Abstract
A relevant, yet little recognized feature of antisense regulation is the possibility of switching roles between regulatory and regulated RNAs. Here we show that induction of a Salmonella gene relies on the conversion of a small RNA from effector to regulatory target. The chiP gene (formerly ybfM), identified and characterized in the present study, encodes a conserved enterobacterial chitoporin required for uptake of chitin-derived oligosaccharides. In the absence of inducer, chiP is kept silent by the action of a constitutively made small RNA, ChiX (formerly SroB, RybC), which pairs with a sequence at the 5′ end of chiP mRNA. Silencing is relieved in the presence of chitooligosaccharides due to the accumulation of an RNA that pairs with ChiX and promotes its degradation. Anti-ChiX RNA originates from an intercistronic region of the chb operon, which comprises genes for chitooligosaccharide metabolism and whose transcription is activated in the presence of these sugars. We present evidence suggesting that the chb RNA destabilizes ChiX sRNA by invading the stem of its transcription terminator hairpin. Overall, our findings blur the distinction between effector and target in sRNA regulation, raising the possibility that some of the currently defined targets could actually be inhibitors of sRNA function.
Keywords: Antisense regulation, chitoporin, chitobiose, Hfq, small RNA
In the last decade, the study of a growing number of noncoding small RNAs in Escherichia coli and other bacteria has unveiled unsuspected layers of regulation in a variety of cell processes and responses (Gottesman et al. 2006; Vogel 2009; Waters and Storz 2009). The majority of bacterial sRNAs characterized to date are translational regulators, some binding translational regulatory proteins (Babitzke and Romeo 2007; Lapouge et al. 2008); others directly targeting messenger RNAs (mRNAs) (Storz et al. 2004; Aiba 2007). Members of the latter class act by base-pairing with complementary sequences in the proximity of the translation initiation site. In most instances, formation of the RNA duplex—typically <20 base pairs long—sterically interferes with ribosome loading onto the mRNA (Udekwu et al. 2005; Morita et al. 2006; Bouvier et al. 2008). This inhibits translation initiation, rendering the mRNA more susceptible to RNase attack and decay (Massé et al. 2003; Moll et al. 2003; Morita et al. 2005; Rasmussen et al. 2005; Udekwu et al. 2005). There are also cases in which pairing by the sRNA prevents the formation of alternative secondary structures that themselves inhibit ribosome binding. In these situations, the interaction with the sRNA stimulates translation (Majdalani et al. 1998; Majdalani et al. 2005).
The mRNA:sRNA association is assisted by chaperon protein Hfq, which facilitates the encounter/association with the mRNA target and, in some cases, protects the sRNA from degradation (Zhang et al. 2002; Geissmann and Touati 2004). Hfq is thought to bind to AU-rich regions in both the sRNA and its target (Brennan and Link 2007). Mutants lacking Hfq have been isolated in a number of bacterial species; although viable, these strains are debilitated and more susceptible than wild type to stress treatments. Such impairments have multiple causes including the inability of hfq mutants to fully induce the RpoS-dependent general stress response, due to the loss of sRNA-mediated translational activation of rpoS mRNA (Brown and Elliott 1996; Majdalani et al. 1998), and a toxic overexpression of the RpoE regulon ascribable to loss of sRNA-mediated envelope homeostasis (Ding et al. 2004; Figueroa-Bossi et al. 2006; Guisbert et al. 2007; Sittka et al. 2007).
Regulatory sRNAs are thought to play a major role in improving bacterial adaptation to environmental changes. A significant portion of sRNA regulatory activity in E. coli and Salmonella is devoted to modulating the protein composition of the outer membrane, the compartment most directly involved in exchanges with the environment (Guillier et al. 2006; Vogel and Papenfort 2006; Valentin-Hansen et al. 2007). All of the major porins and several outer membrane proteins (OMPs) are targets for regulation by one or more sRNAs. The genes encoding these sRNAs are generally regulated at the transcriptional level, and expressed under specific conditions (Mizuno et al. 1984; Chen et al. 2004; Douchin et al. 2006; Figueroa-Bossi et al. 2006; Papenfort et al. 2006; Bossi and Figueroa-Bossi 2007; Thompson et al. 2007; Udekwu and Wagner 2007; Rasmussen et al. 2009).
An interesting feature in the working mechanism of some sRNAs emerged from the study of RyhB, an sRNA that accumulates in response to iron limitation and down-regulates a number of genes for iron-binding proteins (Massé and Gottesman 2002). RyhB was found to be “consumed” when active in regulation. Apparently, upon pairing with the target mRNA, RyhB sRNA is no longer protected by Hfq and becomes susceptible to degradation by RNase E (Massé et al. 2003). Thus, the RyhB-mediated response relies on the continuous production of the sRNA, which permits rapid reversal of the response when iron concentration increases. The same study showed other sRNAs to act by a similar stoichiometric mechanism (Massé et al. 2003).
In the present study, we identify a novel OMP, ChiP, involved in the uptake of chitin-derived oligosaccharides. The protein, required for Salmonella to grow on chitooligosaccharides as sole sources of both carbon and nitrogen, is only synthesized in the presence of its substrates. We show that ChiP synthesis results from destabilization of a constitutively made small RNA, ChiX, that normally represses ChiP synthesis by pairing with a sequence in the 5′ untranslated region (UTR) of chiP mRNA. This regulatory response occurs by a novel mechanism involving the production of an RNA sequence that can act as an alternative pairing partner for ChiX sRNA. This alternative base pair interaction is ill fated for ChiX sRNA as it targets the sRNA for nucleolytic decay.
Results
Novel sRNA-regulated OMP in Salmonella
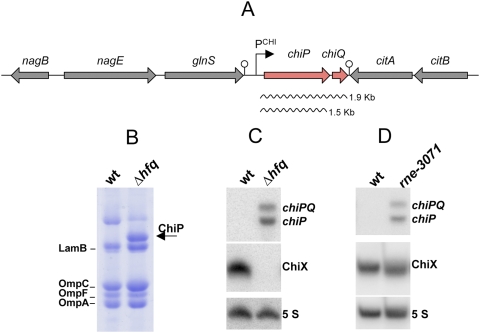
To identify potential targets for regulatory sRNAs in Salmonella, in a previous study we screened a library of random MudK(lac) chromosomal insertions, searching for isolates whose lacZ levels varied in the presence or absence of Hfq. One fusion, strongly up-regulated in the Δhfq background, had the MudK element inserted in the uncharacterized ybfM locus (Fig. 1A; Figueroa-Bossi et al. 2006). Analysis of Salmonella OMP preparations in polyacrylamide gels showed that the extract from the Δhfq strain included a band that was missing in the wild-type sample (Fig. 1B). When eluted from the gel and subjected to MALDI-TOF analysis, the protein was found to correspond to YbfM. Subsequent analysis (described below) revealed that YbfM is a chitoporin; hence, it was renamed ChiP.
Figure 1.
Small RNA-mediated silencing of a Salmonella omp gene. (A) Schematic diagram showing the gene organization of the segment between bp 744131 and 753872 of the Salmonella chromosome (McClelland et al. 2001). The transcription pattern of chiP (ybfM) and chiQ (ybfN) genes was established by Northern blot hybridization with promoter-proximal and promoter-distal probes (pp977 and pA83, respectively) (see the Supplemental Material). (B) Analysis of OMP preparations from wild-type and Δhfq mutant strains. Cultures of strains MA8740 (ompD∷Tn10 hfq+) and MA8741 (ompD∷Tn10 Δhfq) were grown in LB to an OD600 of 0.35 and processed for the OMP preparation. (C,D) Northern blot analysis of chiPQ and ChiX RNAs from wild-type, Δhfq and rne mutant strains. RNA was extracted from strains MA3409 (wt), MA7791 (Δhfq), and MA9816 (rne-3071). In the experiment in D, cells were incubated at 43°C for 15 min prior to RNA extraction. RNA was separated on a 1.3% agarose-formaldehyde gel (top panels) or on an 8% polyacrylamide/8 M urea gel (middle and bottom panels). Blots were hybridized with oligonucleotides pp977 (chiPQ, chiP), ppB7 (ChiX), and ppB10 (5S). The rne-3071 allele was constructed by site-directed mutagenesis as described in the Supplemental Material.
