Abstract
Intracellular heme levels must be tightly regulated to maintain proper mitochondrial respiration while minimizing toxicity, but the homeostatic mechanisms are not well understood. Here we report a novel negative feedback mechanism whereby the nuclear heme receptor Rev-erbα tightly controls the level of its own ligand. Heme binding to Rev-erbα recruits the NCoR/histone deacetylase 3 (HDAC3) corepressor complex to repress the transcription of the coactivator PGC-1α, a potent inducer of heme synthesis. Depletion of Rev-erbα derepresses PGC-1α, resulting in increased heme levels. Conversely, increased Rev-erbα reduces intracellular heme, and impairs mitochondrial respiration in a heme-dependent manner. Consistent with this bioenergetic impairment, overexpression of Rev-erbα dramatically inhibits cell growth due to a cell cycle arrest. Thus, Rev-erbα modulates the synthesis of its own ligand in a negative feedback pathway that maintains heme levels and regulates cellular energy metabolism.
Keywords: Rev-erbα, PGC-1α, heme homeostasis, mitochondria, energy
Heme is an essential molecule for many biological functions (Furuyama et al. 2007). In hemoproteins, such as hemoglobin and cytochromes, heme functions as a prosthetic group serving physiological functions as a transporter for oxygen and electrons (Terwilliger 1998). Ironically, heme can cause oxidative stress when it reacts with molecular oxygen, which in turn causes DNA damage, lipid peroxidation, and protein denaturation (Levin et al. 1973; Chiu and Lubin 1989; Vile et al. 1994; Rahman et al. 1997). Consequently, cellular heme homeostasis is tightly controlled at the level of its biosynthesis, which is rate-limited by ALAS1 (δ-aminolevulinic acid synthase 1), as well as its degradation by HMOX1 (Heme oxygenase 1) (Maines 1988; Furuyama et al. 2007). Heme-induced expression of Hmox1 is an acute and robust response, mainly via the function of transcriptional repressor Bach1 (Sun et al. 2002). However, little is known about how physiological concentrations of heme feedback to maintain heme homeostasis.
Circadian rhythm plays an important role for many metabolic processes, such as sleep–wake cycle, blood pressure, and glucose metabolism (Lowrey and Takahashi 2004; Stratmann and Schibler 2006; Kohsaka and Bass 2007). The molecular clock is driven by an interlocked transcriptional and translational feedback loop. BMAL1 and CLOCK, two positive regulators, activate the expression of negative regulators including Cry, Per, and Rev-erbα (Thresher et al. 1998; Lucas and Foster 1999; Preitner et al. 2002; Yin and Lazar 2005). As CRY and PER reach critical levels, they then bind directly to BMAL1/CLOCK and repress their own transcription (Darlington et al. 1998; Shearman et al. 2000; Reppert and Weaver 2001). Rev-erbα directly represses transcription of Bmal1 and therefore serves as an additional feedback loop of circadian circuitry (Preitner et al. 2002; Ueda et al. 2002; Yin and Lazar 2005; Yang et al. 2007). In addition to Bmal1, Rev-erbα regulates metabolic genes, including glucose 6-phosphatase (Yin et al. 2007), ApoCIII (Coste and Rodriguez 2002), and ElovI3 (Downes et al. 1995), suggesting that this nuclear receptor (NR) links circadian rhythm to metabolism (Duez and Staels 2008).
Rev-erbα, also known as NR1D1, is a member of the NR superfamily of ligand-regulated transcription factors (Yang et al. 2007; McKenna et al. 2009). Unlike most other NRs, Rev-erbα does not recruit coactivators and functions primarily as a repressor by interacting with the NR corepressor NCoR (Harding and Lazar 1995; Zamir et al. 1996). Recently, we and others identified heme as a physiological ligand for Rev-erbα (Raghuram et al. 2007; Yin et al. 2007; O'Malley 2008). Heme binds reversibly to Rev-erbα and stimulates its interaction with NCoR, enhancing repression of its gene targets (Raghuram et al. 2007; Yin et al. 2007; O'Malley 2008; Rogers et al. 2008).
Heme biosynthesis is also subjected to circadian control (Zheng et al. 2001; Kaasik and Lee 2004; Burris 2008; Rogers et al. 2008). The mRNA of ALAS1 shows a robust circadian oscillation (Panda et al. 2002). NPAS2, a homolog of Clock (Dudley et al. 2003), binds to the promoter of Alas1 and may contribute to the circadian expression of Alas1 (Kaasik and Lee 2004). PGC-1α, a key metabolic transcriptional regulator, can induce the expression of Alas1 in a NRF1- and FOXO1-dependent manner (Handschin et al. 2005). PGC-1α itself is also subject to circadian regulation (Liu et al. 2007), suggesting it may also be important for driving oscillatory expression of Alas1 and heme biosynthesis. However, the circadian machinery for PGC-1α oscillation is unknown.
Here we show that Rev-erbα regulates intracellular heme levels by directly repressing the expression of PGC-1α via recruitment of NCoR in a heme-responsive manner. Manipulation of heme levels thus regulates PGC-1α in the direction opposite to that of the change in heme levels in a classic feedback loop. Heme deficiency induced by overexpression of Rev-erbα also restricts mitochondrial respiration-driven oxygen consumption, and therefore causes cell cycle arrest. Together, these data demonstrate the existence of a negative feedback loop that regulates heme levels and represents a novel link between the circadian clock and energy homeostasis.
Results
Manipulation of Rev-erbα changes intracellular heme levels
Feedback regulation by cognate ligands has been shown to be an important mechanism for modulating NR activity (Chin and Gharib 1986; Therrien and Drouin 1993; Chiamolera and Wondisford 2009). Since heme is the physiological ligand for Rev-erbα and its biosynthesis is subjected to circadian control, we postulated that Rev-erbα might directly regulate heme level via a negative feedback loop. We first validated our heme measurement system by examining heme levels in HepG2 human liver cells treated with succinylacetone, a potent inhibitor of heme synthesis (Ebert et al. 1979). Indeed, we observed a dose-dependent drop in the total heme level following succinylacetone treatment (Supplemental Fig. S1). To study the effect of Rev-erbα on heme biosynthesis, we generated cell lines expressing full-length human Rev-erbα under the control of a doxycycline-repressible promoter in NIH3T3 fibroblasts (“REV-3T3 cells”). Since Rev-erbα protein is unstable in many cellular environments, we used a stabilized form (S55D/S59D) to avoid this confounding factor (Yin et al. 2006). The increased expression of total Rev-erbα protein was confirmed by Western blot (Fig. 1A). Remarkably, heme levels were markedly decreased in the REV-3T3 cells (Fig. 1B). In contrast, depletion of Rev-erbα by siRNA led to an increased heme level (Fig. 1C). Efficiency of Rev-erbα knockdown was confirmed by the down-regulation of Rev-erbα mRNA (Fig. 1D). Taken together, these results suggest that Rev-erbα negatively regulates the level of its own ligand and thus engages in a feedback loop regulating its own activity.
