Abstract
A petrous apex cholesterol granuloma (PACG) is the most common lesion of the petrous apex mass. Affected patients present with various symptoms such as hearing loss, vertigo, headache, tinnitus, facial spasms, and diplopia. We report the case of a 32-yr-old man with a PACG, who was first misdiagnosed with Ménière's disease. He was placed on a low-salt diet, and prescribed medication from another hospital, for several months, but the symptoms persisted and worsened. The patient presented to the emergency room complaining of left facial twitching and numbness. To rule out a central neurological lesion, temporal bone magnetic resonance imaging was carried out and a 2.5 cm mass with high signal intensity on T1- and T2-weighted imaging, without gadolinium enhancement, was found. Because of the hearing and facial problems, we drained cholesterol-bearing material via an infralabyrinthine approach using a computer aided image-guided surgical device, the BrainLAB®. After the operation, the vertigo and hearing loss were no longer present. It is likely that the patent's Ménière's disease-like symptoms were due to the compression of the endolymphatic sac by a PACG.
Keywords: Cholesterol granuloma, Petrous apex, Endolymphatic hydrops
INTRODUCTION
A petrous apex cholesterol granuloma (PACG) is a very common lesion of the petrous apex. Muckle et al. (1) reported hearing loss as the most common symptom of petrous apex lesions, followed by vestibular dysfunction, headache, tinnitus, facial spasms, and diplopia. However, Castillo et al. (2) reported headache and facial nerve weakness as the most common symptoms, and that most patients presented with more than one symptom. Thus, there are no disease specific symptoms specific to PACG; symptoms depend on the size and site of the PACG. Here, we describe a case of PACG that manifested with a whirling-type vertigo and fluctuating hearing loss, initially mistaken for Ménière's disease.
CASE REPORT
A 32-yr-old, previously healthy man visited the International Clinic of Asan Medical Center in May 2008, complaining of intermittent whirling-type vertigo. At that time, no obvious neurological deficit or nystagmus was found on examination. Previously, he had been prescribed medication from other hospitals. He took dimenhydrinate for about 2 weeks, but the vertigo worsened. He was referred to our Department of Otolaryngology. Each vertigo attack lasted about 10-15 min, and the episodes occurred twice a month. In addition, he complained of right sided tinnitus, a sense of ear fullness, and had a fluctuating hearing disturbance. Both tympanic membranes were intact and otorrhea was absent. Pure tone audiometry, speech audiometry, vestibular evoked myogenic potential (VEMP), electrocochleography, and caloric testing, were planned, and a low-salt diet was recommended (3).
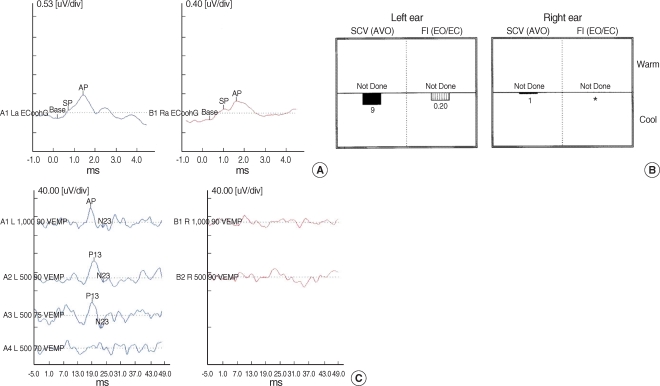
Bone and air conduction values on the right side were 12 dB and 15 dB, respectively, with up-sloping patterns. The cochlear summating potential to auditory nerve action potential ratio (SP/AP ratio) of the electrocochleogram was 0.43-0.56 (Fig. 1A) (4). Video caloric testing demonstrated a right unilateral weakness of 79% (Fig. 1B). The VEMP response was absent on the right side (Fig. 1C). We diagnosed the patient with Ménière's disease of the right side and prescribed ginkgo biloba extract and betahistidine mesylate for 2 weeks. However, 4 days later the patient presented to our emergency room because of muscle twitching and numbness of the right side of the face. The patient was referred to a neurosurgeon.
