Abstract
Batches of commercial fetal bovine serum, described by the suppliers as antibody-free, all contained antibody to bovine syncytial virus (BSV) when tested by indirect immunofluorescence. Antibody to bovine respiratory syncytial virus (RSV) was not detected in these sera. Twenty-four percent of individual fetal bovine sera contained antibody to BSV, and 14% contained antibody to RSV when tested by indirect immunofluorescence. BSV antibody titers in fetal sera from dams with high BSV antibody levels were variable but always higher than RSV antibody titers. Radial immunodiffusion studies with BSV-positive sera revealed the presence of immunoglobulin M (IgM), IgG, and IgA, but the quantity of these immunoglobulins was not directly related to the BSV antibody titers. The evidence suggests that the antibody present in fetal sera arose as the result of infection rather than from maternal transfer across the placenta.
Full text
PDF

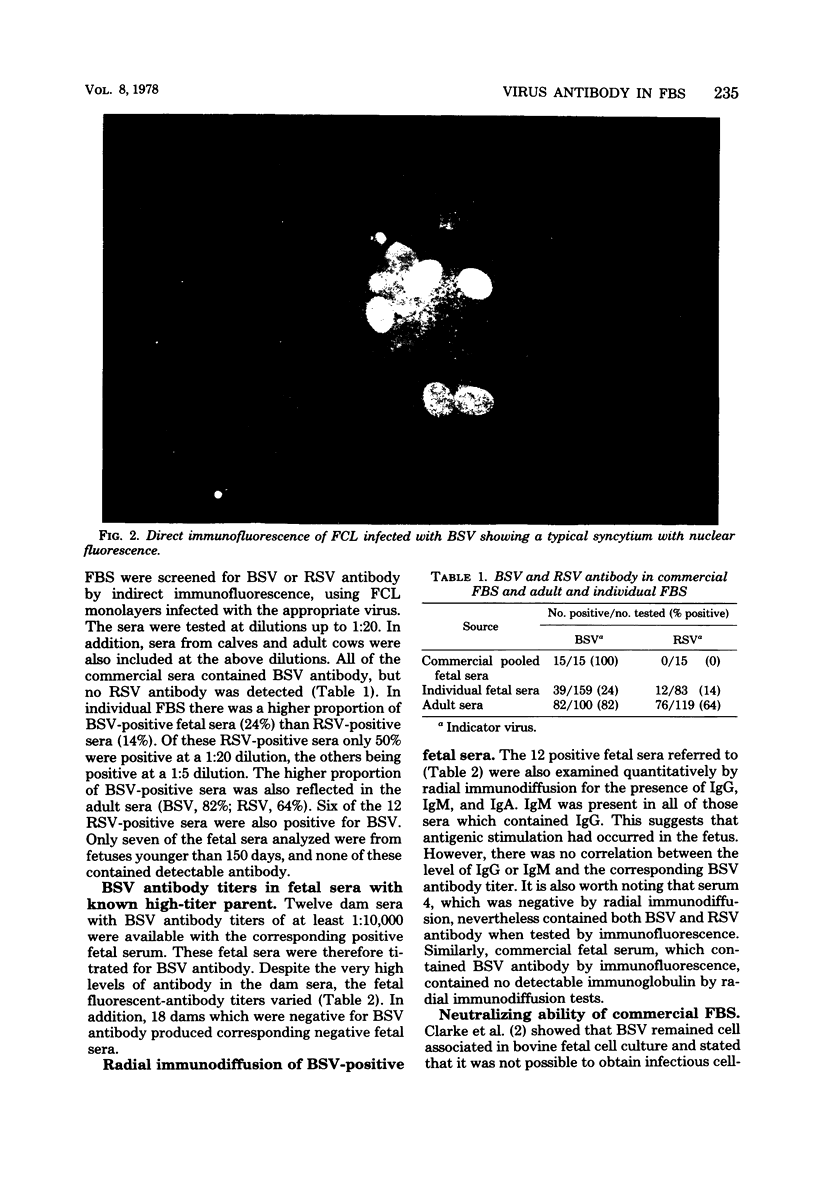

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke J. K., McFerran J. B., Nelson R. T. The isolation of a train of bovine syncytial virus in Northern Ireland. Res Vet Sci. 1973 Jan;14(1):117–119. [PubMed] [Google Scholar]
- Classick L. G., Fernelius A. L. Bovine viral diarrhea virus: neutralizing antibodies in a calf obtained by cesarean section from cow which was infected. Am J Vet Res. 1970 Feb;31(2):393–395. [PubMed] [Google Scholar]
- Dermott E., Clarke J. K., Samuels J. The morphogenesis and classification of bovine syncytial virus. J Gen Virol. 1971 Aug;12(2):105–119. doi: 10.1099/0022-1317-12-2-105. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- FENNESTAD K. L., BORG-PETERSEN C. Leptospira antibody production by bovine foetuses. Nature. 1957 Nov 30;180(4596):1210–1211. doi: 10.1038/1801210a0. [DOI] [PubMed] [Google Scholar]
- Fleming W. A., Clarke J. K. Fluorescence assay of foamy virus. J Gen Virol. 1970 Feb;6(2):277–284. doi: 10.1099/0022-1317-6-2-277. [DOI] [PubMed] [Google Scholar]
- Horner G. W., Johnson R. H., Dennett D. P., Lane W. R. A serological study of bovine foetal immunoglobulins. Aust Vet J. 1973 Jul;49(7):325–329. doi: 10.1111/j.1751-0813.1973.tb06821.x. [DOI] [PubMed] [Google Scholar]
- Hubbert W. T., Bryner J. H., Estes P. C., Foley J. W. Bovine bluetongue: viral isolation from placentome and serologic survey in pregnant cows. Am J Vet Res. 1972 Sep;33(9):1879–1882. [PubMed] [Google Scholar]
- Hubbert W. T., Bryner J. H., Fernelius A. L., Frank G. H., Estes P. C. Viral infection of the bovine fetus and its environment. Arch Gesamte Virusforsch. 1973;41(1):86–98. doi: 10.1007/BF01249933. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Malmquist W. A., Van der Maaten M. J., Boothe A. D. Isolation, immunodiffusion, immunofluorescence, and electron microscopy of a syncytial virus of lymphosarcomatous and apparently normal cattle. Cancer Res. 1969 Jan;29(1):188–200. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Todaro G. J., Scolnick E. M., Aaronson S. A. RNA dependent DNA polymerase in primate syncytium-forming (foamy) viruses. Nature. 1971 Jan 22;229(5282):258–260. doi: 10.1038/229258a0. [DOI] [PubMed] [Google Scholar]
- Scott F. W., Shively J. N., Gaskin J., Gillespie J. H. Bovine syncytial virus isolations. Arch Gesamte Virusforsch. 1973;43(1):43–52. doi: 10.1007/BF01249347. [DOI] [PubMed] [Google Scholar]
- Swift B. L., Kennedy P. C. Experimentally induced infection of in utero bovine fetuses with bovine parainfluenza 3 virus. Am J Vet Res. 1972 Jan;33(1):57–63. [PubMed] [Google Scholar]
- Van der Maaten M. J., Malmquist W. A., Cheville N. F. Susceptibility of calves to bovine syncytial virus given by different inoculation routes. Am J Vet Res. 1972 Jun;33(6):1157–1160. [PubMed] [Google Scholar]