A strain carrying chiP∷MudK is phenotypically Lac− and becomes Lac+ when Hfq is inactivated. We reasoned that mutations affecting other cell components involved in chiP silencing might similarly result in a Lac+ phenotype. Such mutants were sought using growth on lactose as positive selection. The Lac+ mutations mapped at three different loci: (1) the hfq gene; (2) the chiP∷MudK locus, and (3) the gene encoding the Salmonella ortholog of SroB (RybC) sRNA (Vogel et al. 2003; Zhang et al. 2003). The latter findings suggested that SroB, renamed ChiX, might participate together with Hfq in chiP down-regulation.
ChiX sRNA is readily detectable in Northern blots of RNA from wild-type cells (Fig. 1C). In contrast, the sRNA is not detected in cells lacking Hfq. Disappearance of ChiX in the hfq mutant correlates with the appearance of two chiP-related RNAs: a longer species extending to the end of the ybfN gene (renamed chiQ) downstream from chiP (Fig. 1A), and a shorter species terminating within the intercistronic region (ICR) between chiP and chiQ. The same two species accumulate in a RNase E temperature-sensitive (ts) mutant incubated at the restrictive temperature (Fig. 1D). Taken together, these data allow us to infer that normally, ChiX activity leads to RNase E-dependent degradation of chiP transcripts.
ChiP is an inducible chitoporin
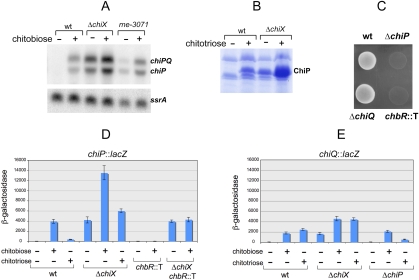
In an attempt to identify additional players involved in ChiP regulation, we searched for mutations conferring a conditional Lac+ phenotype. The lac fusion used in these experiments, chiP91∷pCE40(lac), carries the lacZ coding sequence of plasmid pCE40 (Ellermeier et al. 2002) joined to the 9th codon of chiP. This construct expresses lacZ at much higher levels than the original chiP∷MudK fusion. Mutants selected at 30°C were screened for the presence of isolates that did not form colonies on lactose plates at 42°C. Two such mutants were identified. Conceivably, they carried ts alleles of a factor involved in the regulation of chiP, or chiX, or both. Genetic mapping and DNA sequence analysis identified this factor as ChbR, the AraC-type activator of the chitobiose operon, chb (Keyhani and Roseman 1997). The chb operon (chbBCARFG) includes genes for transport and utilization of chitobiose, along with the chbR regulatory gene (Keyhani and Roseman 1997; Plumbridge and Pellegrini 2004). Chitobiose (the dimer of N-acetylglucosamine) and other chitooligosaccharides (e.g., chitotriose) are produced by the enzymatic breakdown of chitin, the main component of the exoskeleton of arthropods. The involvement of ChbR in chiP regulation, combined with the outer membrane localization of the ChiP protein, suggested that the latter was a porin dedicated to the uptake of chitin-derived oligosaccharides. Consistent with this hypothesis, we observed that both chitobiose and chitotriose effectively induce chiP mRNA synthesis as well as ChiP protein accumulation in the outer membrane (Fig. 2A,B). Furthermore, the chiP gene is absolutely required for Salmonella to be able to grow on chitotriose as the sole source for both carbon and nitrogen (Fig. 2C). A similar requirement is observed with chitobiose, but only at low concentrations, suggesting the existence on an additional, lower affinity uptake system for the disaccharide (data not shown). Existence of this system could explain why chitobiose, but not chitotriose, induces expression of the chiP-lac fusion in spite of chiP being disrupted by the lac sequence (Fig. 2D). In contrast, both sugars are equally efficient at inducing expression of a chiQ-lac fusion, provided that chiP is functional (Fig. 2E). The latter data indicate that chiQ and chiP genes are coordinately regulated. ChiQ, however, appears dispensable for growth on either chitobiose or chitotriose (Fig. 2C; data not shown).
Figure 2.
Regulation of the chiPQ Operon. (A) Northern blot analysis of chiPQ RNA. Cultures from strains MA3409 (wt), MA8933 (ΔchiX), and MA9816 (rne-3071) were grown in LB or in LB supplemented with chitobiose (2 mM; added at an OD600 of 0.2) to an OD600 of 0.35. RNA was extracted and analyzed as described in the legend to Figure 1C. MA9816 cultures were shifted to 43°C 15 min prior to RNA extraction. RNA blots were hybridized to 32P-labeled oligonucleotides ppB68 (chiPQ and chiP) and pp813 (ssrA). (B) OMP patterns in the absence and presence of chitotriose. Salmonella OMPs were prepared from 2 mL cultures of strains MA3409 (wt) and MA8933 (ΔchiX) grown overnight in LB without or with 2 mM chitotriose. (C) Genetic requirements for Salmonella growth on chitotriose. Cultures from strains MA3409 (wt), MA9131 (chiP∷scar), MA9654 (chiQ∷scar), and MA9843 (chbR∷Tn5-TPOP) were grown overnight in NCE medium supplemented with 0.2% glycerol were spotted on an NCN plate containing 2 mM chitotriose. Strains MA9131 and MA9654 carry small deletions in the 5′-end portions of chiP and chiQ genes, respectively. (D) Expression of chiP91-lacZ fusion. Cultures were grown overnight in NCE 0.2% glycerol medium without or with 2 mM chitobiose or 2 mM chitotriose added. β-Galactosidase activity was measured in strains MA9132 (chiP-lacZ), MA9184 (chiP-lacZ ΔchiX), MA9844 (chiP-lacZ chbR∷Tn5-TPOP), and MA10049 (chiP-lacZ ΔchiX chbR∷Tn5-TPOP). (E) Expression of chiQ92-lacZ fusion. Strains used were MA9655 (chiQ-lacZ), MA9841 (chiQ-lacZ ΔchiX), and MA9923 (ΔchiP chiQ-lacZ).
In chiX+ strains, chiPQ levels are negligible in the absence of inducers and rise >200-fold upon induction (Fig. 2A–C). In contrast, in strains deleted for the chiX gene, chitobiose exposure causes chiPQ expression to increase approximately fourfold over an already high basal level (Fig. 2D). The increase depends on the ChbR protein, and may reflect a direct stimulation of chiPQ transcription. Thus, ChiX tightens the response and amplifies the regulatory range. Failure of chitobiose or chitotriose to induce the chiPQ operon in a strain in which the chbR gene is disrupted by a transposon insertion (Fig. 2D) confirms the ChbR requirement for induction. ChbR is thought to undergo an allosteric change upon binding to a chitobiose-derived metabolite (Plumbridge and Pellegrini 2004; Kachroo et al. 2007). Conceivably, the two conditional ChbR alleles described above (S135L and N238Y) may mimic the allosteric change at low temperature in the absence of inducers.
Mutational analysis of ChiX sRNA
The Lac-based screen proved suitable to a genetic dissection of ChiX sRNA function. Lac+ mutants arising spontaneously, or generated by mutagenizing the chiX gene with the PCR, were isolated from the strain carrying the chromosomal chiP∷MudK(lac) insert. The effects of mutations on chiP expression were quantified measuring chiPQ mRNA in Northern blots and assaying β-galactosidase activity in strains with the chiP91∷pCE40(lac) fusion.
