Abstract
Canonical Notch signaling is thought to control the endocrine/exocrine decision in early pancreatic progenitors. Later, RBP-Jκ interacts with Ptf1a and E12 to promote acinar differentiation. To examine the involvement of Notch signaling in selecting specific endocrine lineages, we deregulated this pathway by targeted deletion of presenilin1 and presenilin2, the catalytic core of γ-secretase, in Ngn3- or Pax6-expressing endocrine progenitors. Surprisingly, whereas Pax6+ progenitors were irreversibly committed to the endocrine fate, we discovered that Ngn3+ progenitors were bipotential in vivo and in vitro. When presenilin amounts are limiting, Ngn3+ progenitors default to an acinar fate; subsequently, they expand rapidly to form the bulk of the exocrine pancreas. γ-Secretase inhibitors confirmed that enzymatic activity was required to block acinar fate selection by Ngn3 progenitors. Genetic interactions identified Notch2 as the substrate, and suggest that γ-secretase and Notch2 act in a noncanonical titration mechanism to sequester RBP-Jκ away from Ptf1a, thus securing selection of the endocrine fate by Ngn3 progenitors. These results revise the current view of pancreatic cell fate hierarchy, establish that Ngn3 is not in itself sufficient to commit cells to the endocrine fate in the presence of Ptf1a, reveal a noncanonical action for Notch2 protein in endocrine cell fate selection, and demonstrate that acquisition of an endocrine fate by Ngn3+ progenitors is γ-secretase-dependent until Pax6 expression begins.
Keywords: Endocrine progenitor, Notch, pancreatic development, Presenilins, cell fate, lineage tracing
The pancreas develops from Pdx1- and Ptf1a-expressing progenitors that emerge from the foregut endoderm to form ventral and dorsal buds. Shortly thereafter, these buds will fuse and form a complex organ composed of endocrine, acinar, and ductal cells. The steps involved in establishing the identity of the endocrine progenitors and the molecular steps involved in organogenesis have been the subjects of many studies, several identifying Notch signaling as a master regulator controlling the initial endocrine versus acinar decision (for review, see Jørgensen et al. 2007). The Notch genes encode conserved, membrane-bound receptors mediating short-range cell–cell communications during embryonic development and in the adult. Notch proteins are proteolytically processed in response to ligand binding (for review, see Kopan and Ilagan 2009). Intramembrane proteolysis of all Notch receptors is mediated by the enzyme γ-secretase with Presenilin proteins (Ps1 or Ps2) forming its catalytic core. Following γ-secretase cleavage, the released Notch intracellular domain (NICD) enters the nucleus, where it binds to RBP-Jκ, recruits Mastermind, and assembles an activator complex.
Recent lineage studies with neurogenin3-Cre transgenic lines exclusively marked the endocrine tissue, suggesting that expression of Ngn3 identified committed endocrine progenitors (Gu et al. 2002, 2003). Deletion of Ngn3 compromised endocrine differentiation, indicating that this transcription factor is required (Gradwohl et al. 2000; Lee et al. 2001). Overexpression of Ngn3 in transgenic mice blocked exocrine development and accelerated endocrine differentiation (Apelqvist et al. 1999), demonstrating that Ngn3 by itself is sufficient to drive the endocrine fate at this stage. Ngn3 regulates endocrine differentiation in part through the activation of Pax6, a transcription factor that bears both a paired box and a homeodomain (for review, see Jørgensen et al. 2007). A few Ngn3+ progenitor cells are detected in the adult pancreas (Gu et al. 2002) where, under specific regeneration conditions, they can proliferate and differentiate into pancreatic β cells (Xu et al. 2008), further establishing Ngn3 as a marker of endocrine progenitors.
Although Notch signaling is thought to antagonize Ngn3 expression through activation of HES1 (Esni et al. 2004), allowing the Ptf1a/p48 complex to direct the cells toward acinar differentiation (Obata et al. 2001; Beres et al. 2006; Fujikura et al. 2007; Masui et al. 2007), HES1 can also be activated by several other signaling pathways (Kageyama et al. 2007). Expression of receptors (Notch1–3), ligands (Jag-1, Jag-2, Dll-1, and Dll-3), and targets (HES1) was observed in cells that did not yet appear to be differentiated, but not in more mature endocrine cells within the embryonic pancreas (Jensen et al. 2000). Complicating matters are observations identifying an essential role for RBP-Jκ as a cofactor for Ptf1a/p48, thus directly promoting acinar differentiation independent of Notch (Obata et al. 2001; Beres et al. 2006; Fujikura et al. 2007; Masui et al. 2007). Since earlier studies were not aware of this dual function of RBP-Jκ, and thus did not account for it in the experimental design and interpretation of results, ambiguity surrounds the exact role of Notch signaling throughout pancreatic development. Disruption of Notch signaling by removing the ligand (Dll1) or RBP-Jκ resulted in decreased pancreatic bud size along with expansion of endocrine cells (Apelqvist et al. 1999); however, loss of p48 activity in RBP-Jκ-null cells was not considered. In contrast, Pdx1-controlled overexpression of NICD3, an ineffective HES1 activator that binds to RBP-Jκ with the same affinity as NICD1 (Lubman et al. 2007), led to reduction in HES1 and Ptf1a expression, with a subsequent increase in Ngn3 expression (Apelqvist et al. 1999). Again, the possible negative impact on Ptf1a function was not considered. Although recent studies have implicated Notch2 as a susceptibility locus for type 2 diabetes (Zeggini et al. 2008), confidence in an early role for Notch signaling during pancreas development has eroded further since removal of both Notch1 and Notch2 still permitted formation of a functional pancreatic organ, whereas removal of RBP-Jκ did not (Nakhai et al. 2008). Whatever their role, Notch receptors are required only transiently, as highlighted by the observation that the constitutively active form of Notch1 expressed under the control of the Pdx1 promoter caused marked reduction in both exocrine and endocrine differentiation (Hald et al. 2003; Murtaugh et al. 2003).
To investigate whether Notch signaling was still involved after the initial endocrine/acinar decision, we inactivated Notch signaling by removal of presenilin1 and presenilin2 after the establishment of Ngn3 progenitors with an Ngn3-Cre line and, in parallel, traced the fate of presenilin-deficient cells with an EGFP. Unexpectedly, we discovered that Ngn3 progenitors, originally thought to be committed to the endocrine fate in the absence of Notch signals, selected instead the acinar fate even when presenilin dose was only reduced, not eliminated. The number of Ngn3 progenitors entering the acinar fate was inversely correlated with Presenilin activity. The phenotype associated with presenilin-nulls can be attributed in most cases to loss of Notch signaling (Struhl and Greenwald 2001; Pan et al. 2004, 2005); however, in addition to their role in Notch, ErbB, and Ryk proteolysis (Kopan and Ilagan 2004; Lyu et al. 2008), Presenilins have been implicated in multiple additional processes: transport vesicles (Sisodia and St George-Hyslop 2002; Pigino et al. 2003; Wood et al. 2005), scaffolds for AKT activation (Baki et al. 2004), β-catenin degradation (Kang et al. 1999), and Erk activation (Kim et al. 2005; Wines-Samuelson and Shen 2005). Since pharmacological inhibition of the γ-secretase function in vitro also diverted Ngn3 progenitors to the acinar fate, we concluded that γ-secretase activity was involved. Finally, genetic interaction with Notch2 indicates that Notch is a relevant substrate in this process.
Once formed, we observed that Ngn3-derived, presenilin-deficient acinar cells undergo proper acinar differentiation, but display enhanced proliferation compared with control cells. Importantly, proper organ size is maintained by a corresponding increase in apoptosis. In addition, we found that whereas Ngn3+ cells are still bipotential, endocrine progenitors become irreversibly committed to the endocrine fate as soon as Pax6 is expressed and can no longer switch to acinar fates in the absence of presenilins. This study thus exposed a default (acinar) fate of Ngn3 progenitors that persists during a narrow developmental window. We propose that, within this window, the activity of γ-secretase generates NICD2 that sequesters RBP-Jκ away from p48 to promote, rather than inhibit, the endocrine fate. A similar noncanonical Notch function may also participate in controlling the glutaminergic/GABAergic decision within the CNS (Hori et al. 2008), and may be also involved in controlling horizontal/amacrine versus ganglion cell fate decisions in the retina (Fujitani et al. 2006).
