Abstract
Objectives
Sublingual immunotherapy (SLIT) has recently received much attention around the world as a treatment for allergic rhinitis. This study aimed to investigate the efficacy and adverse effects of SLIT in Korean patients with allergic rhinitis caused by house dust mites. The treatment compliance and the patient satisfaction with SLIT were also assessed.
Methods
The patients who were sensitized to Dermatophagoides pteronyssinus and Dermatophagoides farinae and who started SLIT between November 2007 and July 2008 were included in this study. The symptom questionnaires, which included items on rhinorrhea, sneezing, nasal obstruction, itchy nose, olfactory disturbance, eye discomfort and sleep disturbance, were obtained before and 6 months after SLIT. The patient satisfaction and the adverse effects were also investigated.
Results
One hundred forty-two patients started SLIT and 98 of them continued SLIT for 6 months or more. Ninety-two of the 98 patients completed the questionnaires. The duration of receiving SLIT was 9.8 months on average (range, 6 to 13 months). All the symptoms of allergic rhinitis were improved with SLIT. Forty-five percent of the patients were satisfied for SLIT, while 12% were unsatisfied. The incidence of adverse effects was 12% during maintenance therapy, although it was 48% during the up-dosing phase. The drop-out rate of SLIT was 31.0%.
Conclusion
The subjective symptoms were improved with SLIT in Korean patients with allergic rhinitis for house dust mites. Yet the drop out rate was high despite of the symptomatic improvement.
Keywords: Allergic rhinitis, Immunotherapy, Compliance
INTRODUCTION
The treatment of allergic rhinitis (AR) includes avoidance, pharmacologic treatment and immunotherapy. Contrary to other treatment modalities, immunotherapy is the only therapeutic option that modifies the basic allergic mechanism by inducing desensitization and an anergy state for the allergen. Immunotherapy by subcutaneous allergen injection (subcutaneous immunotherapy, SCIT) has been used since Noon's first report in 1911 and it has been demonstrated to be a clinically effective treatment for allergic disorders such as rhinoconjunctivitis or asthma. However, due to the inconvenience, invasiveness and severe systemic adverse effects of SCIT, other methods of administering allergen have been developed such as the oral, sublingual, nasal or bronchial routes (1, 2). Among them, the sublingual route is now widely used to replace the subcutaneous route and it was acknowledged by the WHO Allergic Rhinitis and Its Impact on Asthma (ARIA) group that sublingual immunotherapy (SLIT) could be effective for pollen or mite AR (3). However, there have been no studies on Asian patients sensitized to Dermatophagoides pteronyssinus (Dp) and Dermatophagoides farina (Df).
SLIT has recently been introduced and it is available for treating AR in Korea. This study was conducted to investigate the short-term efficacy, the adverse effects and the patient satisfaction with SLIT for Korean patients with AR that is caused by house dust mites.
MATERIALS AND METHODS
Patient selection
The patients who were diagnosed with AR at Seoul National University Hospital or Seoul National University Bundang Hospital and who were sensitized to Dp and Df were indicated for SLIT. The patients who started to receive SLIT between November 2007 and July 2008 were included in this study. The patients who had a history of asthma, atopic dermatitis or bronchial hyper-responsiveness, but who didn't need regular medication were enrolled, while the patients with symptomatic asthma, atopic dermatitis or bronchial hyper-responsiveness that required regular medication such as oral steroid, steroid inhaler or anti-histamine were excluded. The subjects who suffered from immunologic or hematologic disorder were also excluded. Sensitization to Dp and Df was defined as 1) a serum specific IgE level for Dp and Df ≥0.7 UI/mL on multiple allergen simultaneous tests (MAST) or 2) the wheal diameters for Dp and Df were equal to or greater than that of the positive control (histamine) on skin prick tests. The Institutional Review Board of the Clinical Research Institute at Seoul National University Hospital approved the study protocol (H-0811-034-262).