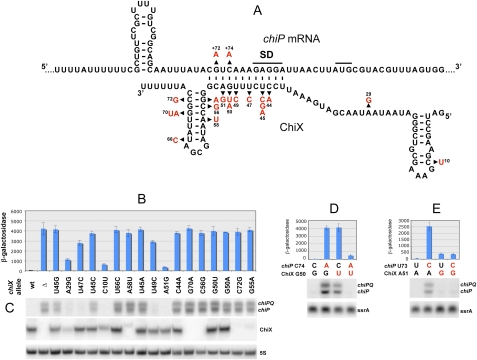
Several chiX mutations affected a 12-base segment complementary to a sequence spanning the Shine-Dalgarno motif of the chiP gene (Fig. 3A). This suggests a base pair interaction to be responsible for ChiX-mediated repression. Most of the changes relieve repression completely, while some have less pronounced effects (Fig. 3B,C). The differences can be ascribed to the nature of the change (i.e., A51G replaces a U:A base pair by a U:G pair) or to its position in the duplex. Mismatches in the middle of the segment (U47C, U49C) appear less detrimental to regulatory proficiency than the ones on the sides (e.g., U45C). Mutants with a weaker phenotype express significant LacZ activity and yet show little or no chiPQ RNA in the Northern blots (Fig. 3, cf. B and C). This discrepancy could originate from RNA cleavage events occurring in between rounds of translation initiation. Even though some ribosomes can reach the end of the message (yielding a protein product and measurable LacZ activity), no chiP RNA molecule ever exists in its full length. Northern blot analysis of processing intermediates from the 3′ end of the chiQ gene supports this interpretation (Supplemental Fig. S2).
Figure 3.
Analysis of ChiX sRNA mutations relieving chiP silencing. Mutants were all derived from strain MA8110 (chiP∷MudK) either selecting for growth on NCE-minimal lactose plate (spontaneous Lac+ mutants) or by PCR mutagenesis of the chiX gene. In this second procedure, mutants were identified as blue colonies on LB plates supplemented with X-gal. Due to the sensitivity of the colorimetric screen, the latter method yielded some alleles causing more subtle alterations. Following isolation, the chiX mutations were moved into the chiP91∷pCE40(lac) background (strain MA9132). (A) Model for the pairing interaction between chiP mRNA 5′ UTR and ChiX sRNA. The base changes conferring a Lac+ phenotype are indicated. The 5′ ends of chiP and chiX transcripts were determined by primer extension (Supplemental Fig. S1). (B) Effect of chiX mutations on expression of the chiP91-lacZ fusion. β-Galactosidase activity was measured on cultures grown overnight in LB medium (OD600 ≈ 3). (C) Northern blot analysis of RNA from chiX mutants. RNA was extracted and analyzed as in Figure 2A. (D,E). Effects of compensatory changes in the chiP/ChiX RNA duplex on chiP expression. The strains used in B and C are listed in Supplemental Table S3, strains used in D and E are listed in Supplemental Table S4.
Two chiP mutations obtained in the initial Lac+ selection fall within the presumptive ChiX pairing window (Fig. 3A). The change in chiP C74A is predicted to restore base-pairing if combined with ChiX G50U. Results in Figure 3D show that chiP C74A disrupts regulation when present alone but not in combination with the ChiX G50U allele. Similar compensatory effects were observed with other chiP changes obtained by site-directed mutagenesis (Fig. 3E; data not shown). These data provide evidence that repression by ChiX sRNA involves base-pairing with chiP mRNA. Likely, formation of the RNA duplex inhibits chiP translation initiation by interfering with ribosome binding.
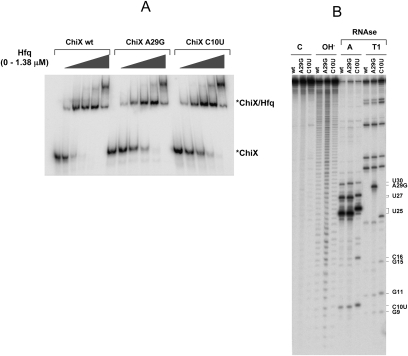
In chiX mutants carrying changes outside the pairing interval, altered chiP regulation correlates with a decrease in ChiX sRNA levels (Fig. 3A–C). Most of these mutations disrupt the Rho-independent terminator hairpin, a structure thought to be important for stability in prokaryotic RNAs (Mott et al. 1985; Aiba et al. 1991; Guarneros and Portier 1991). Therefore, the depletion of ChiX sRNA in these mutants is likely due to increased decay. Two alleles with a weaker phenotype, C10U and A29G, are located in the 5′ half of the molecule. We envisaged that these mutations might affect ChiX–Hfq binding. This idea was tested in vitro comparing the affinities of wild-type and mutant sRNAs for purified Hfq in a gel retardation assay. Results confirmed that both C10U and A29G impair the sRNA's ability to bind Hfq (Fig. 4A). Allele A29G falls within an AU-rich sequence (AAUAAUAAUA), which includes two overlapping AAYAA motifs proposed to play a role in Hfq recognition (Soper and Woodson 2008). Given that the mutation disrupts both repeats (AAUGAUAAUA), the data in Figure 4A further substantiate the role of this sequence in Hfq–RNA interactions.
Figure 4.
Characterization of chiX alleles A29G and C10U. Wild-type and mutant ChiX RNA were synthesized in vitro and labeled at their 5′ with 32P. (A) Gel shift assay with purified Hfq protein. 32P-labeled RNA (4 nM) was incubated at 30°C for 30 min with increasing amounts of purified Salmonella Hfq protein (0, 2, 11, 55, 276, 1380 nM) in 50 mM Tris-HCl (pH 7.8), 50 mM NaCl, 50 mM KCl,10 mM MgCl, and 6 μM yeast tRNA. Samples were loaded on a 5% nondenaturing polyacrylamide gel and radioactivity detected and quantified by phosphorimaging. (B). Structural analysis of wild-type and mutant RNAs. 5′-End-labeled RNAs were subjected to the indicated treatments followed by separation on a denaturating polyacrylamide gel. (C) Untreated control; (OH) alkaline ladder. Note the effect of the C10U mutation on band spacing in the OH lane as well as in the RNase A-treated samples (e.g., U25).
The C10U mutation causes the replacement of a G:C base pair by a G:U base pair in a putative secondary structure at the 5′ end of ChiX sRNA (Fig. 3A). Structural analysis (Fig. 4B) reveals that the mutation affects the reactivity of surrounding bases as well as the spacing of bands in the sequence ladder (cf. OH− lanes in Fig. 4B). These results might indicate that the C10U change destabilizes the top portion of the hairpin structure, causing the expansion of the apical loop. Such a perturbation might interfere with the binding of Hfq protein to the adjacent recognition site.
ChiX sRNA is destabilized during chiPQ induction
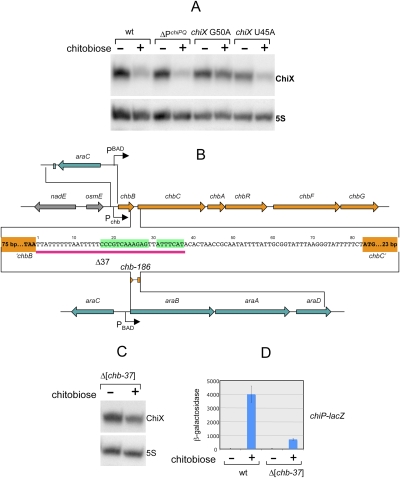
Interestingly, ChiX sRNA levels are drastically reduced in cells growing in the presence of chitobiose (Fig. 5A). Considering that this decrease could be the basis of chiP regulation, we set out to investigate the underlying mechanism. Our initial hypothesis—that activated ChbR protein acted as a repressor at the chiX promoter—was ruled out by the analysis of a lacZ gene fusion to the chiX promoter, which showed lacZ expression to remain unchanged in the presence of chitobiose (data not shown). We then postulated that the accumulation of chiPQ mRNA during induction might be sufficient to titrate out ChiX sRNA and stimulate its decay (Massé et al. 2003). However, ChiX depletion was still observed in a strain where chiPQ transcription cannot take place due to a promoter deletion (Fig. 5A). Nonetheless, the above line of reasoning led us to test whether the ChiX depletion was in some way related to the sRNA's ability to base-pair. Analysis of two of the pairing mutants described earlier (G50A, U45A) revealed ChiX sRNA levels to be significantly less affected in the presence of the G50A allele (Fig. 5A). This suggested that ChiX sRNA undergoes degradation during induction as a result of its participation in a base pair interaction. Having ruled out chiP mRNA involvement, we searched elsewhere for an alternative pairing target. We discovered that the ICR between the first two genes of the chb operon, chbB and chbC, encodes a sequence complementary to most of the pairing segment of ChiX sRNA (Fig. 5B). These findings were all the more interesting as the chb RNA is expected to accumulate in the presence of chitobiose, and thus it could easily become in excess of ChiX sRNA.
