Abstract
Many bacterial small regulatory RNAs (sRNAs) pair with mRNA targets, stimulating or inhibiting mRNA stability and/or translation. Regulation of these sRNAs is usually due to tight transcriptional regulation of synthesis.In this issue of Genes & Development and a related paper in Molecular Microbiology, Figueroa-Bossi and colleagues (pp. 2004–2015) and Overgaard and colleagues report a novel regulatory mechanism in which induction of a competing mRNA acts to titrate away the sRNA, allowing expression of an otherwise strongly inhibited target gene.
Keywords: Antisense regulation, chitoporin, chitobiose, Hfq, small RNA
Bacterial pairing RNAs: ON and OFF switches
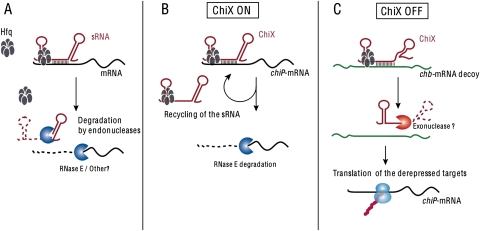
In many bacteria, including Escherichia coli and Salmonella, one large class of small regulatory RNAs (sRNAs) acts by pairing to its mRNA targets and regulating their translation and/or stability (for reviews, see Gottesman 2004; Waters and Storz 2009). Pairing is stimulated in vitro by the RNA chaperone Hfq; in vivo, Hfq acts to both stabilize the sRNA and promote base-pairing. Multiple mRNAs can be targeted by a single sRNA. Target mRNAs carry short regions of complementarity to the sRNA and, in the studied cases, binding sites for Hfq. In a large number of cases, this complementarity lies near to or overlaps the ribosome-binding site, and pairing results in rapid degradation of the mRNA as well as the sRNA (Fig. 1A).
Figure 1.
(A) Classical down-regulation of an mRNA target gene by an sRNA: The base-pairing between the sRNA (red) and the mRNA target (black), usually in a region near or overlapping the ribosome-binding site, is helped by the RNA chaperone Hfq. Outcome is an inhibition of translation of the mRNA concomitant with the degradation of both the sRNA and its target, generally initiated by the endonuclease RNase E, in a stoichiometric manner. Note that Hfq and RNase E likely share similar RNA-binding sites, and Hfq may help recruit RNase E to both the mRNA and the sRNA and/or be displaced by it. In the majority of the cases described so far, regulation of the expression of the sRNA, and thus of its activity, occurs at the level of its transcription initiation. (B) Catalytic down-regulation of the chiP mRNA by the ChiX sRNA: In the case of the down-regulation of the chiP (ybfM) mRNA by ChiX (MicM), the sRNA does not become destabilized after base-pairing to its target, and therefore could act catalytically to destroy multiple mRNAs (Overgaard et al. 2009). ChiX is expressed in what seems to be a constitutive manner, implying that it must be degraded to relieve its inhibition of the chiP target. RNase E may not be able to displace Hfq from this sRNA. (C) Competitive regulation of the ChiX sRNA by an mRNA decoy: Base-pairing of ChiX to an intergenic region of the chb mRNA (green), acting as a decoy, induces degradation of the sRNA (Figueroa-Bossi et al. 2009). Figueroa-Bossi et al. (2009) propose that base-pairing to the chb intergenic region induces a destabilization of the sRNA terminator stem–loop and its subsequent degradation by an as yet unidentified exonuclease. Thus, expression of the chb operon upon induction by chitobiose induces the degradation of ChiX and relieves the inhibition of the chiP mRNA target (black). Note that the fate of the green chb mRNA decoy is not known; possibly it is reused to target multiple ChiX sRNA molecules.