Immunotherapy
Standardized extract of house dust mites (50% Dp/50% Df, Pangramin® SLIT, ALK-Abello, Madrid, Spain) was used for the immunotherapy. During a 4-week up-dosing phase, the participants took daily increasing doses from 1 to 5 drops of 1.6 STU/mL solution from day 1 to 10, 1 to 5 drops of 8 STU/mL solution from day 11 to day 15, 1 to 5 drops of 40 STU/mL solution from day 16 to day 20, 1 to 5 drops of 200 STU/mL solution from day 21 to day 25 and 1 to 5 drops of 1,000 STU/mL solution from day 26 to day 30. After reaching the maintenance dose (5 drops of 1,000 STU/mL solution), the participants took the allergen 3 times a week during the maintenance phase. The patients had to keep the drops of allergen under their tongue for 2-3 min before swallowing. When the symptoms of AR were aggravated during immunotherapy, the patients were allowed to use antihistamine and/or intranasal steroid.
Symptom score and satisfaction
All the patients were asked to complete the questionnaires before SLIT and 6 months after receiving SLIT without medication. The questionnaire included items on rhinorrhea, sneezing, nasal obstruction, itchy nose, olfactory disturbance, eye discomfort and sleep discomfort. Each symptom was graded from 0 to 5 (0=no symptom, 1=very mild symptom, 2=mild, 3=moderate, 4=severe, 5=very severe). The total nasal symptom score (TNSS) was defined as the sum of the scores of five nasal symptoms, to, rhinorrhea, sneezing, nasal obstruction, itchy nose and olfactory disturbance. The patients were also asked to assess their use of anti-allergic medications such as anti-histamine, anti-leukotriene, and intranasal steroid according to 3 categories of, "increased", "similar", and "decreased", comparing months after SLIT to before SLIT. Patient satisfaction was evaluated by 3 caterories of "satisfied", "fair", and "unsatisfied" simultaneously with the symptom evaluation after SLIT. If the participants had discontinued SLIT, the reason of cessation was ascertained by telephone interview.
Adverse effects
To evaluate the adverse effects, the participants recorded the adverse effects related to SLIT everyday on diary cards during the whole period.
Statistical analysis
The symptoms before and after SLIT were statistically analyzed by paired t-tests. Student's t-test, paired t-tests and the Wilcoxon signed rank test were used to compare the symptomatic changes. SPSS ver. 12.0 was used for all the statistical analysis. All of the tests were 2-tailed and the criterion for statistical significance was set at P<0.05.
RESULTS
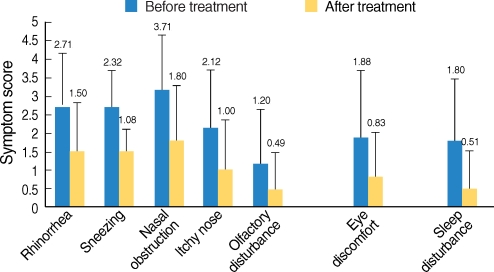
One hundred forty-two patients (mean age, 16.3 yr; range, 5 to 71 yr) were enrolled in this study. Ninety-one of the patients were male and 51 were female. Ninety-eight of 142 patients continued SLIT and 44 discontinued. Ninety-two of the 98 patients who continued SLIT were considered in this study and the other six were excluded due to the lack of data. The mean age of the 92 patients under consideration was 15.8 yr (range, 5 to 53 yr) and the duration of allergic rhinits was a mean of 7.0 yr (range, 1 to 30 yr). The mean duration of receiving SLIT was 9.8 months (range, 6 to 13 months). All the symptoms, including the nasal symptoms, the eye discomfort and the sleep disturbance, were significantly improved after SLIT (Fig. 1). For the questionnaire about the use of anti-allergic medication, 63 patients responded "decreased use", 24 patients responded "similar use", and 6 patients responded "decreased use".
Fig. 1.
Changes of the symptom scores for all the patients who received sublingual immunotherapy (SLIT). All the symptoms were improved after SLIT with statistical significance (P<0.05 by paired t-tests).