Figure 5.
Involvement of the chb operon in chiP regulation. (A) Northern blot analysis of ChiX sRNA. Cultures from strains MA3409 (wt), MA9924 (ΔPchiPQ), MA9010 (chiX G50A), MA9267 (chiX U45A) were grown in NCE 0.2% glycerol medium without or with 2 mM chitobiose to an OD600 of 0.35. RNA was extracted, separated on an 8% polyacrylamide/8 M urea gel, and transferred to Hybond-N+ membranes. Blots were hybridized to 32P-labeled oligonucleotides ppB7 (ChiX), ppB10 (5S). (B) Schematic diagram of the Salmonella chb operon and its ara operon fused derivatives. The ICR between chbB and chbC genes (sense strand) is enlarged, with the ChiX complementary segment boxed in green. The red bar underlining a portion of the chbBC ICR indicates the 37-bp deletion analyzed in this study. A 186-bp segment spanning the entire chbBC ICR (chb-186) was inserted in place of the structural portion of the ara operon. (C) Northern blot analysis of ChiX sRNA from the chbΔ37 mutant. RNA was extracted from strain MA10004 (chbΔ37) and processed as in A. (D) Effect of chbΔ37 on the regulation of the chiP91-lac fusion. β-Galactosidase activity was measured in strain MA10074 (Δchb-37 chiP-lac) as described in Figure 2D.
Transcription of chbBC ICR leads to ChiX sRNA destabilization
A 37-base-pair (bp) segment covering the chbBC ICR portion complementary to ChiX (Fig. 5B) was deleted by replacement with the 75-bp “scar” sequence from plasmid pKD13 (Datsenko and Wanner 2000). The deleted strain still suffered a ChiX sRNA decrease when exposed to chitobiose; however, the variation was significantly less than in wild type (Fig. 5C). Possibly, some ChiX sRNA decay occurs as a result of pairing with chiP mRNA, whose levels increase due to ChbR-mediated transcriptional activation (see above). As one might expect, the chbΔ37 deletion prevents full induction of the chiP-lac fusion (Fig. 5D). Residual induction can be ascribed to the increase in chiP mRNA levels saturating the inhibitory capacity of ChiX sRNA. Similar ChiX titration effects were observed upon raising chiP mRNA levels artificially, as in a strain with a chromosomal PBAD-chiP fusion grown in the presence of arabinose or in a chiP “promoter-UP” mutant isolated in the Lac selection above (data not shown).
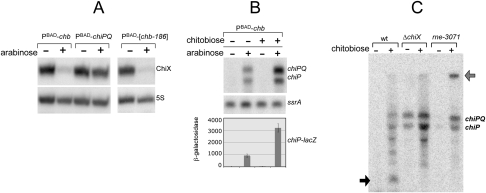
The chb promoter was replaced by a module comprising the araC gene and the PBAD promoter (Fig. 5B). Analysis of the resulting strain showed ChiX sRNA levels to sharply decline when cells were grown in the presence of arabinose (Fig. 6A). In contrast, a similar construct with the PBAD promoter controlling chiPQ transcription had only a modest effect on ChiX sRNA levels (Fig. 6A). Finally, a 186-bp DNA fragment spanning the chbBC ICR was translocated into the ara operon, placed under the control of the PBAD promoter (Fig. 5B). The resulting strain reproduced the effect of arabinose on ChiX sRNA levels (Fig. 6A), confirming the chbBC ICR to be solely responsible for ChiX sRNA decay.
Figure 6.
Role of chbBC ICR in ChiX sRNA turnover. (A) Effect of chbBC ICR transcription on ChiX sRNA levels. RNA was extracted from cells growing in NCE 0.2% glycerol without or with chitobiose (2 mM) and/or 0.02% arabinose at an OD600 of 0.35 and processed for Northern blot analysis. Strains were MA9995 (PBAD-chb); MA9996 (PBAD-chiPQ), and MA10000 (PBAD-[chb-186]). (B) Effect of chbBC ICR transcription on chiP expression. Northern blot analysis was carried out with strain MA9995 (PBAD-chb). β-Galactosidase activity was measured in strain MA10031 (PBAD-chb chiP-lac). (C) Northern blot analysis of chb RNA. The Northern blot in Fig. 2A was hybridized to a 32P-labeled oligonucleotide from the chbBC ICR (ppD96) without stripping from previous chiPQ probe.
As expected, arabinose induction of the PBAD-chb fusion constructs leads to chiPQ derepression. However, the chiPQ increase is significantly less dramatic than normally observed following chitobiose exposure. Ascribing this difference to the lack of ChbR activation, the induction experiments were repeated in the presence of chitobiose (in the strain carrying the entire chb operon fused to the PBAD promoter). Results confirmed that maximal chiPQ derepression is attained when both sugars are concomitantly present (Fig. 6B). These data further support the idea that ChiP induction normally results from coupling ChiX sRNA decay to the stimulation of chiP transcription.
To gather information on the chbBC ICR transcript, the RNA blot in Figure 2A was rehybridized to a probe covering the segment pairing to ChiX. This analysis detected an ∼300-base RNA species accumulating during chitobiose induction (solid arrow in Fig. 6C). Interestingly, this species is not present in the chiX deletion mutant, suggesting that its formation is consequent to pairing with ChiX sRNA. Failure to detect the 300-base RNA in the rne ts mutant, together with the accumulation of a high molecular RNA band in this strain (open arrow in Fig. 6C), points to the involvement of RNase E in the pathway leading to the 300-base RNA.
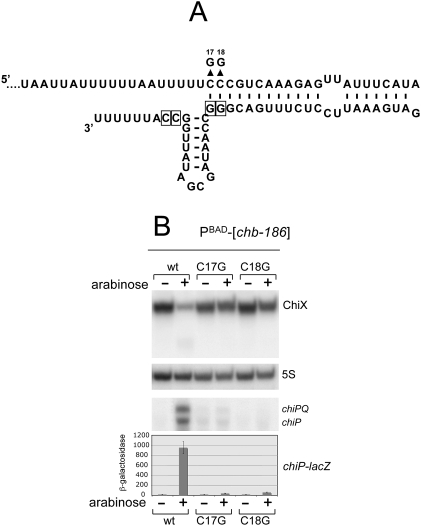
The chbBC ICR RNA invades ChiX sRNA transcription terminator
The data in Figure 6A suggest that ChiX sRNA is far more susceptible to degradation when pairing with the chbBC ICR than with the chiP 5′ UTR. A peculiarity of the chbBC spacer element is that the region of complementarity to ChiX sRNA extends two nucleotides into the transcription terminator hairpin of the sRNA (Fig. 7A). This raises the possibility that the pairing interaction with chbBC ICR RNA disrupts the bottom portion of ChiX terminator stem, causing the stem to become 2 bp shorter. Given the importance of this region for the overall stability of ChiX sRNA (see mutant analysis above) one expects that the loss of 2 bp would dramatically accelerate sRNA decay. The hypothesis predicts that affecting the ability of the chbBC sequence to base-pair with the terminator portion should prevent its degradation. This prediction was tested by changing either of the two C residues involved to G residues. Results in Figure 7B show that either change attenuates ChiX sRNA decay following activation of chbBC ICR transcription. Concomitantly, either mutation completely abolishes chiP derepression (Fig. 7B). Therefore, these observations confirm that the first 2 bases on the 5′ side of ChiX attenuator stem participate in chbBC ICR pairing. Shortening of the stem might make ChiX sRNA more susceptible to degradation.
Figure 7.