Figure 1.
Rev-erbα regulates intracellular heme levels. (A) Overexpression of Rev-erbα. Expression level of total Rev-erbα protein in NIH3T3 cells stably transfected control vector (Control-3T3) or vector expressing Rev-erbα SD55/59 (REV-3T3). (B) Rev-erbα reduces heme levels. Intracellular heme levels were measured in NIH3T3 cells stably expressing either control (Control-3T3) or Rev-erbα SD55/59, a stable form of Rev-erbα (REV-3T3). Mean ± SEM (n = 3). (*) P < 0.05 versus control cells expressing empty vector. (C) Depletion of Rev-erbα increases heme levels. Intracellular heme levels were measured in either control or Rev-erbα-deficient HepG2 cells. Mean ± SEM (n = 3). (*) P < 0.05 versus control shRNA β-galactosidase. (D) Depletion of Rev-erbα. shRNA knockdown of either β-galactosidase (control) or human Rev-erbα in HepG2 liver cells. Rev-erbα mRNA was quantitated by QPCR and normalized to those of GADPH level. Mean ± SD (n = 3). (*) P < 0.05 compared with control by Student's t-test.
Rev-erbα reduces heme levels by repressing PGC-1α
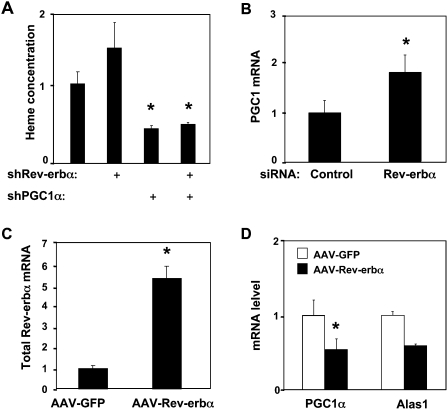
We next searched for the molecular target of Rev-erbα regulation of heme metabolism, turning our attention to PGC-1α, a circadian gene that positively regulates Alas1 and heme synthesis (Zheng et al. 2001; Panda et al. 2002; Handschin et al. 2005). As expected, heme levels were reduced by depletion of PGC-1α from HepG2 cells (Fig. 2A). Importantly, under these conditions, depletion of Rev-erbα did not significantly increase heme levels, while the knockdown of Rev-erbα alone increased the heme concentration (Fig. 2A). These data show that elevation of intracellular heme levels by Rev-erbα requires PGC-1α.
Figure 2.
Rev-erbα represses PGC-1α gene expression. (A) PGC-1α is required for Rev-erbα regulation of heme levels. HepG2 cells were infected with adenovirus expressing Rev-erbα shRNA and/or PGC-1α shRNA as indicated. Mean ± SEM is shown. (*) P < 0.05 versus control adenovirus. (B) Rev-erbα depletion induces PGC-1α. PGC-1α gene expression was measured after siRNA knockdown of either scrambled sequence (control) or human Rev-erbα in HepG2 liver cells. (C) Overexpression of Rev-erbα. Rev-erbα was determined by QPCR in mouse livers with tail-injected AAV-GFP (control) and AAV-Rev-erbα 55/59SD. (*) P < 0.05 versus control. (D) Rev-erbα represses PGC-1α expression. PGC-1α and Alas1 were measured in livers from mice transduced by tail injection of AAV-GFP (control) and AAV-Rev-erbα 55/59SD.
To determine if Rev-erbα directly affects PGC-1α gene expression, we depleted Rev-erbα in HepG2 human hepatoma cells (Supplemental Fig. S2) and observed a significant increase in the total level of PGC-1α (Fig. 2B). In addition, using adeno-associated virus (AAV)-mediated transduction, we overexpressed the stabilized form of Rev-erbα in mouse liver (Fig. 2C). PGC-1α expression was markedly diminished in livers of mice expressing ectopic Rev-erbα (Fig. 2D). Consistent with the role of PGC-1α in regulating the rate-limiting enzyme in heme biosynthesis, Alas1 mRNA was also down-regulated in the livers with ectopic expression of Rev-erbα.
Rev-erbα directly represses PGC-1α transcription via functional retinoic acid receptor-related orphan receptor response elements (ROREs)
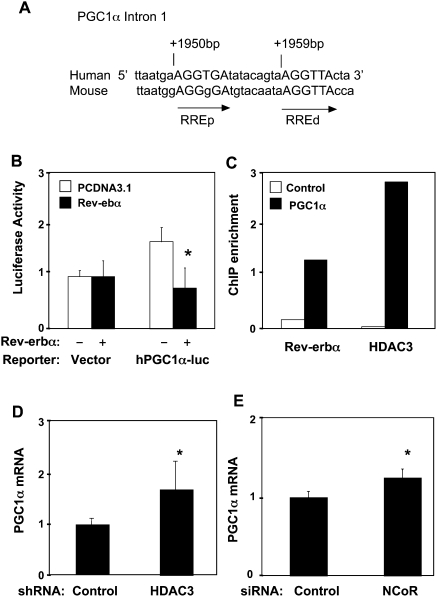
Analysis of the PGC-1α gene promoter region up to −3.1 kb from the transcriptional start site failed to identify functional Rev-erbα response elements (Czubryt et al. 2003; data not shown). However, the importance of intronic NR response elements has been increasingly recognized (Carroll and Brown 2006; Carroll et al. 2006; Lefterova et al. 2008; Schupp et al. 2009). Indeed, we identified two putative Rev-erbα monomer-binding sites (ROREs) spaced by 6 base pairs (bp) within the first intron region of the PGC-1α gene and conserved in human and mouse (Fig. 3A). Rev-erbα overexpression repressed the luciferase activity of a reporter driven by the PGC-1α intron sequence (Fig. 3B). This repression was dose-dependent, as the increasing amounts of the cotransfected Rev-erbα expression plasmid further reduced the luciferase activity (Supplemental Fig. S3A). Moreover, consistent with the requirement for two Rev-erbα monomers to recruit corepressor and inhibit transcription (Zamir et al. 1997; Yin and Lazar 2005; Wang et al. 2006), mutation of either RORE led to marked reductions in the ability of Rev-erbα to repress the luciferase, and mutation of both sites abolished repression (Supplemental Fig. S3B).