For eukaryotic siRNAs and microRNAs (miRNAs), precursor RNAs are subject to complex processing and export from the nucleus, followed by further processing. Here, each step is a possible target for regulation of RNA activity (for review, see Winter et al. 2009). In contrast, bacterial sRNA action is thought to depend mainly on abundance, which is tightly controlled at the level of transcription initiation. Thus far, a dozen of these trans-encoded sRNAs, defined by their dependence on Hfq for stability and function in vivo, have been studied sufficiently to define transcriptional regulatory signals that induce the synthesis of the sRNA and its ability to regulate at least one target mRNA. Environmental signaling proceeds, at least in large part, via classic transcriptional regulators that control the initiation of transcription of given sRNAs, providing the ON signal for regulation of targets.
The OFF signal is provided by the reversal of ON signaling upon adaptation, and by the observed rapid turnover of the existing sRNA molecules (Fig. 1A). Under biologically relevant conditions, many Hfq-dependent sRNAs turn over relatively rapidly (within minutes) (Massé et al. 2003). However, if all cellular transcription is inhibited with rifampicin, the sRNA is then stable. Our interpretation of these results has been that sRNAs pair stoichiometrically with their targets and are degraded as a result of pairing, suggesting that Hfq protection is lost after pairing. When rifampicin inhibits all transcription, no mRNA transcripts are available to pair and the sRNA remains stable. It is also known that Hfq binding stabilizes these sRNAs and that, in the cases tested, the endonuclease RNase E degrades either the naked sRNA (in the absence of Hfq) or the sRNA involved in pairing (in the presence of Hfq). This stoichiometric destruction of an sRNA by pairing with a target raises the intriguing possibility that one target, made in high amounts, might titrate away an sRNA from other targets by causing degradation. The work by Figueroa-Bossi et al. (2009) and Overgaard et al. (2009) demonstrates a number of new twists on this idea.
Discovery of the ChiX sRNA
Initially, ChiX, the Salmonella sRNA under study by Figueroa-Bossi et al. (2009) fulfilled most of the expectations for a classical trans-encoded sRNA. It was discovered in E. coli in two different global searches for sRNAs—first as an sRNA binding to Hfq in an immunoprecipitation experiment and named RybC (Zhang et al. 2003), and as part of a cloning-based sRNA identification project and named SroB (Vogel et al. 2003). Earlier this year, it made its next appearances in studies defining its ability to regulate two different specific targets. In one study, it was found to regulate ybfM, encoding a predicted outer membrane protein (OMP) of unknown function (Rasmussen et al. 2009). Because the sRNAs regulating other OMP genes are named mic (mRNA-interfering complementary RNA) followed by the last letter of the OMP gene they regulate, the ybfM-regulating sRNA was renamed MicM (Rasmussen et al. 2009). In a second study, the same sRNA was identified in a screen of an overexpression library for down-regulation of the dpiBA operon, which encodes a two-component regulatory system involved in SOS induction in response to sublethal concentration of antibiotics (Mandin and Gottesman 2009). However, neither study reported a transcriptional regulator of this sRNA. Furthermore, deletion analysis of the promoter failed to define any sites upstream of the −35 important for expression (Mandin and Gottesman 2009), and attempts to find a transcriptional regulator by various genetic screens also failed.
The interest of Bossi and coworkers (Figueroa-Bossi et al. 2006, 2009) in this sRNA also arose from studies of the YbfM target. In previous work designed to identify genes subject to sRNA regulation in Salmonella, translational fusions to ybfM were found to show a dramatic increase in expression in the absence of hfq (Figueroa-Bossi et al. 2006). Figueroa-Bossi et al. (2009) then proceeded to search for other mutations that could similarly induce the fusion, which is Lac− (tightly repressed) in an hfq+ host, by selecting or screening for Lac+ colonies. This powerful selection uncovered the same sRNA regulator found by Rasmussen et al. (2009), as well as the network that provides both the biological rationale for the regulation of ybfM and the novel mechanism for regulation of this sRNA. Figueroa-Bossi et al. (2009) renamed the sRNA ChiX based on their findings (see below). In a related but complementary approach, Valentin-Hansen and colleagues (Overgaard et al. 2009) carried out a genetic screen in E. coli for genes that, in multicopy, relieved the very tight regulation of a ybfM translational fusion by the sRNA (called by them MicM); their results uncovered the same unique regulatory network.