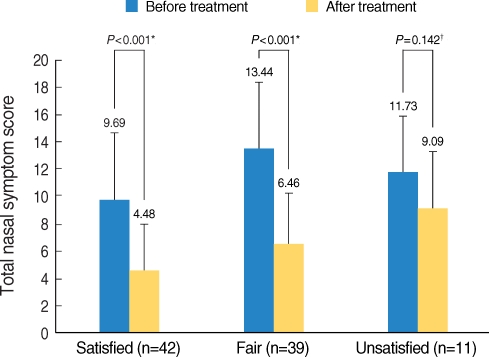
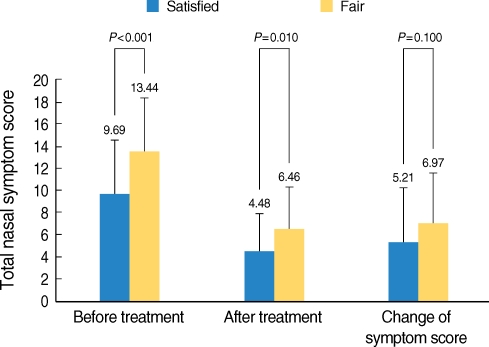
When the patient satisfaction was assessed, forty-two patients (45.7%) answered as "satisfied", 39 patients (42.4%) answered as "fairly satisfied" and 11 patients (12%) answered as "unsatisfied". When the TNSS was compared before and after SLIT according to the satisfaction, respectively, the patients who were "satisfied" or "fairly satisfied" had significant improvement of their TNSS. However, the TNSS was not significantly changed for the unsatisfied patients (Fig. 2). When comparing symptoms between the patients who were "satisfied" and "fairly satisfied", the latter had more severe symptoms before and after SLIT. However, the degree of changes of the TNSS was not different for each group (Fig. 3).
Fig. 2.
Comparing the total nasal symptom scores (TNSSs) before and after sublingual immunotherapy (SLIT) in the satisfied, the fairly satisfied and the unsatisfied groups. The satisfied and the fairly satisfied group got better, with statistical significance. However, the NTSS of the unsatisfied group was not improved after SLIT.
*statistical analysis using paired t-tests; †statistical analysis using the Wilcoxon signed rank test.
Fig. 3.
Comparing the total nasal symptom scores (TNSSs) of the satisfied group with those of the fairly satisfied group. The fairly satisfied group had higher NTSSs before and after sublingual immunotherapy than the satisfied group. However, the change of the TNSS in the satisfied group didn't differ from that in the fairly satisfied group.
*P<0.05 (Student's t-test).
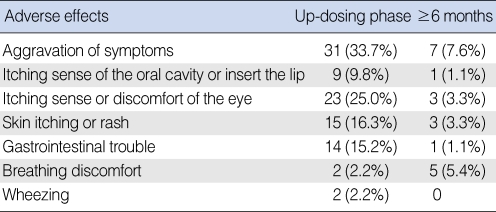
The incidence of adverse effects of SLIT was 52.1% (48 of 92 patients) during first 30 days, which is the up-dosing phase. Aggravation of the AR symptoms was the most common adverse effect during the up-dosing phase (Table 1). After 6 months or more, 13 patients (14.1%) had experienced temporary adverse effects (Table 1). However, these adverse effects, including wheezing and breathing discomfort, were temporary and they subsided spontaneously without medication. None of the patients needed to visit an emergency room due to adverse effects.
Table 1.
Adverse effects of sublingual immunotherapy
The numbers are not mutually exclusive.
Forty-four patients (30.1%) with mean age of 17.3 yr (range, 5 to 71 yr) discontinued SLIT. The male to female ratio was 26:18. Among these patients, 36 patients responded to our telephone interview. Twenty-one of 36 patients ceased SLIT after 3 months and 8 patients ceased SLIT after 4-6 months and 7 patients ceased SLIT after 7 months. The reasons for discontinuation of SLIT were inability to take medication according to schedule (n=10, 27.8%), ineffectiveness (n=8, 22.2%), the discomfort of regularly visiting the hospital regularly (n=6, 16.7%) and adverse effects (n=5, 13.9%). The adverse effects causing discontinuance included aggravation of the symptoms of AR (n=2), fever (n=1), gastrointestinal trouble (n=1) and skin rash (n=1). High cost (n=2), the problem of storing the allergen (n=1) and pregnancy (n=1) were also reported. Three patients answered that they thought SLIT was not useful any more due to the improvement of symptoms.