Analysis of mutations affecting pairing of chbBC ICR RNA with bases in ChiX sRNA transcription terminator stem. (A) Model for chbBC ICR pairing to ChiX sRNA. chbBC ICR bases are numbered starting to the right of the translation stop codon (UAA) of the chbB gene. (B) Analysis of the effects of the mutations on ChiX sRNA levels and on chiPQ regulation. RNA and β-galactosidase measurements were carried out under the same conditions as in Figure 6. Strains used in the Northern blot analysis of ChiX sRNA (top and middle panels) and for the β-galactosidase determinations were MA10043 (PBAD-[chb-186] chiP-lac), MA10044 (PBAD-[chb-186 C17G] chiP-lac), and MA10045 (PBAD-[chb-186 C18G] chiP-lac). Strains used for Northern blot analysis of chiPQ mRNA were MA10051 (PBAD-[chb-186]), MA10052 (PBAD-[chb-186 C17G]), and MA10053 (PBAD-[chb-186 C18G]).
Discussion
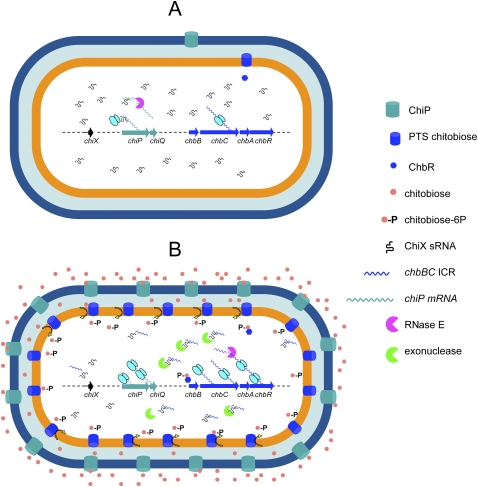
In this study, we describe the existence of a chitoporin (ChiP) in Salmonella, as well as the mechanism by which synthesis of this protein is regulated as a function of substrate availability. ChiP synthesis is induced in the presence of chitooligosaccharides as a result of two combined events: stimulation of chiP gene transcription and relief of chiP translational repression by ChiX small RNA. Both events are triggered by the transcriptional activation of the chb operon, which encodes the components for chitobiose (and chitotriose) phosphoenolpyruvate-dependent phosphotransferase system (PTS) along with the ChbR regulator protein (Plumbridge and Pellegrini 2004). Binding of a chitobiose/chitotriose-derived metabolite to ChbR causes this protein to activate chb transcription, thus increasing ChbR levels. Inducer-bound ChbR stimulates chiP gene transcription. Concomitantly, an RNA sequence processed from the polycistronic chb transcript (the chbBC ICR element) targets ChiX sRNA for degradation. Altogether, these coordinate events may allow coupling ChiP-mediated uptake of chitooligosaccharides to their transport across the inner membrane (Fig. 8).
Figure 8.
Model for chiP regulation. (A) In the absence of inducers, the chiX gene (black arrow) and the chiPQ operon (green arrows) are transcribed constitutively. Pairing of ChiX sRNA with the 5′ UTR of chiPQ mRNA inhibits ChiP synthesis, promoting cleavage of the chiP mRNA by RNase E. The chb operon (blue arrows; only three genes shown) is expressed just to the level needed to prime the system. (B) In the presence of chitooligosaccharides, inducer binding to ChbR causes this protein to activate transcription of the chb operon and to further stimulate chiPQ transcription. Processing of the chb transcript (initiated by RNase E) releases chbBC ICR RNA. This RNA base-pairs with ChiX sRNA making it susceptible to the action of a ribonuclease. The drop in ChiX levels, combined with the increase in chiPQ mRNA, relieves chiP repression leading to a burst of ChiP synthesis. ChiP assembles in the outer membrane resulting in more inducer uptake.
The mechanism by which the chbBC ICR RNA promotes the destruction of ChiX sRNA is unprecedented. ChiX degradation appears to result from chbBC ICR RNA pairing with the portion of the sRNA that makes up the Rho-independent transcription terminator. This suggests that degradation is caused by disruption/destabilization of the terminator stem–loop structure. Thus, the action of chbBC ICR RNA would be equivalent to the effect of mutations that destabilize the terminator, a number of which were described here. Similar mutations in a phage λ terminator were shown to make the RNA transcript susceptible to 3′ exonucleolytic degradation (Cisneros et al. 1996). It seems possible that a 3′ exonuclease could intervene in the chbBC ICR-dependent degradation of ChiX sRNA.
We found a 300-base RNA containing the chbBC ICR sequence to accumulate as a result of pairing with ChiX sRNA. Whether this RNA species is produced by a processing pathway specifically activated by the formation of the duplex, or simply reflects the stabilization of a short-lived intermediate remains to be determined. In either case, the accumulation of the presumptive chb primary transcript in an RNase E mutant leaves little doubt that this activity is responsible for the initial cleavage events. An implication of the rapid turnover is that the regulatory action of chbBC ICR element must depend on continuous chb operon transcription. Conceivably, this allows the system to quickly readjust once the inducer concentration falls and, with it, the requirement for ChiP activity. One might wonder about what makes such a complex transcriptional-translation control to be preferred to a more classical regulation mediated by the ChbR activator protein only. Particularly counterintuitive in the current mechanism is that chiP repression under uninduced conditions relies on both chiP and chiX genes to be continuously transcribed. Perhaps the answer lies in the timing of the induction response. Having the chiP mRNA already present when ChiX sRNA concentration begins to fall (concomitant with the appearance of the chbBC ICR RNA) might allow ChiP synthesis to start before the chb operon is fully induced; namely, before accumulation of the ChbR activator. The ChiX component of the regulatory mechanism could therefore allow for more efficient priming of the induction switch.
The ChiP protein is highly conserved in enteric bacteria with sequence identities exceeding 90% in the Escherichia and Shigella orthologs. The protein belongs to the OprD family and is phylogenetically unrelated to the chitoporin from Vibrio species (Keyhani et al. 2000). Chitin is the most commonly occurring nitrogen-containing polysaccharide in nature. Since Salmonella and E. coli do not naturally secrete chitinases (Francetic et al. 2000), these organisms probably rely on other microorganisms producing the ChiP substrates in the environment. This could occur through the activity of other, chitinase-proficient microorganisms or metabolic processes in eucaryotic hosts. It is relevant in this respect that an increasing number of chitinases have been identified recently in a variety of mammalian species including humans (Escott and Adams 1995; Zhu et al. 2004).
After this work was completed, a report has appeared describing the silencing of ChiP (YbfM) by ChiX (named MicM) in E. coli (Rasmussen et al. 2009). Rasmussen et al. (2009) presented evidence for the base-pairing interaction between the sRNA and the chiP 5′ UTR and substantiated the importance of Hfq as a catalyst of the interaction. However, the study did not elucidate the function of ChiP nor the basis for ChiX (MicM)-dependent regulation. At the same time, a separate report identified ChiX (named RybC) as an sRNA down-regulating the dpiB gene for the sensor component of a two-component system (DpiA/B) required for citrate fermentation and linked to various phenotypes including SOS induction and plasmid stability in E. coli (Mandin and Gottesman 2009). While the role of ChiX in any of these phenotypes remains elusive, the presence of citrate carrier/utilization genes, citA/citB, immediately adjacent to the chiPQ operon in Salmonella (see Fig. 1A) may suggest a link between ChiP and citrate metabolism. (Note that no citA/citB orthologs exist in the E. coli, where, somewhat confusingly, CitA/B is an alternative designation for the DpiA/B system) (Kaspar and Bott 2002). We notice that pairing of dpiB 5′ UTR with ChiX RNA extends 1 base (2 bases allowing a G:U pair) into the ChiX transcription terminator (Mandin and Gottesman 2009). This raises the possibility that in addition to, or rather than, being a regulatory target, the dpiB 5′ UTR is itself a down-regulator of ChiX sRNA. Transcription of dpiB (citA) in E. coli is activated by citrate during anaerobic growth (Yamamoto et al. 2009). Under these conditions, citrate utilization depends upon the presence of an oxidizable cosubstrate, usually a carbohydrate (Lutgens and Gottschalk 1980). Perhaps raising the basal levels of ChiP protein helps meet such a requirement in the event that bacteria come in contact with chitooligosaccharides.