Regulation of ybfM (chiP) and ChiX involves an induced response to chitin breakdown products
What was found? Figueroa-Bossi et al. (2009) isolated mutations inactivating hfq, mutations inactivating the sRNA, and mutations in the fusion, within the pairing region with the sRNA, that all relieved ybfM repression, as expected. In the overproduction screen, genes for the 5′ pyrophosphohydrolase rppH and the polyA polymerase pcnB were identified; these should lead to increased sRNA degradation (Overgaard et al. 2009). Overproduction of the ybfM 5′ untranslated region (UTR), presumably binding to the sRNA and titrating it from the fusion, also led to increased expression of the fusion (Overgaard et al. 2009).
However, it was the final class of mutations and plasmids that relieve ybfM translational repression that provided the critical insight into the system. Figueroa-Bossi and colleagues (Figueroa-Bossi et al. 2009) identified mutations in a gene named chbR, encoding an AraC family regulator of the chitobiose (chb) operon. The chb operon contains genes for the uptake and degradation of breakdown products of chitin, including chitobiose. ChbR is a dual regulator that represses expression of the chb operon in the absence of chitibiose but activates it in the presence of the inducer (Plumbridge and Pellegrini 2004). Based on this observation, Figueroa-Bossi et al. (2009) tested chbR+ cells for induction with chitobiose, and that, too, led to induction of the reporter fusion.
Why does induction of the chb operon lead to increased expression of a ybfM fusion? The answer has two parts: modest ChbR stimulation of transcription of ybfM, and ChbR induction of the chb operon mRNA, which in turn relieves the stringent ybfM translational repression. All of this makes regulatory sense if the YbfM porin protein is part of the chitobiose utilization pathway. In fact, YbfM is necessary for growth on some chitin breakdown products, and thus was renamed ChiP (chitoporin) (Figueroa-Bossi et al. 2009). In the absence of the ChiX (MicM) sRNA, the basal level of chiP (ybfM) expression is high, but induction by chitobiose increases expression an additional fourfold, consistent with direct action by ChbR on chiP transcription initiation. However, this cannot be the whole story. chiP (ybfM) is induced >200-fold when the ChiX (MicM) sRNA is present. Thus, under these induction conditions, ChiX repression is also relieved (Figueroa-Bossi et al. 2009). Furthermore, ChiX sRNA levels decreased significantly during chitobiose induction, apparently as a result of rapid degradation of the ChiX RNA.
Overgaard et al. (2009) also identified the chb operon, overexpressed on their plasmids. Thus, high-level expression of the chb operon relieves chiP (ybfM) repression and leads to ChiX (MicM) degradation, as well as leading to increased transcription of chiP. Upon inspection, both groups identified a region of complementarity to the ChiX sRNA lying within an intergenic region of the chb operon, providing a possible alternative target for ChiX.
Abundance of the ChiX sRNA is regulated by a target decoy, an inducible OFF switch
What is the basis for this chitobiose-induced relief of chiP repression? The answer lies in the characteristics of degradation of this sRNA, and uncovers a unique OFF switch for an sRNA whose default expression appears to be ON.
As noted above, for many sRNAs, down-regulation of the promoter is sufficient to lead to rapid degradation of the sRNA (Massé et al. 2003). However, this does not seem to be the case for ChiX. The ChiX RNA is quite abundant under a variety of growth conditions, and no evidence of a regulated promoter has been found (Vogel et al. 2003; Mandin and Gottesman 2009; Rasmussen et al. 2009). If expressed from an inducible promoter, its half-life in wild-type cells is long (27 min) (Overgaard et al. 2009). Even under conditions in which the chiP (ybfM) target is overproduced, ChiX (MicM) remains stable (Overgaard et al. 2009). Based on these observations and calculations of the levels of ChiX in the cell, Overgaard et al. (2009) argue that ChiX (MicM) is used catalytically, targeting degradation of multiple chiP (ybfM) target mRNAs before it is itself destroyed(Fig. 1B). If so, this would be the first example of catalytic regulation by sRNAs in bacteria, although this is the usual case for eukaryotic miRNAs (for review, see Carthew and Sontheimer 2009). Constitutive expression of a reusable sRNA leads to continuous and tight down-regulation of the chiP mRNA target. Therefore, regulation by the ChiX sRNA can be considered constitutively ON under noninducing growth conditions.