Results
Presenilins control in vivo the terminal differentiation of Ngn3 progenitors
To assess the involvement of Notch signaling in the Ngn3 lineage, we exploited the universal dependence of all Notch receptor paralogs on γ-secretase activity and devised a strategy to create transgenic animals in which only Ngn3+ progenitors became deficient in Presenilin proteins. Mice homozygous for a null mutation in Ps2 and heterozygous for a null mutation in Ps1 are viable and display no apparent phenotype (Donoviel et al. 1999; Hartmann et al. 2002; Pan et al. 2004) until the age of 6 mo (Qyang et al. 2004; Tournoy et al. 2004). These mice were crossed with mates that were trans-heterozygous for a conditional (floxed) allele and a null allele of Ps1 (Saura et al. 2004) to generate Ps1f/−; Ps2−/− breeders. These mice were then crossed with Ngn3-Cre (Gu et al. 2002), Z/EG reporter mice (Fig. 1A; Novak et al. 2000). This breeding schema allowed the generation of Ngn3-Cre+/−; Ps1f/−; Ps2−/−; Z/EG offspring. Ngn3 activation drives the expression of Cre recombinase, leading to the deletion of the remaining Ps1 allele and, independently, to the expression of the EGFP (used for lineage tracing). After deletion of the presenilin allele and some delay due to depletion of existing mRNA and protein, the cells no longer have γ-secretase-dependent or -independent presenilin activity.
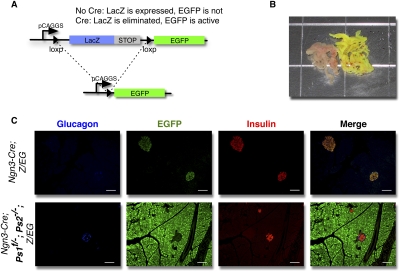
Figure 1.
Elimination of the Presenilins in vivo in Ngn3-progenitors. (A) Schematic representation of the Z/EG reporter. (B) Gross morphology of control (Ngn3; Z/EG) (left) and Presenilin-deficient (Ngn3-Cre; Ps1f/−; Ps2−/−; Z/EG) (right) pancreata of adult mice. (C) Immunostaining for glucagon (blue), EGFP (green), and insulin (red) in Ngn3; Z/EG (top) and Presenilin-deficient Ngn3-Cre; Ps1f/−; Ps2−/−; Z/EG (bottom) adult mice. Bars, 50 μm.
Breeding was designed to generate Ngn3-Cre; Z/EG mice with all possible allelic combinations ranging from wild type (Ps1+/+; Ps2+/+, used as controls) to Ps1f/−; Ps2−/−. Mice from all genotypes were born at expected Mendelian frequencies and all survived to adulthood, indicating sufficient pancreatic function. Eight to 10 mice from each genotype were dissected at 12 wk of age. Wild-type animals or animals heterozygous for Ps1 and Ps2 (Ps1+/f; Ps2+/−) appeared normal with respect to weight and fertility, with no visible alteration of pancreatic morphology (Fig. 1B, left). Mice lacking Ps2 and Ps1 in Ngn3 progenitors (Ngn3-Cre; Ps1f/−; Ps2−/−; Z/EG) presented with unexpected minor defects in somitogenesis (e.g., “curly tails”), but were otherwise indistinguishable from their littermates. The γ-secretase-deficient pancreas' size and weight was not significantly different from the controls, but strikingly, even under normal light, EGFP expression was clearly visible in all Ps1, Ps2-deficient animals throughout the entire pancreas (Fig. 1B, right). In contrast, mice heterozygous for Ps1 and Ps2 (Ps1+/f; Ps2+/−) displayed no visible alteration of pancreatic morphology (Fig. 1B, left).
To identify the lineage of EGFP-expressing cells, sections were analyzed by immunofluorescence for endocrine markers (insulin and glucagon). As described previously (Gu et al. 2002, 2003), Ngn3 progenitors in the control Ngn3-Cre; Z/EG mice gave rise only to the endocrine lineage, contributing to ∼2%–3% of the organ (Fig. 1C). Interestingly, one of four compound heterozygotes (Ngn3-Cre; Ps1+/f; Ps2+/−; Z/EG) contained a few EGFP+ cells within acinar tissue, suggesting that presenilin activity within few progenitors may have become limiting even in the presence of two alleles (Supplemental Fig. 1). When three Presenilin alleles were deleted (Ngn3-Cre; Ps1+/f; Ps2−/−; Z/EG or Ngn3–Cre; Ps1f/-; Ps2+/−; Z/EG), EGFP+ cells were located exclusively in the acini (Fig. 1C, bottom panel), consistent with preferential selection of the acinar fate by Ngn3 cells as the dose of presenilin becomes limiting. Since only one of four Ngn3-Cre; Ps1+/f; Ps2+/−; Z/EG animals had labeled acini, it is possible that genetic background modified the islet/acini choice in these mice.
Acinar cells were infrequently labeled with another Ngn3-Cre transgenic line (Schonhoff et al. 2004), prompting us to confirm that our observations did not reflect aberrant behavior of the Ngn3-Cre transgene. We therefore repeated the experiments with a knocked-in Ngn3-Cre line (a generous gift from Dr. D. Melton). These mice, which are also heterozygous for Ngn3, exhibited an identical phenotype to the transgenic line we used, with EGFP-labeled Ngn3 progenitors confined to the acinar lineage (Supplemental Fig. 2). Thus, we conclude that Ngn3+ progenitors were bipotential and have not yet committed to any lineage. In addition, these results demonstrate that Presenilin dose sufficient for Notch activity elsewhere was limiting for selection of the endocrine fate by these progenitors. Since Ptf1a and Ngn3 compete for E12 proteins, we wished to avoid possible complications due to Ngn3 haploinsufficiency, which may further enhance the acinar fate selection. We thus performed all subsequent experiments in the Ngn3-Cre transgene, using the Cre knock-in line as a control.
Presenilin-deficient Ngn3 cells retain progenitor characteristics; the bulk of the acinar tissue is produced by a late proliferative burst in their descendents
Lineage tracing revealed that, in the adult, all Presenilin-deficient cells (PsLow) contributed to the exocrine mass instead of to the endocrine fate selected by Ngn3+, PsHigh cells. In order to analyze both the timing and mechanism of this fate switch, we stained for EGFP and various differentiation markers at different developmental stages (n = 28–10 per genotype and stage). During organogenesis, expression of Ngn3 peaks around 15.5 d of development (embryonic day 15.5 [E15.5]) (Apelqvist et al. 1999). At E14.5, EGFP expression can be observed in nonendocrine cells and in a few endocrine hormone-expressing cells in control Ngn3-Cre, Z/EG or PsLow mice (Fig. 2A, panels 1,4). PsHigh, EGFP+ cells became progressively restricted to the islets, and at E18.5 (Fig. 2A, panel 2) only a small number of undifferentiated EGFP+ cells remained (data not shown). At birth, all EGFP+ cells were located within the islets and were positive for endocrine markers (Fig. 2A, panel 3).
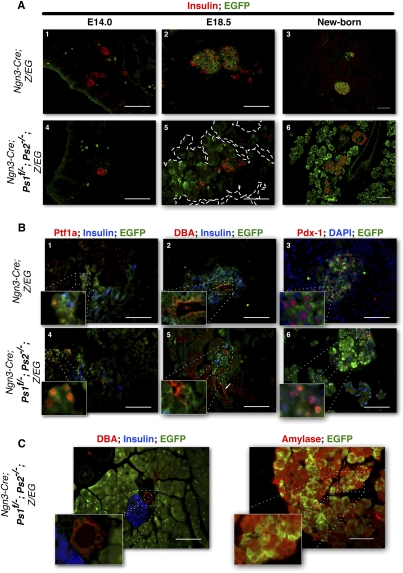
Figure 2.
Presenilin-deficient pancreatic progenitors increase in numbers late during embryonic development. (A) Immunostaining for insulin (red) and EGFP (green) at E14.0 (left) and E18.5 (middle) and in the newborn (right) in control (Ngn3-Cre; Z/EG) (top) and presenilin-deficient (Ngn3-Cre; Ps1f/−; Ps2−/−; Z/EG) (bottom) mice. EGFP-positive cells are found outside of the endocrine compartment in Presenilin mice throughout development and expand around birth. (B) Coimmunostaining for Ptf1a (red), insulin (blue), and EGFP (green) (middle); DBA (red), insulin (blue), and EGFP (green) (right panel); or Pdx1 (red) DAPI (blue), and EGFP (green) (left panel) in sections of E18.5 control (Ngn3-Cre; Z/EG) (top) and Presenilin-deficient (Ngn3-Cre; Ps1f/−; Ps2−/−; Z/EG) (bottom) mice. Pslow, nonendocrine cells still express Pdx1, Ptf1a, and DBA at E18.5. (C) Coimmunostaining for DBA (red), Insulin (blue), and EGFP (green) (left panel), or Amylase (red) and EGFP (green) (right panel). Pslow cells exclusively express Amylase in the adult. Normal, EGFP-negative acinar cells form clusters n the periphery of the tissue. Bars, 50 μm.