An implication from our findings is that sRNA targets identified on the basis of sequence complementarity may actually include modulators or competitive inhibitors of sRNA function activity (Seitz 2009). It seems reasonable to predict that additional sRNAs subjected to this type of control will be found in the future. Best candidates are sRNAs that, like ChiX, are synthesized constitutively or semiconstitutively under standard growth conditions. A number of such molecules have been identified in global searches and are still awaiting characterization (Wassarman et al. 2001; Vogel et al. 2003; Zhang et al. 2003; Padalon-Brauch et al. 2008).
Materials and methods
Strains and growth conditions
Strains used in this study were all derived from Salmonella enterica serovar Typhimurium strain MA3409, a strain LT2 derivative cured for the Gifsy-1 prophage (Figueroa-Bossi et al. 1997). The genotypes of the relevant strains used are shown in Supplemental Table S1. The construction of these strains is detailed in the Supplemental Material. Bacteria were cultured at 37°C in liquid media or in media solidified by the addition of 1.5% Difco agar. LB broth (Bertani 2004) was used as complex medium. Carbon-free medium (NCE) and carbon-free and nitrogen-free medium (NCN) (Maloy and Roth 1983), supplemented with the appropriate carbon and/or nitrogen sources, were used as minimal media. Typically NCE medium was supplemented with 0.2% glycerol. Antibiotics (Sigma) were included at the following final concentrations (in LB): chloramphenicol, 10 μg mL−1; kanamycin monosulphate, 50 μg mL−1; sodium ampicillin, 75 μg mL−1; spectinomycin dihydrochloride, 80 μg mL−1; tetracycline hydrochloride, 25 μg mL−1. LB plates containing 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal; from Sigma), 40 μg mL−1 were used to monitor lacZ expression in bacterial colonies. Liquid cultures were grown in New Brunswick gyrotory shakers and growth was monitored by measuring the optical density at 600 nm with a Milton-Roy Spectronic 301 spectrophotometer.
Enzymes and chemicals
Restriction enzymes, T4 polynucleotide kinase and Taq DNA polymerase were from New England Biolabs, Pfu-Turbo DNA polymerase was from Stratagene, T4 DNA ligase was from USB. DNA oligonucleotides were obtained from Sigma Aldrich. A list with all oligonucleotides used in this work is in Supplemental Table S2. Acrylamide-bisacrylamide (30%, 29:1) and other electrophoresis reagents were from Bio-Rad. Agarose was from Invitrogen. Hybond-N+ membranes and hybridization buffer used for Northern blot analysis were from GE Healthcare and from Applied Biosystems-Ambion, respectively. MegashortScript T7 kit, Kinase Max kit, RNases A and T1, yeast tRNA were all from Applied Biosystems-Ambion.
Genetic techniques
Generalized transduction was perfomed using the high-frequency transducing mutant of phage P22, HT 105/1 int-201 (Schmieger 1972) as described (Lemire et al. 2008). Chromosomal engineering was carried out by the λ red recombination method (Datsenko and Wanner 2000; Yu et al. 2000) as implemented by Datsenko and Wanner (2000) and Uzzau et al. (2001).
Donor DNA fragments were generated by PCR using plasmid or chromosomal DNA templates. Preparation of recipient bacteria, DNA electroporation, and isolation and processing of recombinant clones were carried out as described (Lemire et al. 2008).
β-Galactosidase assays
β-Galactosidase activity was measured in toluene-permeabilized cells as described by Miller (1992), and is expressed in Miller units throughout this work.
OMP preparation
Salmonella OMP fraction was purifed by the method of Santiviago et al. (2003) and analyzed by polyacrylamide gel electrophoresis as described previosuly (Bossi and Figueroa-Bossi 2007).
RNA extraction and Northern analysis
RNA was prepared by the acid-hot-phenol method from exponentially growing cells (OD600 of 0.35) as described previously (Bossi and Figueroa-Bossi 2007). RNA was separated on a 1.3% agarose-formaldehyde gel or on an 8% polyacrylamide/8 M urea gel and transferred to Hybond-N+ membranes. Blots were hybridized to DNA oligonucleotides labeled at the 5′ ends with T4 polynucleotide kinase.
In vitro RNA synthesis, purification, and labeling
A template DNA carrying the chiX gene fused to the T7 promoter was generated by PCR from genomic DNA. To produce wild-type and chiX A29G mutant templates, amplification was with primers ppC19 and ppC20 on genomic DNA from wild-type and from strain MA9220, respectively. For the chiX C10U template, primers were ppC60 (carrying the C10U change) and ppC20, and genomic DNA was from strain MA9226 (see Supplemental Tables S1, S2). In vitro transcription was performed with the MegashortscriptT7 kit (Ambion AM1354) following the manufacturer's protocol. After incubation for 2 h at 37°C, DNase was added and incubation continued for further 15 min, followed by phenol extraction and overnight precipitation in ethanol/sodium acetate/glycogen. RNA was recovered by centrifugation, resuspended in nuclease-free water, and its concentration determined by nanodrop reading. The RNA solution was adjusted to 5 pmol/μL. RNA (10 pmol) was dephosphorylated and labeled at its 5′ end with [γ−32P]ATP (3000 Ci/mmol from Perkin-Elmer) using the KinaseMax kit (Ambion AM1520). Labeled RNA was purified by 8% PAGE. After extraction from gel, RNA was phenol-extracted, ethanol precipitated, and resuspended in nuclease-free water. Before use in binding and structural studies, RNA was heated in refolding buffer (50 mM Tris at pH 8, 0.1 M NaCl, 0.1 M KCl, 1 mM MgCl2) for 3 min at 85°C, followed by 20 min at room temperature, and finally placed on ice.
Hfq protein purification
The Salmonella hfq gene was cloned into plasmid pNFB28, a pET-16b (Novagen) derivative designed to obtain either C-terminal or N-terminal (7×) His-tagged fusions to genes of interest. C-terminally tagged Hfq protein was purified from E. coli BL21 codon+ cells carrying the plasmid clone. Bacteria were grown in 1 L of LB medium supplemented with ampicillin (100 mg/L) at 37°C until OD600 of 0.2; 1 mM isopropyl 1-thio-D-galactopyranoside was added and cells cultured for a further 3 h. Bacteria were harvested by centrifugation at 10,000g for 20 min at 4°C. Pellets were frozen at −20°C for several hours prior to resuspension in 50 mL of 50 mM Tris-HCl (pH 7.8), 0.3 M NaCl, 10% Glycerol, 0.1% Triton X-100, 100 μg/mL lysozyme, and protease inhibitor cocktail from Sigma. Cells were sonicated by 15 rounds of 10-sec pulses/10-sec intervals, on ice. Lysate was incubated for 10 min at 75°C and centrifuged at 11,800g for 40 min. The supernatant was loaded on a 5-mL HiTrap Chelating HP prepacked column from GE Healthcare activated according to the manufacturer's instructions. Column was equilibrated with 25 mL of 50 mM Tris-HCl (pH 7.5), 0.3 M NaCl, and the protein eluted with the same buffer supplemented with 0.5 M imidazole. Following SDS-PAGE electrophoresis of elution aliquots, protein containing fraction was incubated for 10 min at 65°C. Buffer exchange and concentration steps were performed with Centricon 10K Amicon, ultrafiltration unit (Millipore). Protein was kept at 4°C in 50 mM Tris-HCl (pH 7.8), 0.3 M NaCl, 10% glycerol, and 0.1% Triton X-100. The concentration was determined by Bradford assay.
Gel shift assays
Labeled ChiX RNA (4 nM), was incubated with increasing amounts of purified Salmonella Hfq as indicated, in 50 mM Tris-HCl (pH 7.8), 50 mM NaCl, 50 mM KCl,10 mM MgCl, and 6 μM yeast tRNA for 30 min at 30°C. Binding reactions were run on a 5% nondenaturing polyacrylamide gel in 0.5× TBE buffer for 3 h at 4°C at constant current of 15 mA. Results were analyzed by phosphorimaging using ImageQuant software.
