Abstract
AIM: To investigate the effects of Chrysanthemum indicum extract (CIE) on inhibition of proliferation and on apoptosis, and the underlying mechanisms, in a human hepatocellular carcinoma (HCC) MHCC97H cell line.
METHODS: Viable rat hepatocytes and human endothelial ECV304 cells were examined by trypan blue exclusion and MTT assay, respectively, as normal controls. The proliferation of MHCC97H cells was determined by MTT assay. The cellular morphology of MHCC97H cells was observed by phase contrast microscopy. Flow cytometry was performed to analyze cell apoptosis with annexin V/propidium iodide (PI), mitochondrial membrane potential with rhodamine 123 and cell cycle with PI in MHCC97H cells. Apoptotic proteins such as cytochrome C, caspase-9, caspase-3 and cell cycle proteins, including P21 and CDK4, were measured by Western blotting.
RESULTS: CIE inhibited proliferation of MHCC97H cells in a time- and dose-dependent manner without cytotoxicity in rat hepatocytes and human endothelial cells. CIE induced apoptosis of MHCC97H cells in a concentration-dependent manner, as determined by flow cytometry. The apoptosis was accompanied by a decrease in mitochondrial membrane potential, release of cytochrome C and activation of caspase-9 and caspase-3. CIE arrested the cell cycle in the S phase by increasing P21 and decreasing CDK4 protein expression.
CONCLUSION: CIE exerted a significant apoptotic effect through a mitochondrial pathway and arrested the cell cycle by regulation of cell cycle-related proteins in MHCC97H cells without an effect on normal cells. The cancer-specific selectivity shown in this study suggests that the plant extract could be a promising novel treatment for human cancer.
Keywords: Apoptosis, Cell cycle, Chinese traditional medicine, Chrysanthemum indicum, Hepatocellular carcinoma, Herbal medicine
INTRODUCTION
Hepatocellular carcinoma (HCC) is known as a common and aggressive malignant tumor worldwide. In China, HCC accounts for 90% of primary liver cancer, which is the second most common cause of death[1]. Chemotherapy plays an important role in the treatment of cancer, but it is limited to a significant extent by its toxicities, significant resistance to available chemotherapeutic agents and side effects, including myelosuppression, neutropenia and thrombocytopenia[2,3]. One possible way to increase the efficacy of anticancer drugs and to decrease toxicities or side effects is to develop traditional medicines, especially from medicinal plants[4-7].
Natural products have become increasingly important for new pharmaceutical discoveries, and among all the uses for natural products in biomedical science, traditional Chinese herbology has been a pioneering specialty[8]. This is particularly evident in the treatment of cancers, in which more than 60% of drugs are of natural origin[9]. Hence a new medicinal plant with anticancer activities could be a valuable substance in cancer treatment. The flowers of Chrysanthemum indicum (Chrysanthemi Indici Flos), a Compositae plant, is a traditional Chinese medicine and medicinal plant distributed widely in China. Oriental Chrysanthemum indicum traditional medicine has been used to treat vertigo, hypertensive symptoms and several infectious diseases such as pneumonia, colitis, stomatitis and carbuncles[10]. A series of studies have demonstrated that Chrysanthemum indicum possesses antimicrobial[11], antiinflammatory[11-13], immunomodulatory[12], and neuroprotective effects[14]. Recently, much attention has been devoted to the anticancer activity of Chrysanthemum indicum on human PC3, HL 60 and HeLa cancer cells in a dose- and time-dependent manner[15-17]. However, its anticancer mechanism of action is still not clear and needs further investigation.
Apoptosis induced by herbs has become a principal mechanism by which anticancer therapy exerts its effect[7,18]. Upstream initiator caspases including caspase-9 activate downstream effector caspases such as caspase-3, playing a pivotal role in the induction of apoptosis. Caspase-9, triggered by chemotherapeutic drugs, is the apical caspase in the mitochondria-initiated apoptosis pathway, which requires the release of cytochrome C from the mitochondria as well as interaction with Apaf-1[19]. This pathway, associated with changes in the permeability of the outer mitochondrial membrane and the collapse of the membrane potential (Δψm), results in release of cytotoxic proteins and caspase activation[19].