At E14.5, few cells express acinar markers (amylase or carboxy-Peptidase-A) (Pictet and Rutter 1972). Throughout organogenesis and until E18.5, EGFP+ cells in PsLow mice were scattered outside the forming islets (Fig. 2A, panel 5) and did not express endocrine markers (insulin, glucagon, or synaptophysin). We calculated the area covered by EGFP at E18.5 to be on average 35% (six randomly selected slides per mouse, n = 27 mice). At birth, the vast majority of acinar tissue is EGFP+, as seen in the adult (Fig. 2A, panel 6).
Ptf1a is an early marker for pancreatic progenitors. It is rarely found in the nascent islets and becomes progressively restricted to the acinar cells by E18.5. In control mice, only a few EGFP+ endocrine progenitors still expressed Ptf1a (Fig. 2B, panel 1). In contrast, 47% of the EGFP+; PsLow cells were also Ptf1a+ (Fig. 2B, panel 4).
DBA (dolichos biflorus agglutinin), a lectin recognizing the N-acetyl glucosamine cell surface 0-glycans, marks ductal cells, embryonic stem (ES) cells, and pancreatic progenitors during development. These ductal markers are also expressed on ES cells and pancreatic progenitors during development. In both control and PsLow mice, only a few EGFP+, DBA+ cells are derived from Ngn3 progenitors (EGFP+, DBA+ form PsLow mice, as shown in Fig. 2B, panels 2,5).
Pdx1, like Ptf1a, is also a marker for early pancreatic progenitors that later restricts to the β cells. As expected, in control mice at E18.5, Pdx-1 is found exclusively in the forming islets (Fig. 2B, right). Surprisingly, at the same stage in PsLow mice, Pdx-1 expression was still detected in EGFP+ nonendocrine cells (Fig. 2B, panels 3,6). In the adult, both Pdx-1 and DBA expressions were lost in PsLow cells (Fig. 2C, left). All PsLow cells in the adult differentiate into acinar cells that express specific markers like amylase (Fig. 2C, right) and form the vast majority of the acinar compartment, as illustrated in Figures 1 and 2. EGFP-negative acinar cells form a few clusters composing 10%–15% of the pancreatic area and are usually located at the periphery of the sections (Fig. 2C). These results indicate that in the absence of Presenilins, some Ngn3 progenitors retain the expression of Ptf1a, DBA, and Pdx-1 as late as E18.5. At birth, when the EGFP+ acinar cells form the vast majority of the acinar tissue, EGFP+, Pdx1+ DBA+ cells were no longer detectable in the pancreas. It remains difficult to ascertain whether the presence of these markers in the absence of Presenilins reflects dedifferentiation during transition from an endocrine progenitor to an acinar cell or an extended progenitor state.
Presenilin-deficient cells compensate for enhanced proliferation with enhanced apoptosis to ensure normal organ size in the adult
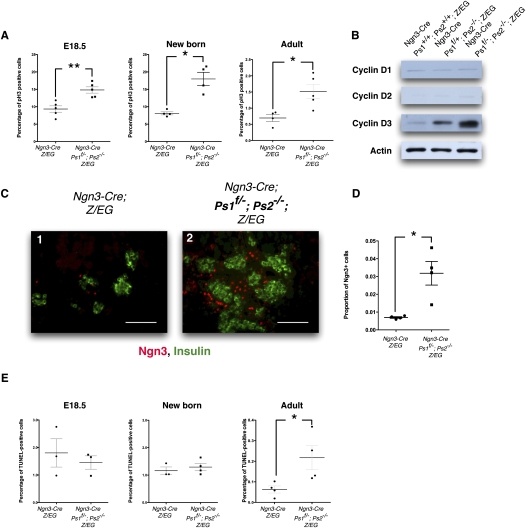
At 18 d of development, PsLow EGFP+ cells represent a third of the total nonendocrine tissue in the pancreas. After birth, this mass dominates the acinar tissue. We therefore analyzed the distribution of Phospho-Histone 3 on sections of E18.5, newborn, or adult pancreases. Throughout development, proliferation rates of nonendocrine cells gradually decrease in control mice; in the adult, very few acinar and islet cells appear to be proliferating at any given moment (Fig. 3A, left columns). In contrast, EGFP+ acinar cells sustain an enhanced rate of proliferation throughout development, and although reduced in the adult, proliferation in the acini remains significantly higher in PsLow mice than in the control mice (P < 0.05) (Fig. 3A). We next examined the relative expression levels of Cyclins D1, D2, and D3 by Western blot analysis of total adult pancreatic protein extracts. In the adult, Cyclins D1 and D2 were expressed at similar levels among the different groups, but Cyclin D3 was increased in Presenilin-deficient animals in a Presenilin dose-sensitive manner, as seen in Figure 3B. In addition, we examined the expression of Ngn3 at E18. In control mice, Ngn3 expression can be seen in only a few cells outside the islets (Fig. 3C, panel 1). In PsLow mice, the number of Ngn3+ cells increased by 4.5-fold (P < 0.05) (Figure 3C, panel 2, quantified in D). Thus, at E18, the Ngn3+ population expanded; these Ngn3+, PsLow progenitors not only preferentially selected the acinar fate, but secondarily produced descendents that proliferate vigorously, a process during which they outcompete any “normal” acinar cell.
Figure 3.
Presenilin-deficient Ngn3-derived progenitors proliferate at a sustained rate and turn over rapidly in the adult. (A) Quantification of the percentage of Phospho-Histone3-positive cells in control and Presenilin-deficient mice at E18.5 (n = 25), newborn mice (n = 24), and adult mice (n = 25). More than 3900 nuclei were counted for each mouse. Presenilin-deficient mice display an increased proliferation rate at all stages. Gray bars represent SEM; (*) P < 0.05; (**) P < 0.01. (B) Western-blot analysis for the expression of Cyclins D1, D2, and D3 in total pancreatic extracts of control (Ngn3-Cre; Z/EG) and Presenilin-deficient (Ngn3-Cre; Ps1+/f; Ps2−/−; Z/EG and Ngn3-Cre; Ps1f/−; Ps2−/−; Z/EG). The immunoblots are normalized over actin and indicate that Cyclin D3 increased concomitantly with the reduction of Presenilins. (C) Expression of Ngn3 around forming islets at E18.5 in control (panel 1) and Presenilin-deficient (panel 2) mice. Ngn3 is stained in red and insulin is stained in green. Bars, 50 μm. (D) Overall proportion of Ngn3+ cells is represented on the right. Gray bars represent SEM; P < 0.05. (E) Quantification of the percentage of TUNEL-positive cells in control and Presenilin-deficient mice at E18.5 (n = 23), newborn mice (n = 24), and adult mice (n = 24); >3400 nuclei were counted for each mouse. The apoptosis rate is indistinguishable between control and Presenilin-deficient mice at E18.35 and at birth, but increases in Presenilin mice in the adult. Gray bars represent SEM; P < 0.05.
The sustained proliferation observed in the PsLow cells did not impact overall acinar mass and the final organ size is not different in weight between adult Presenilin-deficient and control mice (Fig. 1). Since proliferation continues in the adult, we next evaluated if organ size was maintained by an increase in the apoptosis rate in the PsLow cells. At both E18.5 and at birth, PsLow mice display an apoptosis rate similar to that of the control mice. In contrast, numerous EGFP+ Presenilin-deficient (Ngn3-Cre Ps1f/−; Ps2−/−; Z/EG) acinar cells were TUNEL-positive in the adult (P < 0.05) (Fig. 3E). These results indicate that during development, PsLow cells proliferate at a fast pace, outcompeting normal acinar cells not derived from Ngn3 progenitors. As the pancreas reaches the proper size, organ size control is retained through a concomitant increase in apoptosis.