Fig. 1.
(A) This electrocochleogram showed that the patient's ratio of right cochlear summating potential to auditory nerve action potential ratio (SP/AP ratio) was 0.43-0.56. (B) Video caloric testing demonstrated a right unilateral weakness of 79%. (C) Vestibular evoked myogenic potential (VEMP) response was absent on the right side.
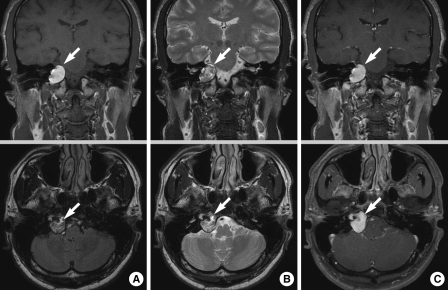
To rule out a central neurological lesion, temporal bone magnetic resonance imaging (MRI) was performed. This showed a 2.5 cm lobulated mass at the right petrous apex. T1-weighted MRI images showed a homogeneous, well-defined, high-intensity mass; T2-weighted images of the same lesion were heterogeneous and of high-intensity, but the area was not enhanced after gadolinium administration (Fig. 2). These findings indicated the presence of a PACG (5). To evaluate the bony structures and assist in the plans for surgery, temporal bone computed tomography (CT) was performed. This showed a 3 cm isodense mass at the petrous apex, with thinning of the bony wall. After consideration of the location of the cyst and the patient's hearing function as well as other possible complications such as contamination of the cranial fossa by cholesterol material during and after a middle fossa surgical approach, excision via an infralabyrinthine approach using a computer-aided image-guided surgical device (BrainLAB®, Heimstetten, Germany) was performed (6-8).
Fig. 2.
Preoperative (A) T1-weighted, (B) T2-weighted, and (C) T1-weighted images with gadolinium-enhanced MRI scans showing a 2.5 cm-sized lobulating mass (arrow) at the right petrous apex.
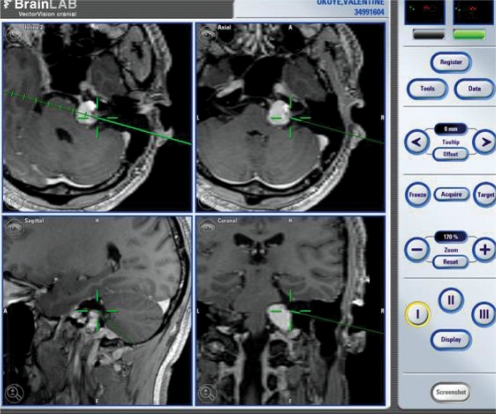
A preoperative contrast-enhanced three-dimensional T1-weighted MRI was conducted to allow installation of the surgical navigation system. The patient's head was fixed and three infrared emitting markers (red dots) and pointers (green dots) were placed (Fig. 3). The instrument recognized not only these markers, but also the surface image of the patient. Paired-point and surface matching were performed to compare the actual position of the skull with the preoperative MRI imaging data. A complete mastoidectomy was performed, with skeletonization of the sigmoid sinus. The mastoid segment of the facial nerve, posterior semicircular canal, and the jugular bulb, were identified. Using a diamond burr, the infralabyrinthine air cell tract was followed anteromedially along the long axis of the temporal bone toward the petrous apex. At the midpoint of the drilling process, a pointer was used to confirm the relationship between the location of the PACG and that of the drilling site (Fig. 3). Shining dark yellowish material emerged from the puncture site (Fig. 4). After suctioning the content of the cyst, a silastic drain was inserted to drain any residue of the mastoid cavity.
Fig. 3.
The computer-aided image-guided surgical device, BrainLAB®. Three infrared emitting fixed markers (red dots) and two pointers (green dots) are seen in the right upper area of the screen.