Figure 3.
Rev-erbα recruits the HDAC3/NCoR corepressor complex to repress the PGC-1α gene through an intronic Rev-erb regulatory element. (A) Schematic presentation of the PGC-1α Intron1 sequence in which two conserved Rev-erbα-binding monomeric sites are closely located. (ROREd) distal RORE; (ROREp) proximal RORE. (B) Rev-erbα regulation of PGC-1α intron luciferase reporter transfected in HEK 293T cells. The control is pGL-3 promoter vector. PGC-1α luciferase reporter plasmid (0.1 μg) was used in transfection mixture along with 2 μg of pCDNA-Flag-Rev-erbα expression vector. The luciferase activities of all experiments are expressed as the mean ± SD (n = 3). (C) ChIP assay for recruitment of Rev-erbα and HDAC3 in 293T cells. (D,E) HDAC3 knockdown (D) or NCoR knockdown (E) induces endogenous PGC-1α gene expression. After shRNA or siRNA transfection, total RNA was prepared and PGC-1α gene expression was analyzed relative to GAPDH control by quantitative real-time PCR. The fold change was calculated as the relative abundance of PGC-1α mRNA in the cells receiving HDAC3 shRNA or NCoR siRNA divided by the relative abundance of PGC-1α mRNA in the cells receiving control shRNA or siRNA, which were set to 1. Results are expressed as mean ± SD. (*) P < 0.05 by paired Student's t-test.
Promoter analysis by chromatin immunoprecipitation (ChIP) in 293T cells revealed the enrichment of Rev-erbα at the implicated region within intron 1 of the endogenous PGC-1α gene, but not at the negative control (Fig. 3C). Histone deacetylase 3 (HDAC3), which is stoichiometrically associated with NCoR (Guenther et al. 2000; Yoon et al. 2003), was also enriched at the Rev-erbα-responsive region of the PGC-1α gene (Fig. 3C), and depletion of Rev-erbα by siRNA in 293T cells led to a loss of recruitment of HDAC3 at the intronic Rev-erbα-binding sites of the PGC1α gene (Supplemental Fig. S4). Furthermore, siRNA depletion of either NCoR or HDAC3 (Supplemental Fig. S5A,B) led to a significant increase in PGC-1α mRNA (Fig. 3C,D). These data indicate that the basal repression of PGC-1α by Rev-erbα requires the NCoR/HDAC3 corepressor complex.
Heme enhances Rev-erbα repression of PGC-1α
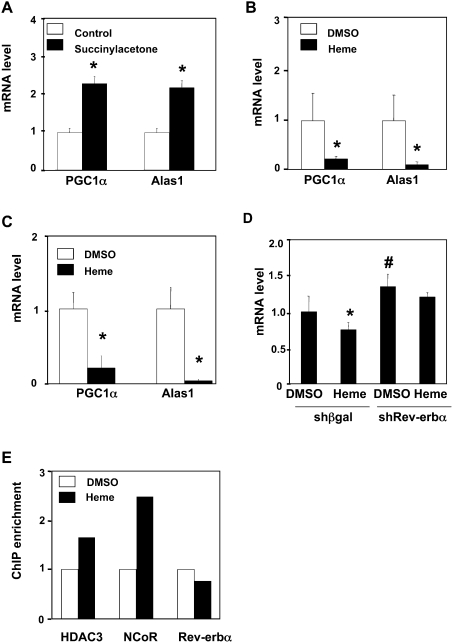
Heme binding stimulates NCoR/HDAC3 recruitment and repression by Rev-erbα (Raghuram et al. 2007; Yin et al. 2007). Having shown that Rev-erbα directly represses PGC-1α, we hypothesized that manipulation of heme levels would regulate PGC-1α expression. Indeed, depletion of heme by succinylacetone treatment led to a significant induction of PGC-1α as well as its target, Alas1 (Fig. 4A). In contrast, the addition of heme suppressed PGC-1α and Alas1 gene expression in human HepG2 cells (Fig. 4B) and mouse primary hepatocytes (Fig. 4C). Additionally, the heme treatment rescued the succinylacetone effect on PGC-1α (Supplemental Fig. S6B). The repressive effect of heme was abrogated when Rev-erbα was depleted (Fig. 4D), demonstrating that heme regulation of PGC-1α is mediated by Rev-erbα. Further supporting this hypothesis, we observed heme-dependent recruitment of NCoR and HDAC3 at the Rev-erbα-responsive region of the endogenous PGC-1α gene, to which Rev-erbα is constitutively bound (Fig. 4E). Taken together, our results support the hypothesis that heme regulates its own synthesis by stimulating Rev-erbα-mediated repression of the PGC-1α gene.
Figure 4.
Intracellular heme concentration modulates PGC-1α gene expression. (A) Succinylacetone induces the expression of PGC-1α and ALAS1. HepG2 cells were treated with succinylacetone (5 mM) for 16 h. mRNA were quantitated by RT–PCR and normalized to GADPH. (B) Heme represses Alas1 and PGC-1α expression in HepG2 cells. Heme treatment was 10 μM for 16 h. (C) Heme represses Alas1 and PGC-1α in primary mouse hepatocytes. Heme treatment was 6 μM for 16 h. (D) Effect of heme on the expression of PGC-1α gene in cells depleted of Rev-erbα in HepG2 cells. Mean ± SD (n = 3); (*) P < 0.05 compared with DMSO-treated cells transfected with control shRNA. (E) Effect of heme on the occupancy of Rev-erbα, HDAC3, and NCoR at the PGC-1α intronic sequence in HepG2 cells. Results of heme treatment are normalized to DMSO results.