It is not clear what characteristics of ChiX protect it from pairing-coupled degradation, therefore allowing it to act catalytically. One possibility, not yet tested, is that Hfq binding to ChiX is particularly tight. Rapid degradation of other sRNAs depends on RNase E and occurs either in the absence of Hfq or after pairing, suggesting that Hfq may be displaced after pairing (Massé et al. 2003). If Hfq protects an sRNA from RNase E cleavage (Moll et al. 2003) and is binding too tightly to be displaced, the sRNA may become long-lived, remaining on its chaperone through multiple rounds of pairing and target mRNA degradation.
However, the fate of ChiX upon pairing with the region within the chb operon is entirely different. Expression of this region, either as part of the chb operon or on its own, leads to the rapid disappearance of the ChiX sRNA and, as a result, the relief of repression of chiP (Fig. 1C). Thus, upon expression of this alternative target mRNA, acting as a decoy, ChiX is turned OFF and chiP expression is derepressed.
Why is degradation of ChiX invoked by one target (the chb decoy RNA), but not another (chiP mRNA)? Further work will be necessary to answer this question, but one possibility suggested by Figueroa-Bossi et al. (2009) is that ChiX becomes particularly unstable upon pairing with the chb decoy target because pairing extends 2 nucleotides into the transcription terminator stem–loop. Consistent with this hypothesis, point mutations in these base pairs and in other nucleotides of the terminator stem also lead to destabilization of ChiX. Figueroa-Bossi et al. (2009) thus suggest that in the case of the pairing with the chb decoy sequence, weakening of this 3′ stem removes a barrier that prevents rapid degradation of the sRNA by 3′ exonucleases. If this proves to be true, then degradation via pairing with the competing target will be by a pathway distinct from that seen for degradation of most sRNAs, which are thought to be initiated by endonucleolytic cleavage.
For this system to work efficiently, it would seem that the induced chb decoy should be a preferred pairing partner for ChiX; it is not yet known if this is the case, but the predicted base-pairing energies of the different RNA hybrids seem to favor ChiX pairing to chb mRNA over pairing to chiP (ChiX/chiP ΔG ≈ −12 kcal/mol; ChiX/chb ΔG ≈ −18 kcal/mol). However, even if it is not a preferred pairing partner, chb pairing should destabilize ChiX, reducing the level of ChiX as long as the chb decoy is being transcribed.
We and Figueroa-Bossi et al. (2009) and Overgaard et al. (2009) have referred to the pairing region within chb as a decoy, a target mimic, or a trap-mRNA; these terms all imply that the chb operon mRNA is not itself a target for regulation—i.e., that ChiX does not affect its expression. This has not yet been examined. Certainly, the biology of the system suggests ChiX should not negatively regulate the chb operon, since the chb mRNA will be made only when the products are needed (chitin degradation conditions), and the position of the pairing region is far from ribosome loading regions for chb products. Possibly, the chb operon mRNA, or at least a fragment of it, might be reused catalytically to induce ChiX degradation. Whether there are other characteristics of this operon that ensure that the chb decoy acts only as a decoy remains to be seen.