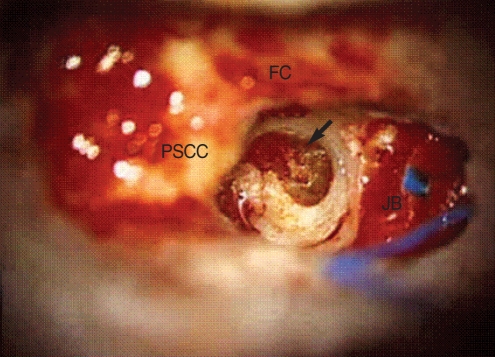
Fig. 4.
Intraoperative findings. After a complete mastoidectomy, we drilled a 1 cm-diameter hole through the petrous apex. This enabled us to locate facial nerve anteriorly, the endolymphatic sac posteriorly, the posterior semicircular canal superiorly, and the jugular bulb inferiorly. The cholesterol granuloma sac was exposed and dark yellow color content was drained (arrow).
FC: facial canal; JB: jugular bulb; PSCC: posterior semicircular canal.
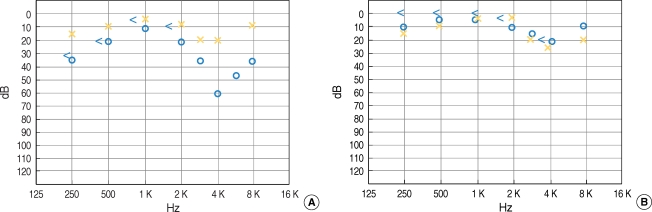
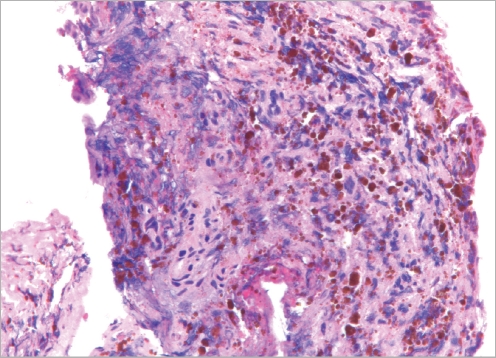
Postoperatively, the patient did not complain of vertigo, headache, or facial palsy. There was no cerebrospinal fluid leakage from the mastoid cavity. The patient was discharged from the hospital on the sixth postoperative day. One month after surgery, the results of the pure tone audiogram returned to the normal range (Fig. 5). A temporal bone CT scan revealed no aeration at the petrous apex and the patient had no vertigo. The patient continued to visit the outpatient clinic for four months after surgery, and remains well. Biopsy was confirmed as cholesterol granuloma (Fig. 6).
Fig. 5.
Pre- and post-operative pure tone audiometry (PTA). (A) Preoperative PTA showed decrease in the right hearing threshold at 0.25, 0.5, and 4 kHz. (B) Postoperative PTA showed improvement at both the lower and higher tone hearing thresholds.
Fig. 6.
Histopathology of the petrous apex cholesterol granuloma. The cholesterol granuloma appeared as empty, irregularly shaped clefts, or as spaces surrounded by a foreign body giant cell reaction; fresh hemorrhage and hemosiderin pigment are apparent.
DISCUSSION
PACG is a reaction to a foreign body (cholesterol deposits). Classically, the pathogenesis of PACG has been described as being due to occlusion of the mastoid air cells. Absorption of trapped air causes hemorrhage into the air cells and subsequent degradation of accumulated hemosiderin to cholesterol, which causes an inflammatory reaction and progressive granuloma formation. Recently, a novel pathogenesis has been suggested; hemorrhage from the exposed bone marrow of the hyper-pneumatized petrous apex is currently thought to be the etiology of PACG (9). Treatment of a PACG is symptomatic or when there are signs of a growing lesion. A variety of surgical approaches are available to resect and drain the lesion, with stenting. To determine the optimal surgical approach, the patient's hearing status, the tumor size and the anatomical relationship between the tumor location and the petrous apex bone, must be established. For a deaf patient the translabyrinthine approach is preferred because this provides good exposure. However, an infralabyrinthine approach is better if the hearing is to be preserved; however, this approach is limited to patients with high jugular bulbs. The best approach to prevent recurrence of a PACG is unclear. Giddings recommended drainage for permanent aeration of a cholesterol-based cyst, and observed that total removal was unnecessary (10, 11). On the other hand, Eisenberg recommended complete surgical extirpation with obliteration of the PACG cavity (12).