RNA structural analyses
End-labeled RNA was refolded as described above. Enzymatic treatments were performed in 10 μL of reaction mix containing 0.2 pmol of RNA, 1 μg of yeast tRNA, 1× Structure buffer (Ambion), and 0.01 ng of RNase A (1 mg/mL; Ambion AM 2274) or 0.1 U of RNase T1 (1 U/mL; Ambion AM 2283). Incubation was for 12 min at 37°C. Reactions were stopped by addition of 20 μL of completed Precipitation/Inactivation buffer from the same manufacturer. Partial alkaline hydrolysis was performed according to Ambion's protocol as follows: 10 μL of reaction mix containing 0.2 pmol of RNA, 1 μg of yeast tRNA, 1× Alkaline Hydrolysis buffer, were incubated for 8 min at 95°C, placed on ice, and 20 μL of completed Precipitation/Inactivation buffer (Ambion) added. After recovery from precipitation, all samples were run on a 10% sequencing polyacrylamide gel in 0.5× TBE. Results were analyzed by phosphorimaging.
Acknowledgments
We thank Nicolas Villagra and Francesca Fiorini for participating in the experiments in Figures 2B and 4, respectively. Furthermore, we thank Francesca Fiorini for purifying Hfq and for technical assistance with the in vitro RNA analyses. We are very grateful to Jackie Plumbridge and Pepe Casadesús for critical reading of the manuscript and for insightful feedback. This work was made possible by a grant from the French National Research Agency (ANR; BLAN07-1_187785).
Footnotes
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.541609.
Supplemental material is available at http://www.genesdev.org.
References
- Aiba H. Mechanism of RNA silencing by Hfq-binding small RNAs. Curr Opin Microbiol. 2007;10:134–139. doi: 10.1016/j.mib.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Aiba H, Hanamura A, Yamano H. Transcriptional terminator is a positive regulatory element in the expression of the Escherichia coli crp gene. J Biol Chem. 1991;266:1721–1727. [PubMed] [Google Scholar]
- Babitzke P, Romeo T. CsrB sRNA family: Sequestration of RNA-binding regulatory proteins. Curr Opin Microbiol. 2007;10:156–163. doi: 10.1016/j.mib.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Bertani G. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol. 2004;186:595–600. doi: 10.1128/JB.186.3.595-600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi L, Figueroa-Bossi N. A small RNA downregulates LamB maltoporin in Salmonella. Mol Microbiol. 2007;65:799–810. doi: 10.1111/j.1365-2958.2007.05829.x. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Sharma CM, Mika F, Nierhaus KH, Vogel J. Small RNA binding to 5′ mRNA coding region inhibits translational initiation. Mol Cell. 2008;32:827–837. doi: 10.1016/j.molcel.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Brennan RG, Link TM. Hfq structure, function and ligand binding. Curr Opin Microbiol. 2007;10:125–133. doi: 10.1016/j.mib.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Brown L, Elliott T. Efficient translation of the RpoS σ factor in Salmonella typhimurium requires host factor I, an RNA-binding protein encoded by the hfq gene. J Bacteriol. 1996;178:3763–3770. doi: 10.1128/jb.178.13.3763-3770.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang A, Blyn LB, Storz G. MicC, a second small-RNA regulator of Omp protein expression in Escherichia coli. J Bacteriol. 2004;186:6689–6697. doi: 10.1128/JB.186.20.6689-6697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros B, Court D, Sanchez A, Montanez C. Point mutations in a transcription terminator, λ tI, that affect both transcription termination and RNA stability. Gene. 1996;181:127–133. doi: 10.1016/s0378-1119(96)00492-1. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Davis BM, Waldor MK. Hfq is essential for Vibrio cholerae virulence and downregulates σ expression. Mol Microbiol. 2004;53:345–354. doi: 10.1111/j.1365-2958.2004.04142.x. [DOI] [PubMed] [Google Scholar]
- Douchin V, Bohn C, Bouloc P. Down-regulation of porins by a small RNA bypasses the essentiality of the regulated intramembrane proteolysis protease RseP in Escherichia coli. J Biol Chem. 2006;281:12253–12259. doi: 10.1074/jbc.M600819200. [DOI] [PubMed] [Google Scholar]
- Ellermeier CD, Janakiraman A, Slauch JM. Construction of targeted single copy lac fusions using λ Red and FLP-mediated site-specific recombination in bacteria. Gene. 2002;290:153–161. doi: 10.1016/s0378-1119(02)00551-6. [DOI] [PubMed] [Google Scholar]
- Escott GM, Adams DJ. Chitinase activity in human serum and leukocytes. Infect Immun. 1995;63:4770–4773. doi: 10.1128/iai.63.12.4770-4773.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Bossi N, Coissac E, Netter P, Bossi L. Unsuspected prophage-like elements in Salmonella typhimurium. Mol Microbiol. 1997;25:161–173. doi: 10.1046/j.1365-2958.1997.4451807.x. [DOI] [PubMed] [Google Scholar]
- Figueroa-Bossi N, Lemire S, Maloriol D, Balbontín R, Casadesús J, Bossi L. Loss of Hfq activates the σE-dependent envelope stress response in Salmonella enterica. Mol Microbiol. 2006;62:838–852. doi: 10.1111/j.1365-2958.2006.05413.x. [DOI] [PubMed] [Google Scholar]
- Francetic O, Belin D, Badaut C, Pugsley AP. Expression of the endogenous type II secretion pathway in Escherichia coli leads to chitinase secretion. EMBO J. 2000;19:6697–6703. doi: 10.1093/emboj/19.24.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann TA, Touati D. Hfq, a new chaperoning role: Binding to messenger RNA determines access for small RNA regulator. EMBO J. 2004;23:396–405. doi: 10.1038/sj.emboj.7600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, McCullen CA, Guillier M, Vanderpool CK, Majdalani N, Benhammou J, Thompson KM, FitzGerald PC, Sowa NA, FitzGerald DJ. Small RNA regulators and the bacterial response to stress. Cold Spring Harb Symp Quant Biol. 2006;71:1–11. doi: 10.1101/sqb.2006.71.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneros G, Portier C. Different specificities of ribonuclease II and polynucleotide phosphorylase in 3′mRNA decay. Biochimie. 1991;73:543–549. doi: 10.1016/0300-9084(91)90021-r. [DOI] [PubMed] [Google Scholar]
- Guillier M, Gottesman S, Storz G. Modulating the outer membrane with small RNAs. Genes & Dev. 2006;20:2338–2348. doi: 10.1101/gad.1457506. [DOI] [PubMed] [Google Scholar]
- Guisbert E, Rhodius VA, Ahuja N, Witkin E, Gross CA. Hfq modulates the σE-mediated envelope stress response and the σ32-mediated cytoplasmic stress response in Escherichia coli. J Bacteriol. 2007;189:1963–1973. doi: 10.1128/JB.01243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo AH, Kancherla AK, Singh NS, Varshney U, Mahadevan S. Mutations that alter the regulation of the chb operon of Escherichia coli allow utilization of cellobiose. Mol Microbiol. 2007;66:1382–1395. doi: 10.1111/j.1365-2958.2007.05999.x. [DOI] [PubMed] [Google Scholar]
- Kaspar S, Bott M. The sensor kinase CitA (DpiB) of Escherichia coli functions as a high-affinity citrate receptor. Arch Microbiol. 2002;177:313–321. doi: 10.1007/s00203-001-0393-z. [DOI] [PubMed] [Google Scholar]
- Keyhani NO, Roseman S. Wild-type Escherichia coli grows on the chitin disaccharide, N,N′-diacetylchitobiose, by expressing the cel operon. Proc Natl Acad Sci. 1997;94:14367–14371. doi: 10.1073/pnas.94.26.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhani NO, Li XB, Roseman S. Chitin catabolism in the marine bacterium Vibrio furnissii. Identification and molecular cloning of a chitoporin. J Biol Chem. 2000;275:33068–33076. doi: 10.1074/jbc.M001041200. [DOI] [PubMed] [Google Scholar]
- Lapouge K, Schubert M, Allain FH, Haas D. Gac/Rsm signal transduction pathway of γ-proteobacteria: From RNA recognition to regulation of social behaviour. Mol Microbiol. 2008;67:241–253. doi: 10.1111/j.1365-2958.2007.06042.x. [DOI] [PubMed] [Google Scholar]
