Google Scholar]
- Fukae M, Shimizu M. (1983). Amino acid sequence of the main component of porcine enamel proteins. Jpn J Oral Biol 25:29 [Google Scholar]
- Fukae M, Ijiri H, Tanabe T, Shimizu M. (1979). Partial amino acid sequences of two proteins in developing porcine enamel. J Dent Res 58(Spec Iss B):1000-1001 [DOI] [PubMed] [Google Scholar]
- Fukae M, Tanabe T, Ijiri H, Shimizu M. (1980). Studies on porcine enamel proteins: a possible original enamel protein. Tsurumi U Dent J 6:87-94 [PubMed] [Google Scholar]
- Fukae M, Tanabe T, Uchida T, Lee SK, Ryu OH, Murakami C, et al. (1998). Enamelysin (matrix metalloproteinase-20): localization in the developing tooth and effects of pH and calcium on amelogenin hydrolysis. J Dent Res 77:1580-1588 [DOI] [PubMed] [Google Scholar]
- Gibson CW, Golub EE, Ding W, Shimokawa H, Young M, Termine JD, et al. (1991). Identification of the leucine-rich amelogenin peptide (LRAP) as the translation product of an alternatively spliced transcript. Biochem Biophys Res Commun 174:1306-1312 [DOI] [PubMed] [Google Scholar]
- Hart PS, Hart TC, Michalec MD, Ryu OH, Simmons D, Hong S, et al. (2004). Mutation in kallikrein 4 causes autosomal recessive hypomaturation amelogenesis imperfecta. J Med Genet 41:545-549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CC, Bartlett JD, Zhang CH, Qian Q, Ryu OH, Simmer JP. (1996). Cloning, cDNA sequence, and alternative splicing of porcine amelogenin mRNAs. J Dent Res 75:1735-1741 [DOI] [PubMed] [Google Scholar]
- Hu JC, Ryu OH, Chen JJ, Uchida T, Wakida K, Murakami C, et al. (2000). Localization of EMSP1 expression during tooth formation and cloning of mouse cDNA. J Dent Res 79:70-76 [DOI] [PubMed] [Google Scholar]
- Hu JC, Sun X, Zhang C, Liu S, Bartlett JD, Simmer JP. (2002). Enamelysin and kallikrein-4 mRNA expression in developing mouse molars. Eur J Oral Sci 110:307-315 [DOI] [PubMed] [Google Scholar]
- Ikawa T, Kakegawa A, Nagano T, Ando H, Yamakoshi Y, Tanabe T, et al. (2005). Porcine amelogenin is expressed from the X and Y chromosomes. J Dent Res 84:144-148 [DOI] [PubMed] [Google Scholar]
- Kim JW, Simmer JP, Hart TC, Hart PS, Ramaswami MD, Bartlett JD, et al. (2005). MMP-20 mutation in autosomal recessive pigmented hypomaturation amelogenesis imperfecta. J Med Genet 42:271-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Machule D, Gao C, DenBesten PK. (1999). Activation of recombinant bovine matrix metalloproteinase-20 and its hydrolysis of two amelogenin oligopeptides. Eur J Oral Sci 107:352-359 [DOI] [PubMed] [Google Scholar]
- Li W, Gibson CW, Abrams WR, Andrews DW, DenBesten PK. (2001). Reduced hydrolysis of amelogenin may result in X-linked amelogenesis imperfecta. Matrix Biol 19:755-760 [DOI] [PubMed] [Google Scholar]
- Li W, Gao C, Yan Y, DenBesten P. (2003). X-linked amelogenesis imperfecta may result from decreased formation of tyrosine rich amelogenin peptide (TRAP). Arch Oral Biol 48:177-183 [DOI] [PubMed] [Google Scholar]
- Lu Y, Papagerakis P, Yamakoshi Y, Hu JC, Bartlett JD, Simmer JP. (2008). Functions of KLK4 and MMP-20 in dental enamel formation. Biol Chem 389:695-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu O, Hu JC, Yamakoshi Y, Villemain JL, Cao X, Zhang C, et al. (2002). Porcine kallikrein-4 activation, glycosylation, activity, and expression in prokaryotic and eukaryotic hosts. Eur J Oral Sci 110:358-365 [DOI] [PubMed] [Google Scholar]
- Ryu OH, Fincham AG, Hu CC, Zhang C, Qian Q, Bartlett JD, et al. (1999). Characterization of recombinant pig enamelysin activity and cleavage of recombinant pig and mouse amelogenins. J Dent Res 78:743-750 [DOI] [PubMed] [Google Scholar]
- Simmer JP, Fukae M, Tanabe T, Yamakoshi Y, Uchida T, Xue J, et al. (1998). Purification, characterization, and cloning of enamel matrix serine proteinase 1. J Dent Res 77:377-386 [DOI] [PubMed] [Google Scholar]
- Simmer JP, Sun X, Yamada Y, Zhang CH, Bartlett JD, Hu JC-C. (2004). Enamelysin and kallikrein-4 expression in the mouse incisor. In: Biomineralization: formation, diversity, evolution and application. Kobayashi I, Ozawa H. editors. Hadano, Japan: Tokai University Press, pp. 348-352 [Google Scholar]
- Smith CE. (1998). Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9:128-161 [DOI] [PubMed] [Google Scholar]
- Tanabe T. (1984). Purification and characterization of proteolytic enzymes in porcine immature enamel. Tsurumi U Dent J 10:443-452 [article in Japanese]. [PubMed] [Google Scholar]
- Tanimoto K, Le T, Zhu L, Witkowska H, Robinson S, Hall S, et al. (2008). Reduced amelogenin-MMP20 interactions in amelogenesis imperfecta. J Dent Res 87:451-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakoshi Y, Tanabe T, Fukae M, Shimizu M. (1989). Amino acid sequence of porcine 25kDa amelogenin. In: Tooth enamel V., Suga S, Fearnhead RW, editors. Tokyo: Florence Publishers, pp. 314-321 [Google Scholar]
- Yamakoshi Y, Tanabe T, Fukae M, Shimizu M. (1994). Porcine amelogenins. Calcif Tissue Int 54:69-75 [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y, Hu JC-C, Ryu OH, Tanabe T, Oida S, Fukae M, et al. (2003). A comprehensive strategy for purifying pig enamel proteins. In: Biomineralization: formation, diversity, evolution and application. Kobayashi I, Ozawa H. editors. Hadano, Japan: Tokai University Press, pp. 326-332 [Google Scholar]
- Yuan ZA, Collier PM, Rosenbloom J, Gibson CW. (1996). Analysis of amelogenin mRNA during bovine tooth development. Arch Oral Biol 41:205-213 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.