Cell cycle regulation, a fundamental mechanism determining cell proliferation, is tightly mediated through a complex network of positive factors, such as cyclin-dependent kinases (CDKs) and cyclin, and negative factors, including CDK-inhibitor (CDKI) regulatory molecules. The activated CDK4-cyclin complexes are inactivated by binding to P21, a CDKI[20]. Plant extracts which arrest the cell cycle in cancer cells via regulation of CDK and CDKI proteins also can be used for therapeutic intervention[21-23].
The aim of the present study was to examine the anticancer activities of Chrysanthemum indicum and related mechanisms in MHCC97H cell lines, typical human HCC cell lines, which are commonly used in the study of antitumor cells[24]. In order to compare the actions of Chrysanthemum indicum on normal hepatocytes and endothelial cells, the effects of the extract were examined in rat hepatocytes and a human umbilical vein endothelial cell ECV304 cell line. Furthermore, we investigated the effect of the extract in human MHCC97H cells and the mechanisms underlying its effect on inhibition of proliferation.
MATERIALS AND METHODS
Plant material and extraction
Fresh, ripe fruits of high quality flowers of Chrysanthemum indicum were procured from Xi’an traditional medicine group (Shaanxi, China) in March 2006 and the characteristics were consistent with that described in the Pharmacopeia of the People’s Republic of China. Moreover, Chrysanthemum indicum was also authenticated by Professor Wang Jun-Xian, a taxonomist in the Department of Pharmacy in Xi’an Jiongtong University. The plant materials were air dried at room temperature and then powdered. The dried and powdered fruit of Chrysanthemum indicum (500 g) were extracted with 95% ethanol (EtOH) twice under refluxed temperature. After evaporation of organic solvent under reduced pressure, the resultant Chrysanthemum indicum EtOH extract (CIE) was concentrated under reduced pressure to give 66.5 g (13.3%) EtOH extract. The dry extract was stored in a refrigerator at -20°C until use in the experiments. CIE was dissolved in phosphate buffered solution (PBS) and diluted in cultured medium before use, and the control group was made up of medium, PBS and the cells.
Reagents, antibodies, cells, and culture medium
A human MHCC97H HCC cell line was purchased from the Liver Cancer Institute of Fudan University (Shang Hai, China). A human ECV304 cell line was obtained from the Cell Bank of Academia Sinica (Shang Hai, China). MHCC97H cells and ECV304 cells were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS) in a humidified incubator containing 5% CO2 in air at 37°C before use and subcultured with 0.25% trypsin-0.02% EDTA. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), rhodamine 123 (Rh123), annexin V and propidium iodide (PI) were purchased from Sigma Corporation (Sigma, St.Louis, MO, USA). DMEM, FBS and trypsin were obtained from GibcoBRL, Grand Island, NY, USA. Anti-caspase-3, anti-caspase-9, anti-cytochrome C, and anti-β-actin were purchased from Santa Cruz Company. Anti-CDK4 and P21 were obtained from eBioscience Corporation.
Hepatocyte preparation, culture and viability assay
Hepatocytes were isolated by the collagenase perfusion method[9] from 10-wk-old male Sprague-Dawley rats anesthetized with intraperitoneal administration of ketamine. The viability of the isolated hepatocytes was over 90% as determined by 0.2% trypan blue exclusion. The cells were plated in 35 mm plastic dishes at a density of 3 × 105 cells/mL in 2 mL of Williams’ Medium E supplemented with 10% FBS, and were cultured in a humidified atmosphere of 5% CO2 and 95% air at 37°C overnight. After overnight incubation, the culture medium was changed to fresh medium, and cultures were incubated with varying concentrations of CIE 400, 800 and 1200 μg/mL for 24 h. According to a previous report[25], the cells were then trypan blue stained and a hemocytometer was used to determine the total cell count and viable cell number. Viability of cells were determined as follows: viability (%) = viable cell number/total cell count × 100%.