Presenilin-deficient Ngn3 progenitors can still form islets if their neighbors are equally handicapped
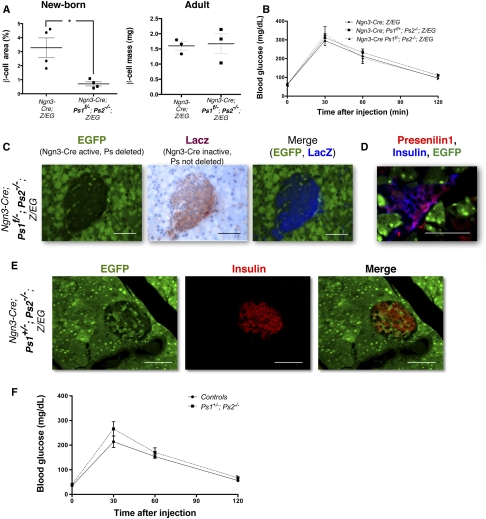
In control mice, all islet cells are derived from Ngn3+ progenitors. We reported above that when presenilins were deleted, Ngn3+ progenitors preferentially selected the acinar fate. Islets, observed in relatively normal numbers in the adults, were exclusively formed from EGFP-negative cells. As shown in Figure 4A, β-cell mass is reduced at birth (P < 0.05), but compensation allows it to catch up and become indistinguishable from that of control mice by 6 wk of age. At this stage, the mice also respond properly to a glucose challenge (Fig. 4B). Consistent with endocrine cells escaping recombination, we examined the tissue for loss of LacZ gene (Fig. 1A). The islets in presenilin-deficient mice were composed of LacZ+, EGFP− cells (Fig. 4C), and immunostaining also confirmed that the EGFP− cells in the islets still expressed Presenilin1 (Fig. 4D). This confirmed that the Cre recombinase was not active in these cells. Mosaicism has been documented previously in Ngn3-Cre; Z/EG mice (Gu et al. 2002) where ∼90%–95% of the cells express the transgene. This implies that during early stages of endocrine development, the few cells that failed to express Cre maintained Presenilin expression and proliferated sufficiently to compensate and form an apparently normal and functional endocrine mass in the pancreas.
Figure 4.
Ngn3-Cre Ps1f/−; Ps2−/−; Z/EG compensate and form normal islets through mosaicism. (A) Morphometric measurement of the β-cell mass of controls (Ngn3-Cre; Z/EG) and Presenilin-deficient (Ngn3-Cre Ps1f/−; Ps2−/−; Z/EG) mice at birth (left) and in the adult (right). The measurements are expressed as a percentage of staining for β cells over the entire area for the pancreas, and is further normalized to the total mass of the pancreas in the adult (n = 23–4). Total pancreas weights are similar between groups. Gray bars represent the SEM for each group. The β-cell mass is significantly reduced in Presenilin-deficient mice at birth, but increases to reach that of the control mice in the adult. (B) Intraperitoneal glucose tolerance test on control (Ngn3-Cre; Z/EG) and Presenilin-deficient (Ngn3-Cre; Ps1+/f; Ps2−/−; Z/EG and Ngn3-Cre Ps1f/−; Ps2−/−; Z/EG) adult mice, indicating no difference in glucose response between the two groups. (C) Endogenous EGFP expression (green) (left) and LacZ staining (red, with hematoxylin counterstain in blue) (right) on Ngn3-Cre; Ps1f/−; Ps2−/−; Z/EG mice. The fields are merged in false color (EGFP in green, LacZ in blue) on the right. (D) Endogenous EGFP expression (green) and immunostaining for Presenilin1 (red) and insulin (blue) on Ngn3-Cre; Ps1+/f; Ps2−/−; Z/EG mice. Presenilin-positive cells are EGFP-negative. (E) Immunostaining for insulin (red) and EGFP (green) in pancreatic sections from Ngn3-Cre; Ps1+/−; Ps2−/−; Z/EG mice. Bars, 50 μm. (F) Glucose tolerance tests on control (wild-type) and Ps1+/−; Ps2−/− mice. n = 25 for each group.
The observation that animals with only one copy of Presenilin1 (Ngn3Cre; Ps1+/f; Ps2−/−; Z/EG) were identical to animals with no presenilins in their progenitors (Ngn3-Cre; Ps1f/−; Ps2−/−; Z/EG) strongly suggested that the presence of only one copy of Presenilin1 is not sufficient to allow selection of endocrine fate by Ngn3 progenitors. However, Ps1+/−, Ps2−/− animals are viable, indicating that pancreas activities remain (Donoviel et al. 1999; Herreman et al. 1999; Pan et al. 2004; Qyang et al. 2004; Saura et al. 2004). In this genetic background where presenilin mutations are inherited and present in every cell, mosaicism in Cre activity could not be evoked to explain islet formation. To understand how islets originate in Ps1+/−, Ps2−/− mice, islet anatomy and function was analyzed in Ngn3-Cretg; Ps1+/−; Ps2−/−; Z/EG, which allows fate mapping of Ngn3 progenitors but in which the presenilin gene dose is independent from Cre activity. Surprisingly, when all cells contain an equal but limiting amount of presenilin, EGFP+ cells reappeared within the islets in addition to a multitude of Ngn3 progenitors that selected the acinar fate (Fig. 4E). The same results were observed in the knock-in lines Ngn3-Creki; Ps1+/−; Ps2−/−; Z/EG (data not shown). Glucose tolerance tests performed at 6 wk of age confirmed normal glucose metabolism and, thus, islet function (Fig. 4F). Therefore, whereas Presenilin deficiency enhanced the competitive advantage in selecting the acinar fate, it reduced, but did not eliminate, the ability to select the endocrine fate. Importantly, this indicates the absence of an intrinsic defect that prevents Ngn3 progenitors from selecting the endocrine fate; they fail to do so only in the presence of cells with wild-type amounts of Presenilin activity, a hallmark of lateral inhibition.
Presenilin control of endocrine fate and proliferation occurs in a γ-secretase-dependent manner
In order to dissect which function of Presenilins was involved in the control of endocrine differentiation, we specifically inhibited γ-secretase activity with a highly selective pharmacological inhibitor, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylgly cine t-butyl ester (DAPT) (Weihofen et al. 2003). DAPT has been used in organ cultures (Doerfler et al. 2001; Hadland et al. 2001; Cheng et al. 2003) and whole animals (Geling et al. 2002; Micchelli et al. 2002), mimicking Notch loss with no general toxicity (van den Brandt et al. 2004; Lanz et al. 2005). For these experiments, we replicated the experimental design that we used previously in our study of the kidney (Cheng et al. 2003) and pancreas (Cras-Méneur et al. 2001). At 13 d of development, most cells in the pancreatic rudiment are still vastly undifferentiated and remain pluripotent. During the following days, some epithelial progenitors begin to express Ngn3, with expression peaking around E15, subsequently differentiating into endocrine cells (Apelqvist et al. 1999). When placed in a three-dimensional collagen matrix for 7 d, the E13 anlagen recapitulates normal organogenesis (Miralles et al. 1999a,b; Cras-Méneur et al. 2001).
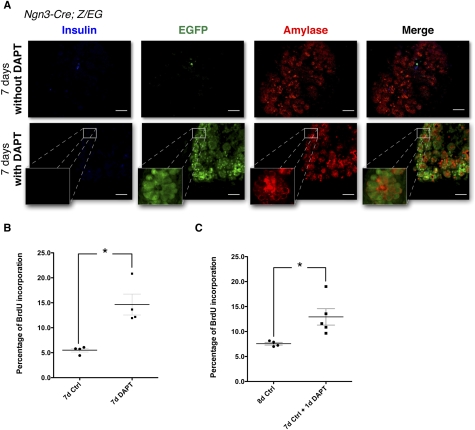
To follow the fate of Ngn3 progenitors, pancreatic rudiments from Ngn3-cre; Z/EG pups were harvested at E13 and placed in culture with or without DAPT. After 7 d of culture in the presence of DAPT, the pancreatic buds remained morphologically similar to the control rudiments. However, immunofluorescence for endocrine and acinar markers revealed that, whereas all Ngn3 progenitors gave rise to endocrine cells under control conditions (EGFP+ cells costained by insulin) (Fig. 5A, top), most EGFP+ cells coexpressed acinar markers when pancreatic rudiments were exposed to DAPT, similar to what we observed in vivo (Fig. 5A, bottom). In order to assess whether inhibition of the γ-secretase also affected the proliferation rate of pancreatic cells, bromo-deoxy-uridine (BrdU) was added to the growth medium during the last hour of culture. Immunodetection for BrdU on control and DAPT-treated buds revealed that inhibition of γ-secretase induced a general threefold increase in proliferation (Fig. 5B). This rate would not be sufficient to allow a small number of abnormal cells to overtake the culture; it is instead consistent with the possibility that, in the absence of γ-secretase activity, Ngn3 progenitors in culture preferentially differentiate into acinar cells followed by moderate expansion. After 7 d of culture, most of the cells growing under control conditions (DMSO) differentiated into acinar cells (Fig. 5A, top panel). If these differentiated cultures are grown for one additional day in culture media supplemented with DAPT, proliferation is significantly increased (Fig. 5C), indicating that γ-secretase activity is required to suppress proliferation in mature acinar cells. These in vitro experiments demonstrated that the control of the endocrine fate and proliferation of the Ngn3 progenitors required γ-secretase activity.
Figure 5.