Rev-erbα inhibits respiration-driven oxygen consumption rate and mitochondrial gene expression
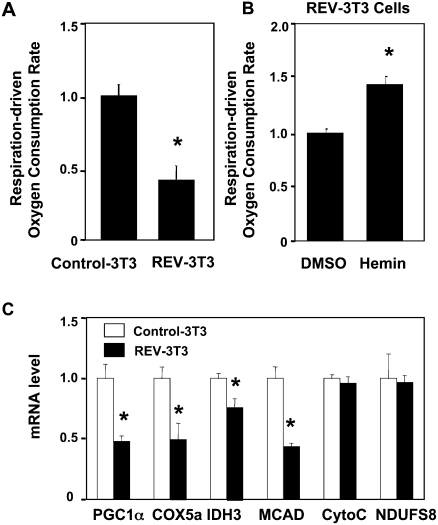
Since stable expression of Rev-erbα lowers intracellular heme levels, and heme deficiency has been shown to adversely affect mitochondrial complex IV activity (Atamna 2004), we suspected that Rev-erbα might affect mitochondrial function. Indeed, cells expressing Rev-erbα exhibited a lower basal respiration-driven oxygen consumption rate, reflecting a lower basal ATP synthesis rate (Fig. 5A). The negative effect of Rev-erbα on respiration was partially reversed by the addition of heme (Fig. 5B). The same concentration of heme had little effect on cells that did not express ectopic Rev-erbα (Supplemental Fig. S7). The incomplete rescue by heme is likely related to other, direct effects on nuclear genes involved in mitochondrial function that are known to be regulated by PGC-1α (Wu et al. 1999; Glass 2006). Indeed, the mRNA level of COX5A, IDH3, and MCAD are decreased in REV-3T3 cells, which could partially contribute to the mitochondrial defect in those cells (Fig. 5C).
Figure 5.
Rev-erbα inhibits respiration-driven oxygen consumption rate and mitochondrial gene expression. (A) Ectopic expression of Rev-erbα inhibits respiration-driven oxygen consumption rate. Oxygen consumption rates and calculations were performed as in the Materials and Methods. Mean ± SEM (n = 15); (*) P < 0.05. (B) Addition of heme (2.5 μM for 6 h) partially rescues the inhibition of oxygen consumption by Rev-erbα. Mean ± SEM (n = 3); (*) P < 0.05. (C) Mitochondrial gene expression in REV-3T3 cells.
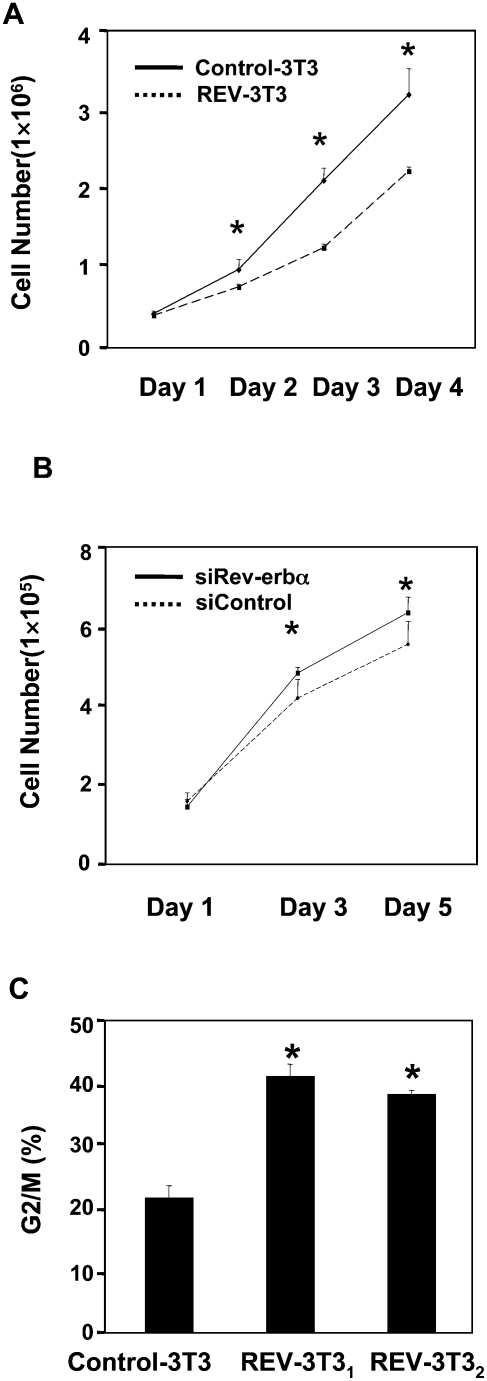
Overexpression of Rev-erbα causes cell growth arrest
As chronic overexpression of Rev-erbα dramatically reduces respiration-driven oxygen consumption, we next determined the effects of this metabolic change on cell growth. We were particularly interested in this issue because we have been unable to generate and passage cell lines that constitutively express Rev-erbα (data not shown). Indeed, induction of Rev-erbα in the REV-3T3 cells markedly slowed cell growth (Fig. 6A). This reduced rate of growth was reversed when doxycycline was added to stop Rev-erbα expression (data not shown), suggesting that Rev-erbα induced a cell cycle block rather than cell death. Conversely, the depletion of Rev-erbα in NIH3T3 led to increased cell growth (Fig. 6B). Flow cytometry performed on two different subclones of the REV-3T3 cells demonstrated that REV-3T3 cells were arrested in G2/M phase (Fig. 6C; Supplemental Fig. S9A,B).
Figure 6.
Ectopic Rev-erbα causes cell cycle arrest and blocks cell growth. (A) Ectopic Rev-erbα blocks cell growth. Cell number experiments are expressed as mean ± SD (n = 3). (*) P < 0.05. (B) The depletion of Rev-erbα promotes cell growth. Cell numbers are expressed as mean ± SD (n = 3). (*) P < 0.05. (C) Ectopic Rev-erbα arrests cells in G2/M phase. The DNA content of NIH3T3 cells was determined by FACS. The percentage of cells in each cell cycle phase was calculated by ModFit Software and is presented in the figure. (*) P < 0.05.