Cell viability assay
ECV304 cells were seeded in a 96-well plate (5 × 104 cells/well). After 24 h seeding, cells were treated with CIE (400, 800 and 1200 μg/mL) for 24 h, in 3 parallel wells each, with untreated cells serving as a control, then the MTT assay as described by Xiao et al[26] was performed. At 24 h, 20 μL MTT solution (5 mg/mL) was added to each well and incubated for a further 4 h. The medium was removed and 200 μL DMSO was added to each well. Absorbance (A) at 570 nm was measured using a microculture reader. The percentage of viable cells was calculated as follows: (A of experimental group/A of control group) × 100%.
MHCC97H cells were seeded into 96-well plates at a density of 5 × 104/well and were then incubated with different concentrations of CIE (400, 800 and 1200 μg/mL) for 24, 48 and 72 h, then the MTT assay was performed. At 24, 48 and 72 h, 20 μL MTT solution (5 mg/mL) was added to each well, and the cells were further incubated at 37°C for 4 h. The MTT assay and calculation of the percentage of viable cells were the same as described above.
Apoptosis assays
In accordance with the study of Chen et al[27], the apoptotic rates were analyzed by flow cytometry using an annexin V-FITC/PI kit. Staining was performed according to the manufacturer’s instructions, and flow cytometry was conducted on a FACS Caliber (Becton Dickinson, Mountain View, NJ, USA). Cells that were annexin V (-) and PI (-) were considered viable cells. Cells that were annexin V (+) and PI (-) were considered early apoptotic cells. Cells that were annexin V (+) and PI (+) were considered late apoptotic cells.
Cell cycle assays
Cell cycle analyses were carried out by the method of Vinodhkumar et al[28]. Briefly, cells were incubated in culture media alone or culture media containing 400-1200 μg/mL of CIE, at 37°C for 48 h. Cells were harvested in cold PBS, fixed in 70% EtOH, and stored at 4°C. Fixed cells were washed with PBS once and suspended in 1 mL of PI staining reagent 50 mg/mL containing 100 μg/mL Rnase, and were then incubated in the dark for 30 min. The distribution of the cell cycle was measured by a Becton Dickinson FACS analysis system and quantitation of cell cycle distribution was carried out using Multicycle Software.
Detection of mitochondrial membrane potential (MMPΔψm)
Loss of MMPΔψm was assessed by flow cytometry, using a fluorescent indicator Rh123, as described by Tang et al[29] and Li et al[30]. Briefly, cells were treated with different concentrations of CIE. Then, Rh123 working solution was added to the culture at a final concentration of 2 μg/mL and then incubated in the dark at 37°C for 30 min. Cells were then washed with PBS, and fluorescence of Rh123 was detected immediately using a FACS Caliber, at an excitation wavelength of 488 nm and emission wavelength of 525 nm.
Western blotting
Cancer cells (2.5 × 107/well) were treated with different concentrations of CIE for 24 or 48 h. To extract cytoplasmic protein as by the method of Li et al[31], cells were collected by centrifugation at 200 r/min for 10 min at 4°C. The cells were washed twice with ice-cold PBS, followed by centrifugation at 200 r/min for 5 min. The cell pellet was then suspended in ice-cold cell extraction buffer for 30 min on ice. In addition, as described by Hsu et al[32], cells were then lysed in a sample buffer, followed by sonication and denaturation. Protein concentrations were measured using DC Protein Assay (Bio-Rad, Hercules, CA) and equal amounts of protein (50 μg) were subjected to SDS-PAGE on 12% gel. The proteins were then electrophoretically transferred to nitrocellulose membranes and processed for immunoblotting. Membranes were first blocked with 5% non-fat dry milk overnight at 37°C and immunolabeled using primary antibodies. Goat anti-rabbit horseradish peroxidase-conjugated antibodies (Cell Signaling Technology, Beverly, MA) were used as secondary antibodies and detected with enhanced chemiluminescence (Amersham, USA). Equal loading of each lane was evaluated by immunoblotting using the same membranes with β-actin antibodies after detachment of previous primary antibodies. The band density for the target protein in each sample was measured with image analysis software (Gene Genus, Gene Company) and normalized to β-actin expression.