Presenilins control the endocrine fate through a γ-secretase cleavage and Notch activation. (A) Cultures of E13.0 pancreatic rudiments from Ngn3; Z/EG rudiments with (bottom) or without (top) a γ-secretase inhibitor (DAPT) for 7 d. Immunostaining for insulin (blue), EGFP (green), and Amylase (red). Insets present higher magnifications for Amylase+, EGFP+ areas, indicating that the Presenilin-deficient phenotype can be recapitulated through γ-secretase inhibition. Bars, 50 μm. (B) BrdU incorporation rate in E13.0 pancreatic rudiments kept in culture for 7 d with (right) or without (left) DAPT. Four rudiments were used for each group, and 3500–4000 cells were counted for each rudiment. Gray bar represents the SEM for each group; P < 0.05. (C) BrdU incorporation rate in E13.0 pancreatic rudiments kept in culture for 8 d in control conditions, or for 7 d in control condition (left, n = 24), then with DAPT for an additional day (right, n = 25). Approximately 4000 cells were counted for each rudiment. Gray bar represents the SEM for each group; P < 0.05.
Presenilins are only required to control the fate of the endocrine progenitors during a narrow developmental window
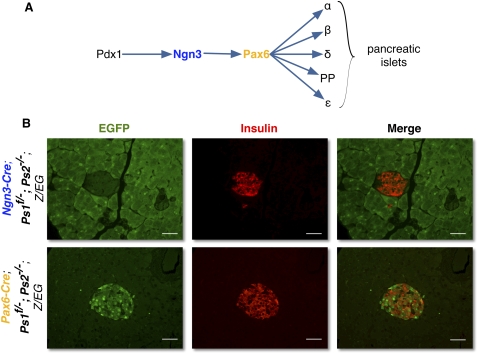
Ngn3 is one of the earliest known markers for endocrine progenitors. In these series of experiments, presenilins are deleted after Ngn3 expression begins, exposing the fact that these cells were not yet fully committed to the endocrine path and preferentially selected the acinar fate if Presenilin were reduced. Pax6 is expressed in endocrine progenitors shortly after Ngn3 (Fig. 6A; Jensen et al. 2000). In order to test whether Pax6 progenitors required Presenilin to maintain an endocrine commitment, we examined Pax6-Cre Ps1f/−; Ps2−/−; Z/EG mice. When presenilins are deleted after the onset of Pax6 expression, EGFP+ progenitors assumed the endocrine fate, contributing only to islets of normal size (Fig. 6B, bottom), proliferation, and apoptosis rates (data not shown). As would be expected, glucose tolerance was not affected (data not shown). These results indicate that Presenilins are only required in a very narrow window to secure endocrine fate selection by Ngn3 progenitors. Once Pax6 is expressed, the cells are committed to the endocrine fate.
Figure 6.
Presenilins are not required for endocrine determination after Pax6 expression. (A) Schematic representation of the differentiation pathway of the pancreatic endocrine progenitors. (B) Immunostaining for insulin (red) and EGFP (green) on Ngn3-Cre; Ps1f/−; Ps2−/−; Z/EG (top) and Pax6-Cre; Ps1f/−; Ps2−/−; Z/EG (bottom) mice. Bars:,50 μm.
Endocrine fate selection is controlled by the amount of NICD
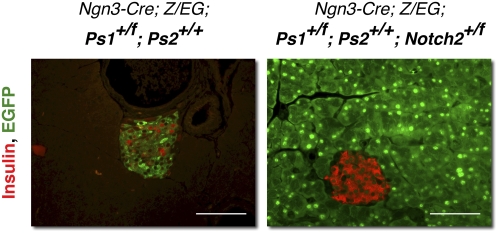
Notch receptors are known targets of Presenilin-dependent γ-secretase. Both Notch1 and Notch2 are expressed in the pancreas at different stages of development without a clear function (Nakhai et al. 2008). We wished to determine if the limiting factor requiring γ-secretase activity was a Notch protein. Because Notch1 was activated infrequently in the pancreas (Vooijs et al. 2007) relative to Notch2 (our unpublished observation), we tested if Notch2 contributed to the selection of endocrine fate by Ngn3 progenitors. Ngn3-Cre; Ps1+/f; Ps2+/+; Z/EG mice are phenotypically normal, with all Ngn3+ cells selecting the endocrine fate (Fig. 7, left). To test if reducing the dose of Notch will alter the fate decision of Ps1+/f; Ps2+/+ cells, we crossed Ngn3-Cre; Ps1+/f; Ps2+/+; Z/EG mice with Notch2 conditional mice. Importantly, when one copy of Notch2 is removed in Ngn3 cells (Ngn3-Cre; Ps1+/f; Ps2+/+; Notch2+/f Z/EG), these cells are once again diverted toward the acinar fate, recapitulating the PsLow phenotype (Fig. 7, right). This result indicates that Presenilin-dependent control of the endocrine fate is mediated through controlling Notch proteolysis; the ability of Notch1/2-deficient cells (Nakhai et al. 2008) and Ps1−/−; Ps2−/− cells to select the endocrine fate in Ngn3-Cre knock-in lines indicates the transcriptional activity of NICD is not required.
Figure 7.
Endocrine fate is controlled by Notch2. Immunostaining for insulin (red) and EGFP (green) in phenotypically wild-type Ngn3-Cre, Ps1+/f; Ps2+/+; Z/EG mice and in Ngn3-Cre, Ps1+/f; Ps2+/+; Notch2+/f; Z/EG mice indicates that eliminating one copy of notch2 in Ngn3 progenitors can enhance the reduction in Presenilins and recapitulate the Pslow phenotype. Bars, 50 μm.
Discussion
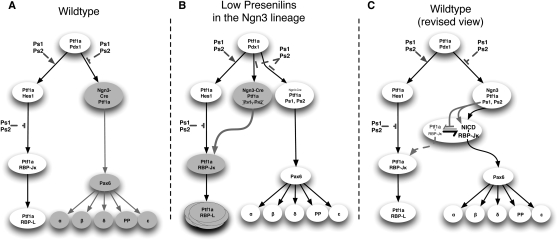
The results of the experiments presented here revise the current view describing the steps involved in the creation of a committed endocrine progenitor. We describe an unappreciated bipotent state of Ngn3+,Pax6− progenitors that default to an acinar fate if Notch proteolysis falls below a threshold (Fig. 8B). This Notch-dependent step along the path to endocrine differentiation is restricted to a narrow developmental window, as Ngn3+ Pax6+ progenitors are fully committed to the endocrine fate in the absence of Notch signaling (Fig. 8C). The number of Ngn3+, Pax6− progenitors entering the acinar fate was inversely correlated with Presenilin activity, indicating a stoichiometric requirement. Reduction in Notch2 in otherwise normal presenilin heterozygotes again resulted in selection of acinar fate, indicating that the amount of NICD2 (but not its activity as a transcription factor, which can be satisfied by the remaining Notch2 allele and the two Notch1 alleles) played a critical role.
Figure 8.
Presenilins are involved in multiple steps of pancreatic development. (A) Representation of the previous view of the control of the fate of the pancreatic progenitors. (B) Representation of the alteration of fate in low abundance of the Presenilins in Ngn3 progenitors. (C) Proposed revised view of the control of the fate of the endocrine progenitors.
In neural (de la Pompa et al. 1997) or muscle (Schuster-Gossler et al. 2007) progenitors, loss of Notch signaling results in premature differentiation; the presence of activator-class basic helix–loop–helix (bHLH) proteins is sufficient to drive the neural or muscle fate. In the pancreas, two bHLH protein, Ngn3 and Ptf1a, compete: Both court E12 to gain transcriptional activity; however, Ptf1a forms a stable complex only if it can also recruit RBP-Jκ. The results reported here demonstrate that although progenitors with reduced presenilin dosage can produce islets (Fig. 4C), they are easily outcompeted by cells with a full complement of Presenilin proteins and, in both cases, differentiate preferentially into acinar cells that exhibit a high turnover rate in the adult, suggesting that Presenilin dose can effect the competition between Ngn3 and Ptf1a. Since the mice do not exhibit any sign of malabsorption (such as weight loss), we can assume that the Presenilin-deficient acinar cells are functional, but we did not rule out the possibility that the bulk of acinar functions are assumed instead by the 10%–15% of acinar cells not derived from Ngn3 progenitors.