CONCLUSION
PACG can be accurately diagnosed by CT and MRI if there are clinical manifestations. However, a PACG may show no definitive disease-specific symptoms or signs, because of the location and size of the PACG. Therefore, the clinician must keep in mind the possibility of a petrous apex lesion in patients that have the clinical features of slowly aggravated endolymphatic hydrops. Temporal bone MRI and CT scans aid in an accurate diagnosis.
References
- 1.Muckle RP, De la Cruz A, Lo WM. Petrous apex lesions. Am J Otol. 1998;19(2):219–225. [PubMed] [Google Scholar]
- 2.Castillo MP, Samy RN, Isaacson B, Roland PS. Petrous apex cholesterol granuloma aeration: does it matter? Otolaryngol Head Neck Surg. 2008 Apr;138(4):518–522. doi: 10.1016/j.otohns.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Sajjadi H, Paparella MM. Meniere's disease. Lancet. 2008 Aug 02;372(9636):406–414. doi: 10.1016/S0140-6736(08)61161-7. [DOI] [PubMed] [Google Scholar]
- 4.Hwang JH, Ho HC, Hsu CJ, Yang WS, Liu TC. Diagnostic value of combining bilateral electrocochleography results for unilateral Meniere's disease. Audiol Neurootol. 2008;13(6):365–369. doi: 10.1159/000136155. [DOI] [PubMed] [Google Scholar]
- 5.Isaacson B, Kutz JW, Roland PS. Lesions of the petrous apex: diagnosis and management. Otolaryngol Clin North Am. 2007 Jun;40(3):479–519. doi: 10.1016/j.otc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Brackmann DE, Toh EH. Surgical management of petrous apex cholesterol granulomas. Otol Neurotol. 2002 Jul;23(4):529–533. doi: 10.1097/00129492-200207000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Caversaccio M, Panosetti E, Ziglinas P, Lukes A, Hausler R. Cholesterol granuloma of the petrous apex: benefit of computer-aided surgery. Eur Arch Otorhinolaryngol. 2009 Jan;266(1):47–50. doi: 10.1007/s00405-008-0719-4. [DOI] [PubMed] [Google Scholar]
- 8.Van Havenbergh T, Koekelkoren E, De Ridder D, Van De Heyning P, Verlooy J. Image guided surgery for petrous apex lesions. Acta Neurochir (Wien) 2003 Sep;145(9):737–742. doi: 10.1007/s00701-003-0054-x. [DOI] [PubMed] [Google Scholar]
- 9.Jackler RK, Cho M. A new theory to explain the genesis of petrous apex cholesterol granuloma. Otol Neurotol. 2003 Jan;24(1):96–106. doi: 10.1097/00129492-200301000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Giddings NA, Brackmann DE, Kwartler JA. Transcanal infracochlear approach to the petrous apex. Otolaryngol Head Neck Surg. 1991 Jan;104(1):29–36. doi: 10.1177/019459989110400107. [DOI] [PubMed] [Google Scholar]
- 11.Fong BP, Brackmann DE, Telischi FF. The long-term follow-up of drainage procedures for petrous apex cholesterol granulomas. Arch Otolaryngol Head Neck Surg. 1995 Apr;121(4):426–430. doi: 10.1001/archotol.1995.01890040050008. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg MB, Haddad G, Al-Mefty O. Petrous apex cholesterol granulomas: evolution and management. J Neurosurg. 1997 May;86(5):822–829. doi: 10.3171/jns.1997.86.5.0822. [DOI] [PubMed] [Google Scholar]