Statistical analysis
All data were expressed as mean ± SE. Statistical analysis was performed with analysis of variance (ANOVA) using the statistical software SPSS 11.0. P-values < 0.05 were regarded as statistically significantly.
RESULTS
Effect of CIE on numbers of viable rat hepatocytes and ECV304 cells
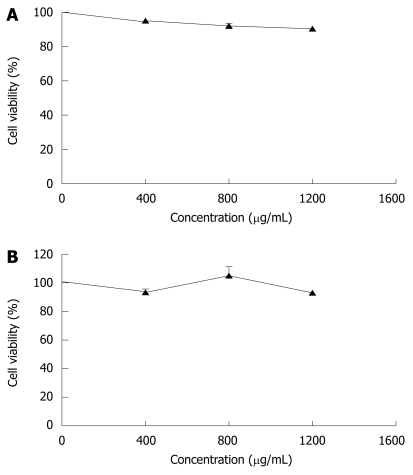
In order to compare the effects of CIE on rat hepatocytes and human ECV304 cells, the numbers of viable cells were measured. As shown in Figure 1A, CIE did not decrease the number of viable rat hepatocytes, used as a normal cell model. To confirm the activity of CIE in human cells, we measured the number of viable cells treated with varying concentrations of CIE in human endothelial cells (ECV304). CIE did not reduce the number of viable ECV304 cells at any dose (Figure 1B). Therefore, the effect of CIE in inhibiting proliferation of MHCC97H cells and its mechanism were examined in subsequent experiments.
Figure 1.
Effects of Chrysanthemum indicum extract (CIE) on cell viability of normal cells. A: Rat hepatocytes; B: Human umbilical vein endothelial cell line ECV304. Various concentrations of CIE were added, and the cells were incubated for 24 h. Cell viability was measured by 0.2% trypan blue exclusion (A) and MTT assay (B) respectively. Results presented are representative of 3 independent experiments.
Cytotoxic activities of CIE against human HCC cells
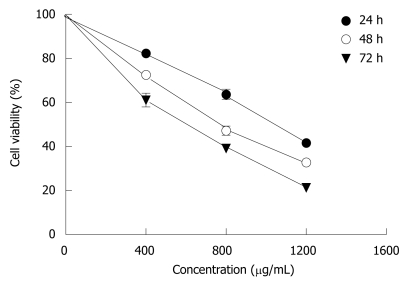
When MHCC97H cells were incubated with 400-1200 μg/mL CIE for 24, 48, 72 h, as shown in Figure 2, there was a significant dose-dependent reduction in cell viability. The IC50 value at 24 h was 1009 ± 130 μg. When 1200 μg/mL CIE was incubated with cancer cells for 72 h, viable cells amounted to only 25% of control. These findings indicated that CIE significantly decreased proliferation of MHCC97H cells in a dose- and time-dependent manner. Hence, the proliferation inhibitory effect of CIE on MHCC97H and its mechanisms were tested in the following experiments.
Figure 2.
Dose-response curves for CIE in MHCC97H cells, following 24 h, 48 h and 72 h incubation, as assessed by MTT assay. CIE produced a concentration- and time-dependent decrease in cellular proliferation. Results presented are representative of 3 independent experiments.