Discussion
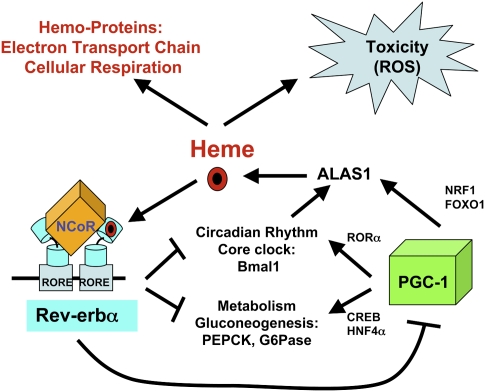
Here we showed that, in addition to regulating circadian rhythms and intermediary metabolism (Preitner et al. 2002; Ueda et al. 2002; Yin and Lazar 2005; Yin et al. 2007; Duez et al. 2008), Rev-erbα reduces the level of its own ligand, heme, in a pathway with the features of a classical negative feedback loop (Fig. 7). This feedback is likely to dampen the magnitude and duration of the Rev-erbα target gene repression. Indeed, we show here that one of these targets is PGC-1α, itself a circadian gene that regulates metabolism (Fig. 7). It is the link between Rev-erbα and PGC-1α that forms the basis of the feedback loop, with heme activating Rev-erbα repression of PGC-1α, thereby reducing heme synthesis and dampening the extent of Rev-erbα repression. Conversely, when heme is reduced, reduced Rev-erbα repression enables PGC-1α to stimulate heme synthesis via transcriptional activation of the rate-limiting enzyme ALAS.
Figure 7.
The Rev-erbα/PGC-1α pathway regulating heme homeostasis. Heme promotes Rev-erbα repression of PGC-1α, thereby reducing ALAS1 gene expression and heme biosynthesis. Conversely, low heme levels reduce Rev-erbα repression, enhancing PGC-1α stimulation of heme synthesis via transcriptional activation of the rate-limiting enzyme ALAS1.
This feedback loop, in addition to dampening Rev-erbα activity, would also tend to reduce the magnitude of fluctuations in heme levels. Our finding that overexpression of Rev-erbα reduces heme levels by ∼50% and depletion of Rev-erbα increase intracellular heme about twofold suggest that the Rev-erbα/PGC-1α cycle functions to keep heme excursions within a fourfold range. The expression of ALAS1 is known to be circadian (Zheng et al. 2001; Kaasik and Lee 2004), regulated by circadian transcription factor NPAS2 (Kaasik and Lee 2004) as well as PGC-1α (Handschin et al. 2005) and, as shown here, Rev-erbα (via PGC-1α). Consistent with our model, a recent study measuring heme levels over a 24-h period indeed found that they fluctuate within this fourfold range (Rogers et al. 2008).
It is well established that, at pathologically high levels of heme, HMOX1 is massively induced, resulting in heme breakdown (Ryter et al. 2006). We suggest that the Rev-erbα/PGC-1α cycle regulates intracellular heme concentration within the physiological range. This negative feedback mechanism would function to help prevent heme concentrations from reaching pathological levels that require detoxification by the HMOX1 system (Furuyama et al. 2007; Jarmi and Agarwal 2009). In addition, and uniquely, the relief of this negative feedback provides a transcriptional sensing mechanism to stimulate heme biosynthesis when its levels are low. Maintenance of heme sufficiency via the Rev-erbα/PGC-1α cycle is likely to be critical to normal cellular function. Heme is a prosthetic group for critical enzymes within metabolically active cells, including cytochromes involved in mitochondrial electron transport and oxidative metabolism (Furuyama et al. 2007). It has long been known that heme is essential for the growth and survival of many cell types, and that inhibition of heme synthesis causes cell cycle arrest (Ebert et al. 1979; Tschudy et al. 1980). We showed here that manipulation of Rev-erbα levels alters endogenous heme levels, in a manner that is opposite the change in Rev-erbα, as would be expected for loss of the heme feedback-sensing mechanism. The repression of Rev-erbα on heme biosynthesis indeed affected mitochondrial respiration in a manner that is partly reversed by heme. Moreover, excess Rev-erbα activity is deleterious to cell growth, due to a G2/M cell cycle arrest.
PGC-1α has been implicated in the pathogenesis of insulin resistance, neurodegeneration, and other disorders associated with impaired mitochondrial function (Mootha et al. 2003; Patti et al. 2003; Lin et al. 2005; Handschin and Spiegelman 2006; Lin 2009). The link discovered here between heme, Rev-erbα, and PGC-1α reveals a molecular pathway coordinating and maintaining cellular energy homeostasis, which may be defective or challenged in pathological states such as obesity, diabetes, and cancer. As an NR regulated by reversible binding of a lipophilic heme ligand (Yin et al. 2007), Rev-erbα may be an excellent target for intervention. Indeed, the recent identification of a nonheme, pharmacological ligand for Rev-erbα (Meng et al. 2008) suggests that novel therapies may emerge from the manipulation of Rev-erbα activity.
Materials and methods
Plasmids and reagents
The PGC-1α luciferase reporter construct was generated by PCR amplification of the 401-bp human PGC-1α Intron1, which was subcloned into the PGL3 promoter vector using both Kpn1 and NheI sites (Promega). RORE mutants were generated by site-directed mutagenesis using the QuikChange kit (Stratagene) and confirmed by sequencing analysis. The expression vectors encoding human Rev-erbα have been described previously (Yin and Lazar 2005). Protein A-Sepharose was obtained from Amersham Biosciences. Heme and succinylacetone were purchased from Sigma.
Mammalian cell culture and transfection
HepG2 and HEK-293T cells were maintained in high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Invitrogen). NIH3T3 cells were maintained in high-glucose DMEM supplemented with 10% bovine serum. NIH3T3 cells stably transfected with stabilized Rev-erbα (S55D/S59D) under the negative control of doxycycline have been described previously (Yin et al. 2006). Cells were grown at 37°C in 5% CO2. All transient transfection assays were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For repression assays, cells were grown in 24-well plates and transfected with 0.1 μg of PGC-1α luciferase reporter, 0.1–2 μg of Rev-erbα expression vector, and 0.1 μg of β-galactosidase expression vector. The total amount of expression plasmid transfected per well was kept constant by adding varying amounts of empty vector. At 48 h posttransfection, cells were lysed and their luciferase activity was assayed using a reporter assay kit (Promega). Luciferase units were normalized to β-galactosidase expression. Fold repression was calculated as the activity of the same reporter in the presence of Rev-erbα expression vector, with the control group (PCDNA3.1) normalized to 1. Each experiment was performed three times in triplicate. In some experiments, succinylacetone was added for 16 h before cell harvesting, and heme was added at various concentrations and times as described (Yin et al. 2007).
Animals and administration of recombinant AAV
All mouse studies were approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Eight-week-old male C57BL/6 mice were purchased from Jackson Laboratories and maintained in 12-h light/dark conditions for 4 wk before experiments. Mice were given unrestricted access to water and maintained on a normal chow diet. AAV viral particles were injected via tail vein at a dose of 1 × 1012 genome copies per mouse. Mice were randomly divided into three groups and injected with AAV-GFP and AAV-Rev-erbα SD55/59. At time of necropsy, the livers were harvested for RNA analysis.