Implications and parallels in other RNA-based regulation
There are many characteristics of the ChiX system that have allowed this unique regulatory cascade to be uncovered (common induction of the target and decoy, catalytic use of ChiX, and its constitutive synthesis). However, it is clear that many of the principles found here will be pertinent to other systems as well. These findings raise the interesting possibility that some of what were believed to be targets of sRNAs may in fact be used to modulate activity. There may well be a continuum of effects in which some targets are truly targets, impinging little on the availability of the sRNA, some mRNAs act only as decoys, regulating sRNA stability and availability, while many mRNAs may do both, setting a hierarchy for sRNA regulation by their ability to compete for a limiting sRNA and destroy it as a result of pairing.
The decoys need not always be mRNAs, and the decoys need not always act directly by pairing. In at least two cases, bacterial sRNAs have been shown to affect the stability and availability of another sRNA, directly or indirectly. RyeB and RyeA are overlapping complementary sRNAs; as one accumulates in stationary phase, the other is processed, presumably as a result of pairing, although the biological roles of neither sRNA nor this regulatory cascade is yet known (Vogel et al. 2003). The second case involves the GlmY/GlmZ sRNAs and their regulation of glmS of E. coli. Like the ChiX story, this also involves regulation of sugar transport and metabolism, a growing theme for sRNAs (Gorke and Vogel 2008). While GlmY and GlmZ sRNAs are homologous and positively regulate the translation of glmS, a gene encoding an enzyme of the biosynthesis pathway of amino sugars, only GlmZ pairs directly to its target. GlmY is also able to activate GlmS synthesis in vivo by counteracting a 3′ RNA processing in GlmZ that otherwise inactivates it (Urban and Vogel 2008). Although the mechanism of this protection is unknown, one interesting possibility is that it involves RNA mimicry, with GlmY competing with the unidentified factors that direct GlmZ cleavage.
What is the OFF switch for eukaryotic miRNAs?
How is the activity of eukaryotic regulatory RNAs regulated? The basic principles of eukaryotic post-transcriptional regulation by miRNAs are similar to the prokaryotic ones discussed here: namely, base-pairing of the regulatory RNA to mRNA targets, followed by translation inhibition and/or mRNA degradation (for review, see Carthew and Sontheimer 2009). However, the miRNAs and siRNAs appear to be reused, and there is very little information about how their action might be turned off. Some proteins have been found to interact with the 3′ UTR of specific target genes under particular conditions, changing the action of miRNAs on these targets (Bhattacharyya et al. 2006; Vasudevan et al. 2007), and it is certainly conceivable that these mechanisms are more widespread. However, such mechanisms, rather than down-regulating a specific miRNA, will be target-specific. Changes in protein components of miRNA complexes may also provide some possibility of an OFF switch (Baumberger et al. 2007; Hammell et al. 2009), although whether these will be specific to a given miRNA, siRNA, or family of RNAs remains to be seen. Thus, it seems highly likely that target decoys might play a role in regulating specific eukaryotic siRNA/miRNA pathways. One example of a very similar control has been described already in the plant Arabidopsis thaliana, where the non-protein-coding gene IPS1 mimics a target of the miRNA miR399 but is not cleaved, sequestering miR399 from its other targets instead (Franco-Zorrilla et al. 2007). Because in this case IPS1 is a noncoding gene that appears to have no other function, Franco-Zorrilla et al. (2007) coined the term “target mimicry” to describe this mechanism. Thus, plants and bacteria have evolved very parallel mechanisms for sequestering and/or destroying regulatory RNAs, providing yet another level of regulation to the growing complexity of RNA-based regulatory networks.
Acknowledgments
We thank P. Valentin-Hansen for sharing his work with us before publication, E. Sontheimer and V. Ambros for advice on the state of knowledge of miRNA turnover, and Aurelia Battesti, Nadim Majdalani, Paola Milanesio, Nicholas De Lay, Gisela Storz, Carin Vanderpool, and Bob Weisberg for comments on the manuscript. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, and Center for Cancer Research.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1846609.