CIE induces MHCC97H cell apoptosis
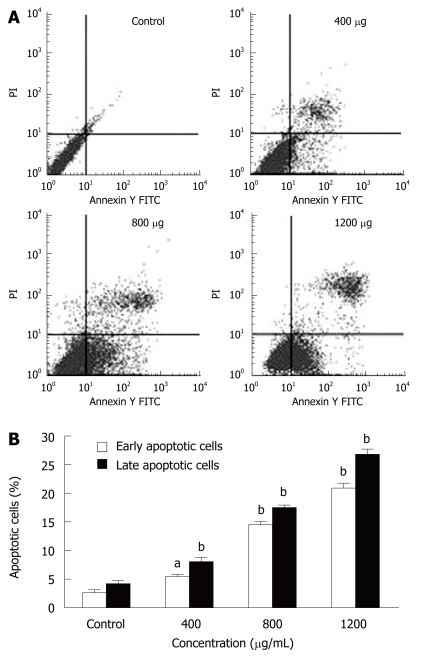
MHCC97H cells were incubated with different CIE concentrations (400, 800 and 1200 μg/mL) for 24 h and were analyzed by flow cytometry. Pretreatment of MHCC97H cells with various concentrations of CIE induced significant apoptosis (Figure 3A). The numbers of early and late apoptotic cells were significantly increased compared with the control group (Figure 3B). The proportion of early and late apoptotic cells in the 1200 μg/mL treatment group was more than 10 times higher than in the drug-free cells.
Figure 3.
Effect of CIE on the induction of MHCC97H cell apoptosis. A: The apoptosis of MHCC97H cells induced by CIE were determined by flow cytometry at 24 h; B: CIE induced a concentration-dependent increase in early and late cellular apoptosis. Results presented are representative of 3 independent experiments. aP < 0.05, bP < 0.01 vs control group.
Effects of CIE on cell morphology
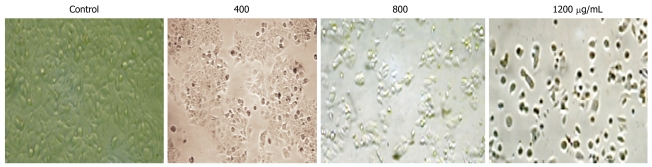
After incubation with CIE at different concentrations (400, 800, 1200 μg/mL), the cells were examined by phase contrast microscopy for evidence of morphological apoptosis induced by CIE (Figure 4). The control cells showed a typical polygonal and intact appearance (control), whereas the CIE-treated cells displayed morphological changes with preapoptotic characteristics, such as cellular shrinkage (400, 800 μg/mL), rounding (800 μg/mL), and poor adherence (1200 μg/mL), as well as round floating shapes (1200 μg/mL).
Figure 4.
Morphological changes in MHCC97H cells with CIE. Cells were observed by phase contrast microscopy in controls and after treatment with 400, 800, 1200 μg/mL CIE (× 200).
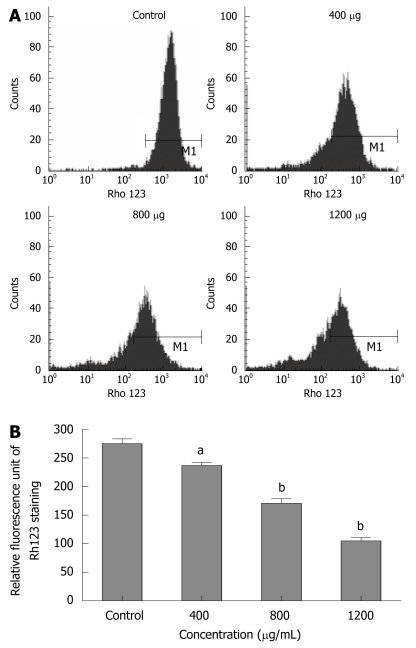
CIE causes loss of MMPΔψm
To explore whether CIE-induced apoptosis involved the MMPΔψm, we used a fluorescent indicator, Rh123 to detect the MMPΔψm when MHCC97H cells were treated with 400-1200 μg/mL of CIE for 24 h. As shown in Figure 5A and B, after exposure to different CIE doses, cells exhibited much lower Rh123 staining (236.7 ± 9.3, 170.7 ± 13.9, 105 ± 10.5) than controls (275 ± 14.5: P < 0.05 or P < 0.01), indicating that CIE can significantly decrease MMPΔψm associated with cancer cell apoptosis.