Isolation of primary mouse hepatocytes
Primary hepatocytes were isolated from 3-mo-old male C57Bl/6 mice. Briefly, following anesthetization with Avertin (1.0 g/kg i.p.;Sigma), the vena cava was cannulated and the liver was perfused with 37°C Liver Perfusion Buffer (Gibco) for 5 min. Next, the perfusion buffer was replaced with 37°C Liver Digestion Buffer (Gibco), supplemented with collagenase 1 (0.2% final concentration; Sigma) for 10 min. Following digestion, the liver was removed and cells were dispersed in DMEM containing 10% FBS and 1% pen per strep. Cells were filtered through a 100-μm mesh, pelleted, and subjected to a Percoll gradient (45% Percoll in DMEM) to separate live and dead cells. Following three washes in DMEM, viable cells were plated at a density of 0.3 × 106 cells per well in 12-well plates coated with collagen 1 (BD BioCoat) in DMEM, 10% FBS, and 1% pen per strep. Five hours later, unattached cells were removed by washing with DMEM. Cells were washed three times with warm phosphate-buffered saline to remove glucose and cultured in the glucose-free medium containing gluconeogenic substrates (20 mM sodium lactate, 2 mM sodium pyruvate). Cells were stimulated with dexamethasone (1 nM) and 8-CPT-cAMP (500 μM) with or without heme.
Transduction of HepG2 cells by adenovirus infection
HepG2 cells (3 × 105) were infected with adenovirus expressing control, shRev-erbα, or shPGC-1α (gift of J. Estall and B. Spiegelman) at a dose of 1 × 108 plaque-forming units.
RNAi
Vectors expressing hairpin siRNAs under the human U6 promoter were constructed by inserting pairs of annealed DNA oligonucleotides into prelinearized pEntry-U6 vector according to the manufacturer's instruction (Invitrogen Life Technologies). Control was pEntry β-galactosidase plus pSilence Scramble siRNA. The target sequences were as follows: for human Rev-erbα, 5′-GGCATGGGTGTTACTGTGTAAA-3′; for β-galactosidase, 5′-GTGCACCTGGTAAATCTTAT-3′; and for HDAC3, 5′-CAGCGCATTGATGACCAGAGTTACA-3′. Double-strand siRNA oligo for NCoR was purchased from Dharmacon. Cells in 12-well plates were transfected twice over a 96-h period with 1.6 μg of siRNA vector per well. After the second transfection, cells were harvested for RNA analysis or protein analysis. In the experiment shown in Figure 2B, double-strand RNAi oligos targeting human Rev-erbα were synthesized by Invitrogen. The control sequence was 5′-GACCCUCGUAAGACGCUUCCAAAGU-3′. The Rev-erbα oligo was 5′-ACUUUGGAAGCGUCUUACGAGGGUC-3′.
ChIP assay
Cells were grown in 10-cm plates and either transfected or treated with 6 μM heme for the indicated experiments. After cross-linking in formaldehyde, cells were lysed in hypotonic buffer (50 mM Tris-HCl, 85 mM KCl, 0.5% Nonidet P-40, 1× protease inhibitor). The nuclear fraction was resuspended in 500 μL of sonication buffer (0.01% SDS, 10 mM EDTA, 50 mM Tris-HCl, 1× protease inhibitor) and sonicated four times for 12 sec each followed by centrifugation at 14,000g for 10 min. Supernatants were collected and diluted in dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 167 mM Tris-HCl, 167 mM NaCl) followed by preclearing with 2 μg of salmon sperm DNA and protein A-Sepharose for 2 h at 4°C. Immunoprecipitation with the following antibodies was performed overnight at 4°C: anti-acetyl histone H4 (Upstate Biotechnologies), anti-NCoR and anti-HDAC3 (Abcam), and anti-Rev-erbα (Cell Signaling). Immunoprecipitated complexes were collected with protein A-Sepharose beads followed by sequential washes in low-salt, high-salt, lithium, and Tris-EDTA buffers. Precipitates were eluted, and 5 M NaCl was added to reverse cross-links for 6 h at 65°C. DNA fragments were column-purified (Qiagen), and 2 μL of purified DNA was used in quantitative PCR (QPCR) using primers encompassing both RORE regions of the human endogenous PGC-1α Intron1 (forward, 5′-TGTTTGCTGTCATCCTAAAACG-3′; reverse, 5′-TGGGGTGTATGTCATGTGAA-3′). An irrelevant region (PGC1α exon 13 coding sequence) worked as a negative control (forward, 5′-GAATTGGCAGGTGGAAAAAA-3′; reverse, 5′-ATGTGAACTGCTGATTTGATGG-3′).
Quantitative RT–PCR
Total mRNA was prepared using the RNeasy kit (Qiagen). Reverse transcription was performed with 1–2 μg of total RNA using a reverse transcription kit (AB high-capacity CDNA reverse transcription kit) according to the manufacturer's instructions. The cDNA was subject to quantitative RT-PCR using SYBR Q-PCR mastermix (Applied Biosystems) on a Prism 7900 HT detection system. The primer pairs for amplifying human Bmal1, mouse GADPH, human PGC-1α, and mouse PGC-1α were purchased from Qiagen. Mouse COX5A, mouse IDH3, mouse MCAD, mouse Cytochrome c, and mouse NDUFS8 primer sets were described previously (Cunningham et al. 2007). The sequences for other primer pairs used in this study were human Alas1 (F, 5′-GGCATCCATTAGCATCTGTCTC-3′; R, 5′-GGCTTCATCTTCACCACCTC-3′), human glyceraldehyde-3-phosphate dehydrogenase (Gapdh) (F, 5′-GAAGGTGAAGGTCGGAGTC-3′; R, 5′-GAAGATGGTGATGGGATTTC-3′), and mouse Alas1 (F, 5′-TGCAGAAGGCAGGAAAGTCT-3′; R, 5′-AGGGGTTTCTTTGACCTGCT-3′).
Target gene expression was normalized to housekeeping gene GAPDH. The average value from each triplicate was used to calculate fold induction of the gene, with the control group normalized to 1.