Presenilins form the catalytic component of the γ-secretase complex. They also participate in additional processes, such as the regulation of the stability of β-catenin, a protein at the crossroad between Wnt and insulin signaling (for review, see Parks and Curtis 2007). The asynchronous nature of pancreatic differentiation dictates that, by E13, the time the cultures are initiated, DAPT-treated pancreata contain both Ngn3+ and Pax6+ progenitors. Since Pax6-expressing cells are committed to the endocrine fate, their descendents develop into endocrine cells even in the presence of DAPT. Nonetheless, when coupled with lineage tracer (Ngn3-Cre; Z/EG), DAPT, a γ-secretase inhibitor with no ability to affect any γ-secretase-independent functions of the Presenilins, was sufficient to divert Ngn3-Cre-expressing cells to the acinar fate. We could also demonstrate (using DAPT) that even “normal” (Ngn3−) acinar cells require γ-secretase to exit from the cell cycle (Fig. 5C). Thus, the mechanism restricting acinar fate selection by Ngn3+ progenitors and their expansion requires γ-secretase. Because RBP-Jk can be removed without consequences for endocrine development (Nakhai et al. 2008), the requirement for presenilin and Notch2 defines a noncanonical contribution by Notch.
We can offer only a speculative explanation as to how Presenilins make a dosage-dependent contribution to endocrine determination in Ngn3 progenitors. These in vitro experiments strongly suggest that the pancreatic phenotypes described herein are γ-secretase-dependent. The γ-secretase complex gained recognition as a Notch and APP protease, but other substrates are steadily being added to a growing list. Some of these, like ErbB1 (EGFR) and ErbB4, are also involved in pancreatic development (Kritzik et al. 2000; Cras-Méneur et al. 2001; Huotari et al. 2002; Koo and Kopan 2004; Lee et al. 2006; Zhang et al. 2007). Nonetheless, here, too, we think that Notch and an activator bHLH (Ptf1a) are involved in a mechanism that differs from classical Notch function. Overexpression of the intracellular domain of Notch1 (NICD1) prevents acinar cells from fully differentiating (Murtaugh et al. 2003), yet Notch2 and Notch1 proteins are not required for pancreatic development (Nakhai et al. 2008). We believe that, under normal circumstances, Ngn3/E12 complexes form preferentially over E12/Ptf1a/RBP-Jκ. It was demonstrated that NICD and Ptf1a compete for RBP-Jκ in acinar progenitors (Beres et al. 2006; Masui et al. 2007) as they might do in GABAergic neural progenitors (Hori et al. 2008). RBP-Jκ is still expressed in Ngn3 progenitors (Beres et al. 2006), and its removal permits their development, indicating no requirement for canonical Notch signals (Nakhai et al. 2008). Previous studies have detected Ptf1a mRNA in Ngn3-derived progenitors (Chiang and Melton 2003), a finding we confirmed by immunostaining in our control mice, using lineage tracing (Fig. 2B-1). We speculate that NICD contributes to Ngn3/E12 complex formation by sequestering RBP-Jκ, thus handicapping Ptf1a. Presenilins/Notch thus control endocrine differentiation of Ngn3+ progenitors by modulating RBP-Jκ availability (Fig. 8C). If NICD levels are sustained, the E12/Ptf1a/RBP-Jκ complex cannot form, Ngn3/E12 complexes form, and no acinar differentiation occurs in Ngn3+ cells. Reduction/loss of Notch proteolysis in presenilin-deficient cells would free RBP-Jκ, allowing the formation of a complex with Ptf1a, lowering the likelihood of Ngn3/E12 complex formation, and pushing the bipotent cells toward the acinar fate. This hypothesis could also explain the reduction seen in both endocrine and exocrine differentiation when Notch1 is constitutively overexpressed under the control of the Pdx1 promoter (Hald et al. 2003; Murtaugh et al. 2003). Importantly, this Notch function goes undetected in the absence of fate mapping because the ability of bipotent Ngn3+ progenitors to make islets is not compromised, and the size of the organ (which we expect was made almost entirely of the Ngn3+ linage) is largely the same.
Although γ-secretase activity appears not to be limiting in most cells, a possible explanation for the stoichiometric effect we see could be that, at a specific cellular location (the plasma membrane, perhaps), γ-secretase is limiting. When two of four Presenilin alleles are present, a few EGFP+ acinar cells were rarely seen, suggesting the genetic background could modify this phenotype. Although one allele of Ps1 is sufficient to support the canonical, transcriptional activity of Notch in most organs (Donoviel et al. 1999; Herreman et al. 1999; Pan et al. 2004; Saura et al. 2004), it is not sufficient to prevent assumption of the acinar fate by Ngn3 progenitors. Myeloid progenitors are also sensitive to Presenilin dose (Qyang et al. 2004), where a multipotent granulocyte/monocyte progenitor expands in the spleen. Similar dose sensitivity was observed in nicastrin heterozygotes (Li et al. 2007). It is possible that in all these cases, a titration mechanism similar to the one described here is in play.
During the late stages of development, the PsLow EGFP+ population increases significantly (Fig. 3). This increase results from a combination of an increase in the Ngn3+ population (probably as a compensation mechanism triggered by the reduced endocrine mass) and the increased proliferation rate of these cells. Presenilin-deficient acinar cells retain faster proliferation rates also in the adult, providing a selective growth advantage (Fig. 3A). The total pancreatic mass was not affected, though, indicating that Presenilins and their substrates are not involved in the regulation of organ size. Organ size is maintained as a balance established between proliferation and apoptosis in the adult, as seen after loss of numb in the neural progenitor stem cell compartment (Petersen et al. 2006). In summary, reduced presenilin activity exposed a strong bias toward the acinar fate in bipotent Ngn3 progenitors. Once they selected the acinar fate, EGFP+ cells gained a proliferative advantage: The total mass of EGFP+ in the PsLow animals was considerably larger than that observed in control animals. The mechanisms by which the Presenilins reduce proliferation rates remain to be elucidated; a contribution from other γ-secretase targets in controlling acinar proliferation cannot be ruled out.
Importantly, when all cells were similarly compromised, Pslow, Ngn3+ progenitors formed islets. In contrast, Pslow, Ngn3+ EGFP+ cells are never found in the islets when the pancreata also contains cells with a higher dose of Presenilin. Collectively, our data strongly suggest that (1) Ngn3+ progenitors are bipotential, (2) γ-secretase and Notch2 act in a noncanonical, RBP-Jκ titration mechanism to permit one bHLH protein (Ngn3) to act in the presence of a dominant bHLH (Ptf1a), (3) this is a competitive selection process that sets aside endocrine progenitors based on the amount of free RBP-Jκ, and (4) presenilin-deficient Ngn3 progenitors outcompete other acinar cells that did not lose Presenilin activity due to a secondary defect in cell cycle exit.
Materials and methods
Transgenic mice
The Ngn3-Cre mice, as well as mice with a knock-in of the Cre in the Ngn3 locus, were a generous gift from D. Melton. Pax6-Cre (P. Gruss; St-Onge et al. 1997F), Presenilin (Ps1f, Ps1−, and Ps2−) (Herreman et al. 1999), Notch2 (Notch2+/f), and the Z/EG reporter mice (Jackson Laboratory) were all maintained in a C57BL/6J background and interbred to obtain the necessary combined genotypes.
Glucose tolerance tests
Glucose levels were assessed on whole blood using AccuChek II glucometer (Roche Diagnostics). Glucose tolerance was challenged after an overnight fast by an intraperitoneal injection of 2 mg/g dextrose as described previously (Bernal-Mizrachi et al. 2001).
Embryonic pancreatic bud cultures
Pancreatic rudiments were dissected from E13.0 embryos (the morning of the vaginal plug discovery was designated as day 0.5) according to the Washington University School of Medicine-approved protocols. For each embryo, tissue was collected for genotyping. Pancreatic rudiments were cultured as described previously (Cras-Méneur et al. 2001). DAPT (γ-secretase, inhibitor IX; Calbiochem) was used at 1 μmol/L. For control conditions, equivalent concentrations of DMSO were added to the media. To label cells in S phase, BrdU (10 μmol/L) was supplemented to the culture media for the last hour of culture.