Figure 5.
Effect of CIE on MHCC97H cellular mitochondrial membrane potential (MMPΔψm). A: The MMPΔψm of MHCC97H cells were determined by flow cytometry at 24 h after CIE; B: Results presented are representative of 3 independent experiments. aP < 0.05, bP < 0.01 vs control group.
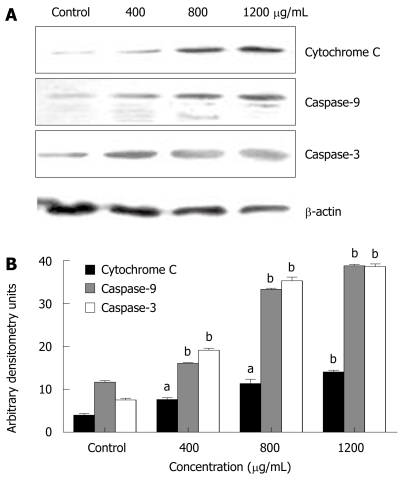
CIE-induced apoptosis is caspase-dependent
To determine whether apoptosis induced by CIE was a mitochondrial-dependent caspase pathway, we further tested whether cytochrome C could be released from the mitochondria into the cytoplasm. As shown in Figure 6A, although there was no detectable cytochrome C in the cytosolic fraction of continuously growing MHCC97H cells, the level of cytochrome C released from the mitochondria increased dose-dependently in the presence of CIE concentrations ranging from 400 to 1200 μg/mL. In accordance with mitochondrial cytochrome C release into the cytoplasm, caspase-9 protein expression was increasingly detected. Accordingly, caspase-3 protein expression was also increased dose-dependently on exposure to CIE (Figure 6A and B). Taken together, these findings suggest that CIE exerted a significant apoptotic effect on MHCC97H cells in a concentration-dependent manner through the mitochondrial pathway, and was accompanied by a decrease in MMPΔψm, release of cytochrome C, and activation of caspase-9 and caspase-3.
Figure 6.
Effect of CIE on apoptosis-related protein expression. A: The expressions of cytochrome C, caspase-9 and caspase-3 were assessed by Western blotting; B: Results presented are representative of 3 independent experiments. aP < 0.05, bP < 0.01 vs control group.
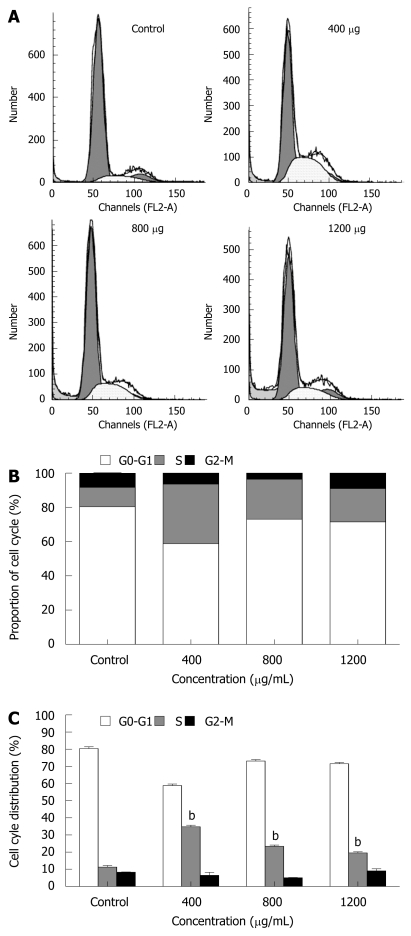
Cell cycle analysis
The cell cycle of cancer cells was also determined by flow cytometry. MHCC97H cells treated with CIE 400, 800, 1200 μg/mL for 48 h showed an accumulation of cells in the S phase of the cell cycle. In contrast, the population of cells in G0-G1and G2/M phases was significantly decreased, especially at 400 μg/mL CIE (Figure 7A-C). In addition, as shown in Figure 7A, cancer cells incubated with higher doses of CIE for 48 h also showed a sub-G1 peak indicating apoptosis. These observations suggest that a small number of cancer cells escape from the S phase and undergo apoptosis, particularly at the 1200 μg/mL CIE concentration. Therefore, with the dose-dependent increase in cancer cell apoptosis, the proportion of cells arrested in the S phase by CIE decreased as the CIE concentration increased from 400 to 1200 μg/mL.