DISCUSSION
Clinical trials for evaluating the safety and efficacy of SLIT were initiated in 1980s and in 2001, and the WHO ARIA group reported that SLIT could be effective for treating AR (3). A meta analysis involving 21 trials and 959 SLIT subjects indicated that SLIT had efficacy against allergic symptoms (4). As the evidence has accumulated, SLIT has received much attention as a valid treatment for AR around the world.
This study revealed that all the symptoms were changed for the better after SLIT in patients with house dust mites AR underwent SLIT. It was not easy to say that house dust mites SLIT had efficacy against AR in our series because the duration of the follow-up was short and our study was not a double-blind, placebo controlled trial. However, placebo-control studies about SLIT have been published since 1990 around the world and many of them reported that SLIT was an effective treatment for pollen-induced allergic airway diseases, and especially seasonal AR (5-10). The effectiveness of SLIT for patients with house dust mites AR has recently been revealed (2, 11). Based on others studies, we anticipate the good long-term results of our series.
Forty six percent of the patients were satisfied with receiving SLIT and 12% were unsatisfied. Forty two percent of the patients answered as fairly satisfied. When comparing the NTSSs before and after SLIT for the unsatisfied patients, the NTSS was not significantly improveder SLIT. Furthermore, although both the satisfied and the fairly satisfied groups got better, the NTSSs before and after SLIT vere higher in the latter group. Considering the relationship between the satisfaction and the symptoms, the degree of improvement of symptoms affected the patient satisfaction. As this study included the patients who were treated with SLIT for 6-13 months and a longer duration of SLIT induced better results (12), it is thought that the proportion of the satisfied group will increase with time. One study that was conducted in Korea and that assessed the patient satisfaction for SCIT revealed that 47.2%, 38.9%, and 13.9% of the patients receiving SCIT felt satisfied, fairly satisfied and unsatisfied, respectively, and the proportion of the satisfied patients was higher in the 3-yr immunotherapy group than that in 1- or 2-yr immunotherapy group (13). The results of that report were similar to ours and the short-term satisfaction with SLIT was not different from that for SCIT.
SLIT induces much fewer systemic side effects and the majority of adverse effects have been local reactions (4, 14). No systemic allergic reactions were reported in the clinical trials of commercial allergen extracts. Three case reports of anaphylaxis after SLIT were published in the English medical literature and none of these reactions occurred with using the commercially available allergen. Two of these case reports described anaphylaxis that was due to a mixture of multiple allergens and the third case was due to latex (15-17). There have been no cases of mortality, and SLIT is known to be safer than SCIT. In our series, 52% of the 92 patients complained of adverse effects. The most common adverse effect was aggravation of the AR symptoms. Although some patients complained of breathing discomfort or wheezing, there was no case requiring other medical intervention or emergency treatment due to adverse effects. Two patients reported wheezing symptoms during the up-dosing phase. These two patients had a history of asthma and positive methacholine provocation test results. Their wheezing symptoms were relieved spontaneously. Two patients complained of breathing discomfort and they didn't have asthma or bronchial hyper-responsiveness and their symptoms resolved without medication. Aggravation of asthmatic symptoms may be caused by allergen application; however, one case-control trial of SLIT reported that the incidence of wheezing, as an adverse effect of SLT, was higher in the control group than that for the patients who received SLIT (18). Therefore, the asthmatic symptoms during SLIT were not only caused by the adverse effect of allergen application, but they were also caused by related factors such as neurosis or environmental effects. The incidence of adverse effects was high during the up-dosing phase in our study, which is similar to the results of other studies (4, 14), and these adverse effects subsided without specific treatment when continuing SLIT. Although the adverse effects subsided without emergency treatment, the incidence of adverse effects in our study was higher than that of other studies. However, the difference of the incidences was not appreciable. While the incidences were reported to be less than 10% in post-marketing surveys (19, 20), one large meta-analytic study showed that the incidence of adverse events in those patients receiving active treatment was 39% compared with approximately 23% in the placebo group (18). The results of that meta-analytic report were similar to ours. Furthermore, we included the patients who were sensitized to house dust mites, which cause perennial allergic disease. Therefore, the symptomatic aggravation of allergic rhinoconjunctivitis, which the participants reported as an adverse effect, involved not only the true adverse effects, but also disease aggravation that was due to environmental allergen.