- Lemire S, Figueroa-Bossi N, Bossi L. A singular case of prophage complementation in mutational activation of recET orthologs in Salmonella enterica serovar Typhimurium. J Bacteriol. 2008;190:6857–6866. doi: 10.1128/JB.00769-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgens M, Gottschalk G. Why a co-substrate is required for anaerobic growth of Escherichia coli on citrate. J Gen Microbiol. 1980;119:63–70. doi: 10.1099/00221287-119-1-63. [DOI] [PubMed] [Google Scholar]
- Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc Natl Acad Sci. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N, Vanderpool CK, Gottesman S. Bacterial small RNA regulators. Crit Rev Biochem Mol Biol. 2005;40:93–113. doi: 10.1080/10409230590918702. [DOI] [PubMed] [Google Scholar]
- Maloy SR, Roth JR. Regulation of proline utilization in Salmonella typhimurium: Characterization of put:Mu d(Ap, lac) operon fusions. J Bacteriol. 1983;154:561–568. doi: 10.1128/jb.154.2.561-568.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandin P, Gottesman S. A genetic approach for finding small RNAs regulators of genes of interest identifies RybC as regulating the DpiA/DpiB two-component system. Mol Microbiol. 2009;72:551–565. doi: 10.1111/j.1365-2958.2009.06665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé E, Escorcia FE, Gottesman S. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes & Dev. 2003;17:2374–2383. doi: 10.1101/gad.1127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- Miller JH. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. [Google Scholar]
- Mizuno T, Chou MY, Inouye M. A unique mechanism regulating gene expression: Translational inhibition by a complementary RNA transcript (micRNA) Proc Natl Acad Sci. 1984;81:1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll I, Afonyushkin T, Vytvytska O, Kaberdin VR, Bläsi U. Coincident Hfq binding and RNase E cleavage sites on mRNA and small regulatory RNAs. RNA. 2003;9:1308–1314. doi: 10.1261/rna.5850703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Maki K, Aiba H. RNase E-based ribonucleoprotein complexes: Mechanical basis of mRNA destabilization mediated by bacterial noncoding RNAs. Genes & Dev. 2005;19:2176–2186. doi: 10.1101/gad.1330405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Mochizuki Y, Aiba H. Translational repression is sufficient for gene silencing by bacterial small noncoding RNAs in the absence of mRNA destruction. Proc Natl Acad Sci. 2006;103:4858–4863. doi: 10.1073/pnas.0509638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JE, Galloway JL, Platt T. Maturation of Escherichia coli tryptophan operon mRNA: Evidence for 3′ exonucleolytic processing after rho-dependent termination. EMBO J. 1985;4:1887–1891. doi: 10.1002/j.1460-2075.1985.tb03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padalon-Brauch G, Hershberg R, Elgrably-Weiss M, Baruch K, Rosenshine I, Margalit H, Altuvia S. Small RNAs encoded within genetic islands of Salmonella typhimurium show host-induced expression and role in virulence. Nucleic Acids Res. 2008;36:1913–1927. doi: 10.1093/nar/gkn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfort K, Pfeiffer V, Mika F, Lucchini S, Hinton JC, Vogel J. σE-Dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol Microbiol. 2006;62:1674–1688. doi: 10.1111/j.1365-2958.2006.05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J, Pellegrini O. Expression of the chitobiose operon of Escherichia coli is regulated by three transcription factors: NagC, ChbR and CAP. Mol Microbiol. 2004;52:437–449. doi: 10.1111/j.1365-2958.2004.03986.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen AA, Eriksen M, Gilany K, Udesen C, Franch T, Petersen C, Valentin-Hansen P. Regulation of ompA mRNA stability: The role of a small regulatory RNA in growth phase-dependent control. Mol Microbiol. 2005;58:1421–1429. doi: 10.1111/j.1365-2958.2005.04911.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen AA, Johansen J, Nielsen JS, Overgaard M, Kallipolitis B, Valentin-Hansen P. A conserved small RNA promotes silencing of the outer membrane protein YbfM. Mol Microbiol. 2009;72:566–577. doi: 10.1111/j.1365-2958.2009.06688.x. [DOI] [PubMed] [Google Scholar]
- Santiviago CA, Toro CS, Hidalgo AA, Youderian P, Mora GC. Global regulation of the Salmonella enterica serovar typhimurium major porin, OmpD. J Bacteriol. 2003;185:5901–5905. doi: 10.1128/JB.185.19.5901-5905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119:75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Seitz H. Redefining MicroRNA targets. Curr Biol. 2009;19:1–4. doi: 10.1016/j.cub.2009.03.059. [DOI] [PubMed] [Google Scholar]
- Sittka A, Pfeiffer V, Tedin K, Vogel J. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol Microbiol. 2007;63:193–217. doi: 10.1111/j.1365-2958.2006.05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper TJ, Woodson SA. The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrA sRNA. RNA. 2008;14:1907–1917. doi: 10.1261/rna.1110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G, Opdyke JA, Zhang A. Controlling mRNA stability and translation with small, noncoding RNAs. Curr Opin Microbiol. 2004;7:140–144. doi: 10.1016/j.mib.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Thompson KM, Rhodius VA, Gottesman S. σE Regulates and is regulated by a small RNA in Escherichia coli. J Bacteriol. 2007;189:4243–4256. doi: 10.1128/JB.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udekwu KI, Wagner EG. σE Controls biogenesis of the antisense RNA MicA. Nucleic Acids Res. 2007;35:1279–1288. doi: 10.1093/nar/gkl1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udekwu KI, Darfeuille F, Vogel J, Reimegard J, Holmqvist E, Wagner EG. Hfq-dependent regulation of OmpA synthesis is mediated by an antisense RNA. Genes & Dev. 2005;19:2355–2366. doi: 10.1101/gad.354405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzzau S, Figueroa-Bossi N, Rubino S, Bossi L. Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci. 2001;98:15264–15269. doi: 10.1073/pnas.261348198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P, Johansen J, Rasmussen AA. Small RNAs controlling outer membrane porins. Curr Opin Microbiol. 2007;10:152–155. doi: 10.1016/j.mib.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Vogel J. A rough guide to the non-coding RNA world of Salmonella. Mol Microbiol. 2009;71:1–11. doi: 10.1111/j.1365-2958.2008.06505.x. [DOI] [PubMed] [Google Scholar]
- Vogel J, Papenfort K. Small non-coding RNAs and the bacterial outer membrane. Curr Opin Microbiol. 2006;9:605–611. doi: 10.1016/j.mib.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Vogel J, Bartels V, Tang TH, Churakov G, Slagter-Jager JG, Huttenhofer A, Wagner EG. RNomics in Escherichia coli detects new sRNA species and indicates parallel transcriptional output in bacteria. Nucleic Acids Res. 2003;31:6435–6443. doi: 10.1093/nar/gkg867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman KM, Repoila F, Rosenow C, Storz G, Gottesman S. Identification of novel small RNAs using comparative genomics and microarrays. Genes & Dev. 2001;15:1637–1651. doi: 10.1101/gad.901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters LS, Storz G. Regulatory RNAs in bacteria. Cell. 2009;136:615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Matsumoto F, Minagawa S, Oshima T, Fujita N, Ogasawara N, Ishihama A. Characterization of CitA–CitB signal transduction activating genes involved in anaerobic citrate catabolism in Escherichia coli. Biosci Biotechnol Biochem. 2009;73:346–350. doi: 10.1271/bbb.80586. [DOI] [PubMed] [Google Scholar]
- Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Wassarman KM, Ortega J, Steven AC, Storz G. The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol Cell. 2002;9:11–22. doi: 10.1016/s1097-2765(01)00437-3. [DOI] [PubMed] [Google Scholar]
- Zhang A, Wassarman KM, Rosenow C, Tjaden BC, Storz G, Gottesman S. Global analysis of small RNA and mRNA targets of Hfq. Mol Microbiol. 2003;50:1111–1124. doi: 10.1046/j.1365-2958.2003.03734.x. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]