Figure 7.
Effect of CIE on MHCC97H cell cycle. A: The cell cycle of MHCC97H cells in the presence of CIE was determined by flow cytometry at 48 h; B and C: Results presented are representative of 3 independent experiments. aP < 0.05, bP < 0.01 vs control group.
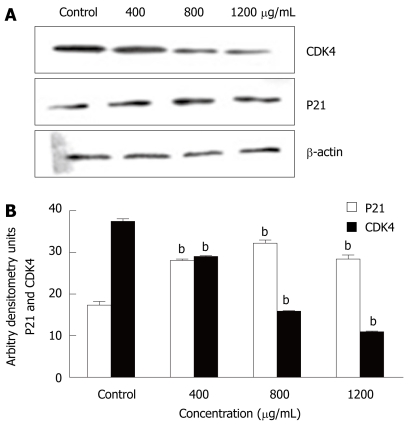
The mechanism of action of CIE on the cell cycle
To determine the mechanism by which CIE arrested the cell cycle in the S phase, Western blotting was used to determine the expression levels of cell cycle-regulating proteins including P21 and CDK4. P21 protein expression was markedly higher than that of the control group at all CIE doses tested. In contrast, CDK4 levels were significantly lower than that of the control group, as shown in Figure 8A and B. The results suggested that CIE could arrest the cell cycle via upregulation of P21 and downregulation of CDK4.
Figure 8.
Effect of CIE on cell cycle-related protein expression. A: The expression of CDK4 and P21 was assessed by Western blotting; B: Results presented are representative of 3 independent experiments. bP < 0.01 vs control group.
DISCUSSION
So far, the underlying mechanisms of the pharmacological effect of Chrysanthemum indicum in cancer therapy have been unclear, and this study examined the effect of CIE and its underlying mechanisms on inhibition of tumor cell proliferation. In the present study, we have demonstrated that CIE potently inhibits the proliferation of MHCC97H cells by inducing apoptosis (Figures 3 and 4) and arresting the cell cycle (Figure 7) but has no cytotoxicity in rat hepatocytes and human endothelial cells (ECV304) that were used as representatives of normal cells (Figure 1).
Morphological changes in apoptotic characteristics, such as cellular shrinkage, rounding, poor adherence, and round floating shapes in CIE-treated cells were also observed by phase-contrast microscopy (Figure 4). The induction of cancer cell apoptosis without side effects is recognized as an important target in cancer therapy. Apoptosis triggered by activation of the mitochondrial-dependent caspase pathway represents the main programmed cell death mechanism[19]. The mitochondrial-dependent apoptosis pathway is activated by various intracellular stresses that induce permeabilization of the mitochondrial membrane, leading to cytochrome C release[33]. Flow cytometry with Rh123 staining showed disruption of MMPΔψm in the CIE-treated cells (Figure 5), indicating that the mitochondrial apoptotic pathway played a pivotal role in CIE-induced apoptosis of MHCC97H cells. Furthermore, cytosolic cytochrome C activates pro-caspase-9 by binding to Apaf-1 and in the presence of dATP, and contributes to activation of caspase-9 and caspase-3, thus triggering apoptosis[34,35]. In this study, caspase-9 and caspase-3 levels reached a maximum at about 24 h in the cells after exposure to CIE 1200 μg/mL (Figure 6). These results indicated that CIE-induced apoptosis of MHCC97H cells was mediated by loss of MMPΔψm, increased cytosolic translocation of cytochrome C, and activation of caspase-9 and caspase-3.