In Western countries, the drop out rate of SLIT was reported to be 7-30% and the main reason for discontinuation was the inability to take the study medication according to schedule or the lack of efficacy (2, 21, 22). The drop-out rate of our series was 31%, which was relative high when considering the short-term follow-up results. The lack of efficacy and the discomfort with taking medication regularly were the main reasons and half of the drop-out patients discontinued in the first 3 months. Because the inconvenient application of allergen was the main reason for drop-out, the drop out rate could be lowered by developing a more convenient application schedule such as a shortened up-dosing phase, a reduced frequency of allergen application during the maintenance therapy and a user-friendly allergen formula. In another study that used a shortened up-dosing phase (10 days) and a convenient allergen formula, (SLITone®, ALK-Abello, Lainate, Italy), which contained 90 monodose containers and counting drops of allergen solution wasn't required, the drop out rate was lower than ours (22). Moreover, it is necessary to educate the patients to understand SLIT properly because the timing of discontinuance was too short to judge the efficacy of SLIT.
In this study, we observed that subjective symptoms were improved with short-term SLIT for house dust mites despite of only 6-13 months of immunotherapy and 45.7% of the patients were satisfied with SLIT. The adverse effects were mostly local reactions and these subsided without other treatment. The incidence of adverse effects was high in the up-dosing phase and this was lowered after continuing immunotherapy. Therefore, we should be alert for adverse effects during the up-dosing phase. The drop out rate was 31%, which is relatively high compared to other studies. Further long-term studies are required to evaluate if the reduced allergic symptoms persist, which was observed during short-term SLIT, and if the symptoms get better in the patients who didn't show symptom improvement with short-term SLIT. Proper and prudent management of the patients is needed to reduce the drop out rate.
Footnotes
This study was conducted without any conflict-of-interest, third-party funding or financial support.
References
- 1.Esch RE. Sublingual immunotherapy. Curr Opin Otolaryngol Head Neck Surg. 2008 Jun;16(3):260–264. doi: 10.1097/MOO.0b013e3282fc706f. [DOI] [PubMed] [Google Scholar]
- 2.Guez S, Vatrinet C, Fadel R, Andre C. House-dust-mite sublingual-swallow immunotherapy (SLIT) in perennial rhinitis: a double-blind, placebo-controlled study. Allergy. 2000 Apr;55(4):369–375. doi: 10.1034/j.1398-9995.2000.00413.x. [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008 Apr;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson DR, Torres LI, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893. doi: 10.1002/14651858.CD002893. [DOI] [PubMed] [Google Scholar]
- 5.Casanovas M, Guerra F, Moreno C, Miguel R, Maranon F, Daza JC. Double-blind, placebo-controlled clinical trial of preseasonal treatment with allergenic extracts of Olea europaea pollen administered sublingually. J Investig Allergol Clin Immunol. 1994 Nov–Dec;4(6):305–314. [PubMed] [Google Scholar]
- 6.Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, et al. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-gamma. J Allergy Clin Immunol. 1996 Jun;97(6):1356–1365. doi: 10.1016/s0091-6749(96)70205-1. [DOI] [PubMed] [Google Scholar]
- 7.Pradalier A, Basset D, Claudel A, Couturier P, Wessel F, Galvain S, et al. Sublingual-swallow immunotherapy (SLIT) with a standardized five-grass-pollen extract (drops and sublingual tablets) versus placebo in seasonal rhinitis. Allergy. 1999 Aug;54(8):819–828. doi: 10.1034/j.1398-9995.1999.00077.x. [DOI] [PubMed] [Google Scholar]