References
- Baumberger N, Tsai C-H, Lie M, Havecker E, Baulcombe DC. The Polerovirus silencing suppressor P0 targets ARGONAUTE proteins for degradation. Curr Biol. 2007;17:1609–1614. doi: 10.1016/j.cub.2007.08.039. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Bossi N, Lemire S, Maloriol D, Balbontin R, Casadesus J, Bossi L. Loss of Hfq activates the σE-dependent envelope stress response in Salmonella enterica. Mol Microbiol. 2006;62:838–852. doi: 10.1111/j.1365-2958.2006.05413.x. [DOI] [PubMed] [Google Scholar]
- Figueroa-Bossi N, Valentini M, Malleret L, Bossi L. Caught at its own game: Regulatory small RNA inactivated by an inducible transcript mimicking its target. Genes & Dev. 2009 doi: 10.1101/gad.541609. (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, Garcia JA, Paz-Ares J. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet. 2007;39:1033–1037. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]
- Gorke B, Vogel J. Noncoding RNA control of the making and breaking of sugars. Genes & Dev. 2008;22:2914–2925. doi: 10.1101/gad.1717808. [DOI] [PubMed] [Google Scholar]
- Gottesman S. The small RNA regulators of Escherichia coli: Roles and mechanisms. Annu Rev Microbiol. 2004;58:303–328. doi: 10.1146/annurev.micro.58.030603.123841. [DOI] [PubMed] [Google Scholar]
- Hammell CH, Lubin I, Boag PR, Blackwell TK, Ambros V. nhl-2 modulates microRNA activity in Caenorhabditis elegans. Cell. 2009;136:926–938. doi: 10.1016/j.cell.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandin P, Gottesman S. A genetic approach for finding small RNA regulators of genes of interest identifies RybC as regulating the DpiA/DpiB two-component system. Mol Microbiol. 2009;72:551–565. doi: 10.1111/j.1365-2958.2009.06665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé E, Escorcia FE, Gottesman S. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes & Dev. 2003;17:2374–2383. doi: 10.1101/gad.1127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll I, Afonyushkin T, Vytvytska O, Kaberdin VR, Blasi U. Coincident Hfq binding and RNase E cleavage sites on mRNA and small regulatory RNAs. RNA. 2003;9:1308–1314. doi: 10.1261/rna.5850703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard M, Johansen J, Moller-Jensen J, Valentin-Hansen P. Switching off small RNA regulation with trap-mRNA. Mol Microbiol. 2009 doi: 10.1111/j.1365-2958.2009.06807.x. (in press). [DOI] [PubMed] [Google Scholar]
- Plumbridge J, Pellegrini O. Expression of the chitobiose operon of Escherichia coli is regulated by three transcription factors: NagC, ChbR and CAP. Mol Microbiol. 2004;52:437–449. doi: 10.1111/j.1365-2958.2004.03986.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen AA, Johansen J, Nielsen JS, Overgaard M, Kallipolitis B, Valentin-Hansen P. A conserved small RNA promotes silencing of the outer membrane protein YbfM. Mol Microbiol. 2009;72:566–577. doi: 10.1111/j.1365-2958.2009.06688.x. [DOI] [PubMed] [Google Scholar]
- Urban JH, Vogel J. Two seemingly homologous noncoding RNAs act hierarchically to activate glmS mRNA translation. PLoS Biol. 2008;6:e64. doi: 10.1371/journal.pbio.0060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: MicroRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Vogel J, Bartels V, Tang HH, Churakov G, Slagter-Jager JG, Huttenhofer A, Wagner EGH. RNomics in Escherichia coli detects new sRNA species and indicates parallel transcriptional output in bacteria. Nucleic Acids Res. 2003;31:6435–6443. doi: 10.1093/nar/gkg867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters LS, Storz G. Regulatory RNAs in bacteria. Cell. 2009;136:615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: MicroRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- Zhang A, Wassarman KM, Rosenow C, Tjaden BC, Storz G, Gottesman S. Global analysis of small RNA and mRNA targets of Hfq. Mol Microbiol. 2003;50:1111–1124. doi: 10.1046/j.1365-2958.2003.03734.x. [DOI] [PubMed] [Google Scholar]