Immunoblotting
Cells were lysed in whole-cell lysis buffer (150 mM NaCl, 10 mM Tris at pH 7.6, 0.1% SDS, 5 mM EDTA) with 1× protease inhibitor. Twenty micrograms of lysates were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Blots were probed with the following antibodies: anti-NCoR (Ishizuka and Lazar 2003), anti-HDAC3 (Abcam), anti-β-actin (Sigma), anti-GSK3β (Abcam), anti-PGC1α (Santa Cruz Biotechnologies), anti-Rev-erbα (Cell Signaling), and anti-Hsp90 (Santa Cruz Biotechnologies).
Heme measurement
Measurement of cellular heme content: Heme in HepG2, NIH3T3 cell lysates was measured by using a modified QuantiChrom Heme Assay (BioAssay Systems) (Raghuram et al. 2007; Rogers et al. 2008). Heme concentrations were normalized to protein. The average heme concentration (micromolar heme per micrograms of total protein) was used to calculate fold changes, with the control group normalized to 1.
Oxygen consumption rate measurement
Cells were seeded at 50,000 cells per well in 24-well XF plates and incubated overnight at 37°C in 5% CO2 incubator. The oxygen consumption rate is measured by using The Seahorse XF24 Analyzer (Seahorse Bioscience). Immediately before measurement, medium was replaced with nonbuffered pH 7.4 speciality medium. Three successive 10-min measurements were performed simultaneously at 5-min intervals in the triplicates wells. Immediately after measurement, the protein concentration was measured by Bio-Rad protein assay. Oxygen consumption rate was normalized to protein. The cells were measured for the basal oxygen consumption rate and also the oxygen consumption rate after the addition of 5 μM oligomycin to inhibit ATP synthase. The difference of oxygen consumption rate between the basal and the oligomycin-treated is considered as the oxygen consumption rate used for ATP synthesis, therefore called “respiration-driven oxygen consumption rate” (Watanabe et al. 2006; Wu et al. 2007; Ferrick et al. 2008). The average “respiration-driven oxygen consumption rate” was used to calculate fold changes, with the control group normalized to 1.
FACS analysis
Cells were trypsinized, washed with PBS, and fixed with ice-cold 70% ethanol overnight at 4°C. Nuclear DNA was stained using a solution with 50 μg/mL propidium iodide (Sigma) and 1 mg/mL RNase A in PBS. The cells were analyzed on FACScalibur (BD Biosciences) using CellQuest and ModFit data analysis software.
Cell growth curve
To compare the cell growth rate, 1 × 105 to 5 × 105 cells of each cell line were seeded in 12-well plates, and the number of cells of each cell line was counted daily by Vi-CELL Cell Viability Analyzers (Beckman). Each experiment was set up in triplicate
Acknowledgments
We thank J. Estall and B. Spiegelman for PGC-1α shRNA adenovirus, R. Bassel-Duby and E. Olson for the PGC-1α-luciferase vector (−3.1 kb), C. Crammer for guidance on mitochondrial assays, E. Yeh for cell growth assay design, C. Khoo for help with flow cytometry assays, and M. Handy for mouse husbandry, We also thank J. Baur, D. Steger S. Mullican, and C. Phelan for helpful discussions. This work was supported by R01 DK45586 (to M.A.L.) and K99/R00 NIH DK 077449 (to L.Y.).
Footnotes
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1825809.
Supplemental material is available at http://www.genesdev.org.
References
- Atamna H. Heme, iron, and the mitochondrial decay of ageing. Ageing Res Rev. 2004;3:303–318. doi: 10.1016/j.arr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Burris TP. Nuclear hormone receptors for heme: REV-ERBα and REV-ERBβ are ligand-regulated components of the mammalian clock. Mol Endocrinol. 2008;22:1509–1520. doi: 10.1210/me.2007-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Brown M. Estrogen receptor target gene: An evolving concept. Mol Endocrinol. 2006;20:1707–1714. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- Chiamolera MI, Wondisford FE. Minireview: Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology. 2009;150:1091–1096. doi: 10.1210/en.2008-1795. [DOI] [PubMed] [Google Scholar]
- Chin WW, Gharib SD. Organization and expression of gonadotropin genes. Adv Exp Med Biol. 1986;205:245–265. doi: 10.1007/978-1-4684-5209-9_11. [DOI] [PubMed] [Google Scholar]
- Chiu D, Lubin B. Oxidative hemoglobin denaturation and RBC destruction: The effect of heme on red cell membranes. Semin Hematol. 1989;26:128–135. [PubMed] [Google Scholar]
- Coste H, Rodriguez JC. Orphan nuclear hormone receptor Rev-erbα regulates the human apolipoprotein CIII promoter. J Biol Chem. 2002;277:27120–27129. doi: 10.1074/jbc.M203421200. [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- Czubryt MP, McAnally J, Fishman GI, Olson EN. Regulation of peroxisome proliferator-activated receptor γ coactivator 1 α (PGC-1α) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci. 2003;100:1711–1716. doi: 10.1073/pnas.0337639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington TK, Wager-Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TD, Weitz CJ, Takahashi JS, Kay SA. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- Downes M, Carozzi AJ, Muscat GE. Constitutive expression of the orphan receptor, Rev-erbAα, inhibits muscle differentiation and abrogates the expression of the myoD gene family. Mol Endocrinol. 1995;9:1666–1678. doi: 10.1210/mend.9.12.8614403. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- Duez H, Staels B. Rev-erbα gives a time cue to metabolism. FEBS Lett. 2008;582:19–25. doi: 10.1016/j.febslet.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Duez H, van der Veen JN, Duhem C, Pourcet B, Touvier T, Fontaine C, Derudas B, Bauge E, Havinga R, Bloks VW, et al. Regulation of bile acid synthesis by the nuclear receptor Rev-erbα. Gastroenterology. 2008;135:689–698. doi: 10.1053/j.gastro.2008.05.035. [DOI] [PubMed] [Google Scholar]
- Ebert PS, Hess RA, Frykholm BC, Tschudy DP. Succinylacetone, a potent inhibitor of heme biosynthesis: Effect on cell growth, heme content and δ-aminolevulinic acid dehydratase activity of malignant murine erythroleukemia cells. Biochem Biophys Res Commun. 1979;88:1382–1390. doi: 10.1016/0006-291x(79)91133-1. [DOI] [PubMed] [Google Scholar]
- Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Furuyama K, Kaneko K, Vargas PD. Heme as a magnificent molecule with multiple missions: Heme determines its own fate and governs cellular homeostasis. Tohoku J Exp Med. 2007;213:1–16. doi: 10.1620/tjem.213.1. [DOI] [PubMed] [Google Scholar]