- 8.Purello-D'Ambrosio F, Gangemi S, Isola S, La Motta N, Puccinelli P, Parmiani S, et al. Sublingual immunotherapy: a double-blind, placebo-controlled trial with Parietaria judaica extract standardized in mass units in patients with rhinoconjunctivitis, asthma, or both. Allergy. 1999 Sep;54(9):968–973. doi: 10.1034/j.1398-9995.1999.00203.x. [DOI] [PubMed] [Google Scholar]
- 9.Dahl R, Kapp A, Colombo G, de Monchy JG, Rak S, Emminger W, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006 Aug;118(2):434–440. doi: 10.1016/j.jaci.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006 Apr;117(4):802–809. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 11.Nuhoglu Y, Ozumut SS, Ozdemir C, Ozdemir M, Nuhoglu C, Erguven M. Sublingual immunotherapy to house dust mite in pediatric patients with allergic rhinitis and asthma: a retrospective analysis of clinical course over a 3-year follow-up period. J Investig Allergol Clin Immunol. 2007;17(6):375–378. [PubMed] [Google Scholar]
- 12.Passalacqua G, Guerra L, Pasquali M, Canonica GW. Non-injection routes for allergen immunotherapy: focus on sublingual immunotherapy. Inflamm Allergy Drug Targets. 2006 Jan;5(1):43–51. doi: 10.2174/187152806775269286. [DOI] [PubMed] [Google Scholar]
- 13.Kim DY, Kwon BW, Son JY. Assessment of satisfaction in patients undergoing immunotherapy for allergic rhinitis using questionnaires. Korean J Otolaryngol-Head Neck Surg. 004 Feb;47(2):132–138. [Google Scholar]
- 14.Frew AJ. Sublingual immunotherapy. N Engl J Med. 2008 May;358(21):2259–2264. doi: 10.1056/NEJMct0708337. [DOI] [PubMed] [Google Scholar]
- 15.Antico A, Pagani M, Crema A. Anaphylaxis by latex sublingual immunotherapy. Allergy. 2006 Oct;61(10):1236–1237. doi: 10.1111/j.1398-9995.2006.01155.x. [DOI] [PubMed] [Google Scholar]
- 16.Dunsky EH, Goldstein MF, Dvorin DJ, Belecanech GA. Anaphylaxis to sublingual immunotherapy. Allergy. 2006 Oct;61(10):1235. doi: 10.1111/j.1398-9995.2006.01137.x. [DOI] [PubMed] [Google Scholar]
- 17.Eifan AO, Keles S, Bahceciler NN, Barlan IB. Anaphylaxis to multiple pollen allergen sublingual immunotherapy. Allergy. 2007 May;62(5):567–568. doi: 10.1111/j.1398-9995.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 18.Canonica GW, Passalacqua G. Sublingual immunotherapy in the treatment of adult allergic rhinitis patients. Allergy. 2006;61(Suppl 81):20–23. doi: 10.1111/j.1398-9995.2006.01161.x. [DOI] [PubMed] [Google Scholar]
- 19.Lombardi C, Gargioni S, Melchiorre A, Tiri A, Falagiani P, Canonica GW, et al. Safety of sublingual immunotherapy with monomeric allergoid in adults: multicenter post-marketing surveillance study. Allergy. 2001 Oct;56(10):989–992. doi: 10.1034/j.1398-9995.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- 20.Rienzo VD, Minelli M, Musarra A, Sambugaro R, Pecora S, Canonica WG, et al. Post-marketing survey on the safety of sublingual immunotherapy in children below the age of 5 years. Clin Exp Allergy. 2005 May;35(5):560–564. doi: 10.1111/j.1365-2222.2005.02219.x. [DOI] [PubMed] [Google Scholar]
- 21.Roder E, Berger MY, de Groot H, Gerth van Wijk R. Sublingual immunotherapy in youngsters: adherence in a randomized clinical trial. Clin Exp Allergy. 2008 Oct;38(10):1659–1667. doi: 10.1111/j.1365-2222.2008.03060.x. [DOI] [PubMed] [Google Scholar]
- 22.Passalacqua G, Musarra A, Pecora S, Amoroso S, Antonicelli L, Cadario G, et al. Quantitative assessment of the compliance with a once-daily sublingual immunotherapy regimen in real life (EASY Project: Evaluation of A novel SLIT formulation during a Year) J Allergy Clin Immunol. 2006 Apr;117(4):946–948. doi: 10.1016/j.jaci.2005.12.1312. [DOI] [PubMed] [Google Scholar]