Tissue preparation and immunostaining
After culture, embryonic rudiments were fixed in 3.7% formalin, then embedded in 10% low-gelling-temperature agarose for paraffin embedding. Other embryonic and adult tissues were isolated and then fixed in either 3.7% formalin for paraffin embedding or in 4% paraformaldehyde, then cryo-protected in 10% sucrose for cryosections depending on the requirements of the antibodies used for immunostaining. Depending on the fixative and duration of the fixation process itself, some misexpression of the EGFP reporter can be observed occasionally in the nucleus. Nonfixed fresh-frozen tissues were used to control for the faithful expression of the endogenous EGFP, and no EGFP was ever observed in the nucleus of these sections. The following primary antibodies were used overnight for immunostaining: anti-insulin (guinea pig, 1:750; Dako, anti-glucagon (mouse, 1:300; Sigma), anti-amylase (rabbit, 1:500; Sigma), anti-Presenilin 1 (R&D Systems), anti-EGFP (rabbit, 1:200; Molecular Probes; when endogenous EGFP was too weak to detect), anti-β-galactosidase (rabbit, 1:1000; Cappel), antiPhospho-Histone 3 (mouse, 1:500; Upstate Laboratories, BrdU (1:2; GE Healthcare), anti-Pdx-1 (1:50; Santa Cruz Biotechnologies, Inc.), and anti-Ptf1a (a gift from C.V. Wright). DBA (Dolichos Biflorus Agglutinin, Vector Laboratories) staining was performed according to the manufacturer's instructions. Sections were incubated with the appropriate secondary antibodies (Jackson Immunoresearch). TUNEL staining was performed using the ApopTag detection kit (Chemicon), according to the manufacturer's instructions. For morphometry, slides were stained for bright-field using the Histomouse SP labeling kit (Zymed Laboratories, Inc.). Fluorescent images were captured using a Leica DM4000B microscope with a Leica DFC 350FX monochromatic camera for fluorescence or a Leica DFC 290FX tri-CCD camera for bright-field images, using the Leica FW400 software version 1.2.1 (Leica Microsystems).
Morphometry
The islet mass was determined on randomly selected sections throughout the entire pancreas after immunostaining for insulin and counterstaining with hematoxylin as described previously (Bernal-Mizrachi et al. 2001). The endocrine area (in square microns) was determined by intensity thresholding using the morphometry measurement tools of ImageJ version 1.39p (National Institutes of Health, freely available at http://rsb.info.nihgov/ij/index.html; Girish and Vijayalakshmi 2004). In adult tissues, the endocrine mass was calculated by a ratio between the weight of the pancreas and the proportion of insulin immunoreactive cells in the samples. In newborns, in order to circumvent imprecision in weight measurements due to the small weight of the tissues, the assessments were kept as a ratio of insulin immunoreactive cells over the total pancreatic areas of the sections.
Western blots
Pancreata from 12-wk-old mice were homogenized and lysed in 3 mL of 10 mM Tris (pH 7.6), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, and a cocktail of protease inhibitors buffer (Complete Mini, Roche Diagnostics). Lysates were subjected to polyacrylamide gel electrophoresis and transferred to PVDF membranes (Bio-Rad Life Sciences). After blocking for 30 min, membranes were incubated for 24 h with the following antibodies: Cyclin D1 (mouse, 1:10,000; Cell Signaling Technology), Cyclin D2 (mouse, 1:10,000; Labvision NeomarkerA), Cyclin D3 (mouse, 1:10,000; Cell Signaling Technology) and actin (rabbit, 1:20,000; Sigma). Immunoblotting experiments were performed in triplicate on lysates from at least three different mice per group. The densitometry of the proteins was determined by pixel intensity using NIH ImageJ software version 1.39p (Girish and Vijayalakshmi 2004) and normalized using actin as a loading control. Secondary antibodies were used according to the manufacturer's instructions (Vector Laboratories).
Statistical analysis
All values are represented as mean ± standard error of the mean. Statistical analyses were conducted using a two-tailed, nonparametric, Mann-Whitney U-test with a confidence interval of 95% using Prism 5.0 (GraphPad Software, Inc.).
Acknowledgments
We are grateful to C.V. Wright for the generous gift of the Ptf1a antibody, and to D. Melton, B. DeStrooper, J. Shen, and T. Gridley for the Ngn3-Cre knock-in, Presenilin, and Notch2 lines, respectively. This study was supported by a pilot grant from the NIH-NIH/NIDDK-sponsored β-Cell Biology Consortium (subcontract: Vanderbilt University); NIH grant 1-RO1-DK073453-01A1 to C.C.M., L.L., R.K., and A.P.; and NIH DK066408 to R.K.; and the mouse phenotyping and morphology cores of the Diabetes Research and Training Center at WUSTL (NIH grant P60-DK020579-31).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1800209.
Supplemental material is available at http://www.genesdev.org.
References
- Apelqvist Å, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: Effects of FAD mutations. EMBO J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres TM, Masui T, Swift GH, Shi L, Henke RM, Macdonald RJ. PTF1 is an organ-specific and Notch-independent basic helix–loop–helix complex containing the mammalian suppressor of hairless (RBP-J) or its paralogue, RBP-L. Mol Cell Biol. 2006;26:117–130. doi: 10.1128/MCB.26.1.117-130.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet β cell expression of constitutively active Akt1/PKB α induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001;108:1631–1638. doi: 10.1172/JCI13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R. γ-Secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development. 2003;130:5031–5042. doi: 10.1242/dev.00697. [DOI] [PubMed] [Google Scholar]
- Chiang MK, Melton DA. Single-cell transcript analysis of pancreas development. Dev Cell. 2003;4:383–393. doi: 10.1016/s1534-5807(03)00035-2. [DOI] [PubMed] [Google Scholar]
- Cras-Méneur C, Elghazi L, Czernichow P, Scharfmann R. Epidermal growth factor increases undifferentiated pancreatic embryonic cells in vitro: A balance between proliferation and differentiation. Diabetes. 2001;50:1571–1579. doi: 10.2337/diabetes.50.7.1571. [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- Doerfler P, Shearman MS, Perlmutter RM. Presenilin-dependent γ-secretase activity modulates thymocyte development. Proc Natl Acad Sci. 2001;98:9312–9317. doi: 10.1073/pnas.161102498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoviel DB, Hadjantonakis AK, Ikeda M, Zheng H, Hyslop PS, Bernstein A. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes & Dev. 1999;13:2801–2810. doi: 10.1101/gad.13.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA, et al. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- Fujikura J, Hosoda K, Kawaguchi Y, Noguchi M, Iwakura H, Odori S, Mori E, Tomita T, Hirata M, Ebihara K, et al. Rbp-j regulates expansion of pancreatic epithelial cells and their differentiation into exocrine cells during mouse development. Dev Dyn. 2007;236:2779–2791. doi: 10.1002/dvdy.21310. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Luo H, Qiu F, Burlison J, Long Q, Kawaguchi Y, Edlund H, MacDonald RJ, Furukawa T, et al. Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Development. 2006;133:4439–4450. doi: 10.1242/dev.02598. [DOI] [PubMed] [Google Scholar]
- Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A γ-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girish V, Vijayalakshmi A. Affordable image analysis using NIH Image/ImageJ. Indian J Cancer. 2004;41:47. [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120:35–43. doi: 10.1016/s0925-4773(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hadland BK, Manley NR, Su D, Longmore GD, Moore CL, Wolfe MS, Schroeter EH, Kopan R. γ-Secretase inhibitors repress thymocyte development. Proc Natl Acad Sci. 2001;98:7487–7491. doi: 10.1073/pnas.131202798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol. 2003;260:426–437. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, von Figura K, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for α-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- Herreman A, Hartmann D, Annaert W, Saftig P, Craessaerts K, Serneels L, Umans L, Schrijvers V, Checler F, Vanderstichele H, et al. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci. 1999;96:11872–11877. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Cholewa-Waclaw J, Nakada Y, Glasgow SM, Masui T, Henke RM, Wildner H, Martarelli B, Beres TM, Epstein JA, et al. A nonclassical bHLH Rbpj transcription factor complex is required for specification of GABAergic neurons independent of Notch signaling. Genes & Dev. 2008;22:166–178. doi: 10.1101/gad.1628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari MA, Miettinen PJ, Palgi J, Koivisto T, Ustinov J, Harari D, Yarden Y, Otonkoski T. ErbB signaling regulates lineage determination of developing pancreatic islet cells in embryonic organ culture. Endocrinology. 2002;143:4437–4446. doi: 10.1210/en.2002-220382. [DOI] [PubMed] [Google Scholar]
- Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic α- and β-cells from neurogenin3-expressing precursors: A role for the notch pathway in repression of premature differentiation. Diabetes. 2000;49:163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- Jørgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Masamizu Y, Niwa Y. Oscillator mechanism of Notch pathway in the segmentation clock. Dev Dyn. 2007;236:1403–1409. doi: 10.1002/dvdy.21114. [DOI] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, Sisodia SS, Leibowitz G, Levine F, Koo EH. Presenilin 1 facilitates the constitutive turnover of β-catenin: Differential activity of Alzheimer's disease-linked PS1 mutants in the β-catenin-signaling pathway. J Neurosci. 1999;19:4229–4237. doi: 10.1523/JNEUROSCI.19-11-04229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Park JH, Choi EJ, Park HS. Presenilin acts as a positive regulator of basal level activity of ERK through the Raf–MEK1 signaling pathway. Biochem Biophys Res Commun. 2005;332:609–613. doi: 10.1016/j.bbrc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Koo EH, Kopan R. Potential role of presenilin-regulated signaling pathways in sporadic neurodegeneration. Nat Med. 2004;10:S26–S33. doi: 10.1038/nm1065. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. γ-Secretase: Proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzik MR, Krahl T, Good A, Gu D, Lai C, Fox H, Sarvetnick N. Expression of ErbB receptors during pancreatic islet development and regrowth. J Endocrinol. 2000;165:67–77. doi: 10.1677/joe.0.1650067. [DOI] [PubMed] [Google Scholar]
- Lanz TA, Fici GJ, Merchant KM. Lack of specific amyloid-β(1–42) suppression by nonsteroidal anti-inflammatory drugs in young, plaque-free tg2576 mice and in guinea pig neuronal cultures. J Pharmacol Exp Ther. 2005;312:399–406. doi: 10.1124/jpet.104.073965. [DOI] [PubMed] [Google Scholar]
- Lee JC, Smith SB, Watada H, Lin J, Scheel D, Wang J, Mirmira RG, German MS. Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes. 2001;50:928–936. doi: 10.2337/diabetes.50.5.928. [DOI] [PubMed] [Google Scholar]
- Lee CS, De Leon DD, Kaestner KH, Stoffers DA. Regeneration of pancreatic islets after partial pancreatectomy in mice does not involve the reactivation of neurogenin-3. Diabetes. 2006;55:269–272. [PubMed] [Google Scholar]
- Li T, Wen H, Brayton C, Laird FM, Ma G, Peng S, Placanica L, Wu TC, Crain BJ, Price DL, et al. Moderate reduction of γ-secretase attenuates amyloid burden and limits mechanism-based liabilities. J Neurosci. 2007;27:10849–10859. doi: 10.1523/JNEUROSCI.2152-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman OY, Ilagan MX, Kopan R, Barrick D. Quantitative dissection of the Notch:CSL interaction: Insights into the Notch-mediated transcriptional switch. J Mol Biol. 2007;365:577–589. doi: 10.1016/j.jmb.2006.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu J, Yamamoto V, Lu W. Cleavage of the Wnt receptor Ryk regulates neuronal differentiation during cortical neurogenesis. Dev Cell. 2008;15:773–780. doi: 10.1016/j.devcel.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Masui T, Long Q, Beres TM, Magnuson MA, Macdonald RJ. Early pancreatic development requires the vertebrate Suppressor of Hairless (RBPJ) in the PTF1 bHLH complex. Genes & Dev. 2007;21:2629–2643. doi: 10.1101/gad.1575207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, The I, Selva E, Mogila V, Perrimon N. Rasp, a putative transmembrane acyltransferase, is required for Hedgehog signaling. Development. 2002;129:843–851. doi: 10.1242/dev.129.4.843. [DOI] [PubMed] [Google Scholar]
- Miralles F, Czernichow P, Ozaki K, Itoh N, Scharfmann R. Signaling through fibroblast growth factor receptor 2b plays a key role in the development of the exocrine pancreas. Proc Natl Acad Sci. 1999a;96:6267–6272. doi: 10.1073/pnas.96.11.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Serup P, Cluzeaud F, Vandewalle A, Czernichow P, Scharfmann R. Characterization of β cells developed in vitro from rat embryonic pancreatic epithelium. Dev Dyn. 1999b;214:116–126. doi: 10.1002/(SICI)1097-0177(199902)214:2<116::AID-AJA2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhai H, Siveke JT, Klein B, Mendoza-Torres L, Mazur PK, Algul H, Radtke F, Strobl L, Zimber-Strobl U, Schmid RM. Conditional ablation of Notch signaling in pancreatic development. Development. 2008;135:2757–2765. doi: 10.1242/dev.013722. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Obata J, Yano M, Mimura H, Goto T, Nakayama R, Mibu Y, Oka C, Kawaichi M. p48 subunit of mouse PTF1 binds to RBP-Jκ/CBF-1, the intracellular mediator of Notch signalling, and is expressed in the neural tube of early stage embryos. Genes Cells. 2001;6:345–360. doi: 10.1046/j.1365-2443.2001.00422.x. [DOI] [PubMed] [Google Scholar]
- Pan Y, Lin MH, Tian X, Cheng HT, Gridley T, Shen J, Kopan R. γ-Secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell. 2004;7:731–743. doi: 10.1016/j.devcel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Pan Y, Liu Z, Shen J, Kopan R. Notch1 and 2 cooperate in limb ectoderm to receive an early Jagged2 signal regulating interdigital apoptosis. Dev Biol. 2005;285:472–482. doi: 10.1016/j.ydbio.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Parks AL, Curtis D. Presenilin diversifies its portfolio. Trends Genet. 2007;23:140–150. doi: 10.1016/j.tig.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Petersen PH, Tang H, Zou K, Zhong W. The enigma of the numb-Notch relationship during mammalian embryogenesis. Dev Neurosci. 2006;28:156–168. doi: 10.1159/000090761. [DOI] [PubMed] [Google Scholar]
- Pictet R, Rutter W. Development of the embryonic pancreas. In: Steiner DF, Frenkel N, editors. Handbook of physiology. The Williams & Wilkins Co; Washington, DC: 1972. pp. 25–66. [Google Scholar]
- Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qyang Y, Chambers SM, Wang P, Xia X, Chen X, Goodell MA, Zheng H. Myeloproliferative disease in mice with reduced presenilin gene dosage: Effect of γ-secretase blockage. Biochemistry. 2004;43:5352–5359. doi: 10.1021/bi049826u. [DOI] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, III, Kandel ER, Duff K, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Schuster-Gossler K, Cordes R, Gossler A. Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proc Natl Acad Sci. 2007;104:537–542. doi: 10.1073/pnas.0608281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia SS, St George-Hyslop PH. γ-Secretase, Notch, Aβ and Alzheimer's disease: Where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing α-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Struhl G, Greenwald I. Presenilin-mediated transmembrane cleavage is required for Notch signal transduction in Drosophila. Proc Natl Acad Sci. 2001;98:229–234. doi: 10.1073/pnas.011530298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournoy J, Bossuyt X, Snellinx A, Regent M, Garmyn M, Serneels L, Saftig P, Craessaerts K, De Strooper B, Hartmann D. Partial loss of presenilins causes seborrheic keratosis and autoimmune disease in mice. Hum Mol Genet. 2004;13:1321–1331. doi: 10.1093/hmg/ddh151. [DOI] [PubMed] [Google Scholar]
- van den Brandt J, Voss K, Schott M, Hunig T, Wolfe MS, Reichardt HM. Inhibition of Notch signaling biases rat thymocyte development towards the NK cell lineage. Eur J Immunol. 2004;34:1405–1413. doi: 10.1002/eji.200324735. [DOI] [PubMed] [Google Scholar]
- Vooijs M, Ong CT, Hadland B, Huppert S, Liu Z, Korving J, van den Born M, Stappenbeck T, Wu Y, Clevers H, et al. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development. 2007;134:535–544. doi: 10.1242/dev.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihofen A, Lemberg MK, Friedmann E, Rueeger H, Schmitz A, Paganetti P, Rovelli G, Martoglio B. Targeting presenilin-type aspartic protease signal peptide peptidase with γ-secretase inhibitors. J Biol Chem. 2003;278:16528–16533. doi: 10.1074/jbc.M301372200. [DOI] [PubMed] [Google Scholar]
- Wines-Samuelson M, Shen J. Presenilins in the developing, adult, and aging cerebral cortex. Neuroscientist. 2005;11:441–451. doi: 10.1177/1073858405278922. [DOI] [PubMed] [Google Scholar]
- Wood DR, Nye JS, Lamb NJ, Fernandez A, Kitzmann M. Intracellular retention of caveolin 1 in presenilin-deficient cells. J Biol Chem. 2005;280:6663–6668. doi: 10.1074/jbc.M410332200. [DOI] [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stangé G, Bonne S, De Leu N, Xiao X, Van De Casteele M, Mellitzer G, Ling Z, Pipeleers DG, et al. β Cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Zeggini E, Scott LR, Saxena R, Voight BF, Marchini JL, Hu T, de Bakker PI, Abecasis GR, Almgren P, Andersen G, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Wang R, Liu Q, Zhang H, Liao FF, Xu H. Presenilin/γ-secretase-dependent processing of β-amyloid precursor protein regulates EGF receptor expression. Proc Natl Acad Sci. 2007;104:10613–10618. doi: 10.1073/pnas.0703903104. [DOI] [PMC free article] [PubMed] [Google Scholar]