- Glass CK. Going nuclear in metabolic and cardiovascular disease. J Clin Invest. 2006;116:556–560. doi: 10.1172/JCI27913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes & Dev. 2000;14:1048–1057. [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Handschin C, Lin J, Rhee J, Peyer AK, Chin S, Wu PH, Meyer UA, Spiegelman BM. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1α. Cell. 2005;122:505–515. doi: 10.1016/j.cell.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Harding HP, Lazar MA. The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol. 1995;15:4791–4802. doi: 10.1128/mcb.15.9.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T, Lazar MA. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol Cell Biol. 2003;23:5122–5131. doi: 10.1128/MCB.23.15.5122-5131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmi T, Agarwal A. Heme oxygenase and renal disease. Curr Hypertens Rep. 2009;11:56–62. doi: 10.1007/s11906-009-0011-z. [DOI] [PubMed] [Google Scholar]
- Kaasik K, Lee CC. Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature. 2004;430:467–471. doi: 10.1038/nature02724. [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Bass J. A sense of time: How molecular clocks organize metabolism. Trends Endocrinol Metab. 2007;18:4–11. doi: 10.1016/j.tem.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ, Jr, Liu XS, et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes & Dev. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin W, Lu AY, Jacobson M, Kuntzman R, Poyer JL, McCay PB. Lipid peroxidation and the degradation of cytochrome P-450 heme. Arch Biochem Biophys. 1973;158:842–852. doi: 10.1016/0003-9861(73)90580-8. [DOI] [PubMed] [Google Scholar]
- Lin JD. Minireview: The PGC-1 coactivator networks: chromatin-remodeling and mitochondrial energy metabolism. Mol Endocrinol. 2009;23:2–10. doi: 10.1210/me.2008-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1α integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: Elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Foster RG. Circadian rhythms: Something to cry about? Curr Biol. 1999;9:R214–R217. doi: 10.1016/S0960-9822(99)80132-8. [DOI] [PubMed] [Google Scholar]
- Maines MD. Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- McKenna NJ, Cooney AJ, Demayo FJ, Downes M, Glass CK, Lanz RB, Lazar MA, Mangelsdorf DJ, Moore DD, Qin J, et al. Minireview: Evolution of NURSA, the Nuclear Receptor Signaling Atlas. Mol Endocrinol. 2009;23:740–746. doi: 10.1210/me.2009-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ, McMaster A, Beesley S, Lu WQ, Gibbs J, Parks D, Collins J, Farrow S, Donn R, Ray D, et al. Ligand modulation of REV-ERBα function resets the peripheral circadian clock in a phasic manner. J Cell Sci. 2008;121:3629–3635. doi: 10.1242/jcs.035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- O'Malley B. The year in basic science: Nuclear receptors and coregulators. Mol Endocrinol. 2008;22:2751–2758. doi: 10.1210/me.2008-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Q, Mahmood N, Khan SG, Arif JM, Athar M. Mechanism of asbestos-mediated DNA damage: Role of heme and heme proteins. Environ Health Perspect. 1997;105:1109–1112. doi: 10.1289/ehp.97105s51109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Rogers PM, Ying L, Burris TP. Relationship between circadian oscillations of Rev-erbα expression and intracellular levels of its ligand, heme. Biochem Biophys Res Commun. 2008;368:955–958. doi: 10.1016/j.bbrc.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Schupp M, Lefterova MI, Janke J, Leitner K, Cristancho AG, Mullican SE, Qatanani M, Szwergold N, Steger DJ, Curtin JC, et al. Retinol saturase promotes adipogenesis and is downregulated in obesity. Proc Natl Acad Sci. 2009;106:1105–1110. doi: 10.1073/pnas.0812065106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- Stratmann M, Schibler U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms. 2006;21:494–506. doi: 10.1177/0748730406293889. [DOI] [PubMed] [Google Scholar]
- Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger NB. Functional adaptations of oxygen-transport proteins. J Exp Biol. 1998;201:1085–1098. doi: 10.1242/jeb.201.8.1085. [DOI] [PubMed] [Google Scholar]
- Therrien M, Drouin J. Molecular determinants for cell specificity and glucocorticoid repression of the proopiomelanocortin gene. Ann N Y Acad Sci. 1993;680:663–671. doi: 10.1111/j.1749-6632.1993.tb19768.x. [DOI] [PubMed] [Google Scholar]
- Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C, Selby CP, Dawut L, Smithies O, Takahashi JS, et al. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science. 1998;282:1490–1494. doi: 10.1126/science.282.5393.1490. [DOI] [PubMed] [Google Scholar]
- Tschudy DP, Ebert PS, Hess RA, Frykholm BC, Weinbach EC. Effect of heme depletion on growth, protein synthesis and respiration of murine erythroleukemia cells. Biochem Pharmacol. 1980;29:1825–1831. doi: 10.1016/0006-2952(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yin L, Lazar MA. The orphan nuclear receptor Rev-erbα regulates circadian expression of plasminogen activator inhibitor type 1. J Biol Chem. 2006;281:33842–33848. doi: 10.1074/jbc.M607873200. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- Yang X, Lamia KA, Evans RM. Nuclear receptors, metabolism, and the circadian clock. Cold Spring Harb Symp Quant Biol. 2007;72:387–394. doi: 10.1101/sqb.2007.72.058. [DOI] [PubMed] [Google Scholar]
- Yin L, Lazar MA. The orphan nuclear receptor Rev-erbα recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol Endocrinol. 2005;19:1452–1459. doi: 10.1210/me.2005-0057. [DOI] [PubMed] [Google Scholar]
- Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbα is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, et al. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- Yoon HG, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J. Purification and functional characterization of the human N-CoR complex: The roles of HDAC3, TBL1 and TBLR1. EMBO J. 2003;22:1336–1346. doi: 10.1093/emboj/cdg120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir I, Harding HP, Atkins GB, Horlein A, Glass CK, Rosenfeld MG, Lazar MA. A nuclear hormone receptor corepressor mediates transcriptional silencing by receptors with distinct repression domains. Mol Cell Biol. 1996;16:5458–5465. doi: 10.1128/mcb.16.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir I, Dawson J, Lavinsky RM, Glass CK, Rosenfeld MG, Lazar MA. Cloning and characterization of a corepressor and potential component of the nuclear hormone receptor repression complex. Proc Natl Acad Sci. 1997;94:14400–14405. doi: 10.1073/pnas.94.26.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]