The inhibition of tumor cell growth without toxicity in normal cells has attracted attention as an important target in cancer therapy. Dysregulation of the cell cycle mechanism has also been shown to play an important role in various cancer cell growths, including HCC. In this study, CIE inhibited MHCC97H cell proliferation partly as a result of accumulation of cells in the S phase of the cell cycle. The present study, to the best of our knowledge, is also the first to demonstrate that CIE induced arrest of the cell cycle in the S phase in HCC cells (Figure 7). The S phase is associated with DNA synthesis and plays a crucial role in cell cycle progression. Recently, a series of S phase chemotherapeutic agents such as Smilax glabra Roxb[36], baicalein from Scutellariae radix roots[37] and others[38] have been found to inhibit cancer cells, including HCC. Furthermore, in accordance with these results, CIE upregulated P21 and downregulated CDK4 (Figure 8), indicating that cell cycle-related proteins were involved in the CIE-induced cell cycle arrest in MHCC97H cells. One of the CDKI proteins, P21 can perform a key function in controlling cell cycle progression by negatively regulating CDK4 activity[38]. Inappropriate expression of cell cycle-related proteins, such as CDK4 and P21, could be one of the major factors contributing to HCC development[39]. Moreover, CDK4 and P21 play important roles in regulation of the S phase of the cell cycle[37,38,40,41]. These findings, taken together with the present study, suggest that upregulation of P21 and downregulation of CDK4 are likely to be involved in the S phase arrest induced by CIE in HCC cells.
Additionally, in clinical studies, Chrysanthemum indicum can be used in combination with other chemotherapeutic agents or traditional Chinese medicines in treatment of other cancers. Xiang et al[42] found that patients with metastatic breast cancer postoperatively receiving Chrysanthemum indicum as one of the main components, in combination with other traditional Chinese medicines, had a 5-year overall survival rate of 70% and a complete response rate of 60%, and in combination with chemotherapeutic agents, had a 5-year overall survival rate of 77% and a complete remission rate of 80%, without adverse effects. Bi et al[43] demonstrated that Chrysanthemum indicum, in combination with traditional Chinese medicines, achieved a response rate of 67% in advanced stage esophageal carcinoma patients, without myelosuppression or toxicities of the liver and kidney.
In conclusion, different effects of CIE treatment were observed in cancer and normal cells. CIE exerted a significant apoptotic effect on MHCC97H cells through the mitochondrial-dependent caspase-3 pathway. It arrested the cell cycle of cancer cells in the S phase by upregulation of P21 and downregulation of CDK4. In addition, the cancer-specific selectivity shown in this study suggests that the herb could be a promising novel plant with potential in the treatment of human cancer without side effects.
COMMENTS
Background
Ethnopharmacology used in folk medicine continues to be an important source of discovery and development of novel therapeutic agents in cancer. The flowers of Chrysanthemum indicum, a Compositae plant, is common in ethnopharmacology, and has long had wide spread use in the treatment of hypertension, colitis, pneumonia and carbuncles by traditional Chinese practitioners. Recently, much attention has been devoted to the anticancer activity of Chrysanthemum indicum, especially in hepatocellular carcinoma (HCC). However, the underlying mechanisms of the pharmacological effect of the plant extract in cancer therapy have been largely undetermined.
Research frontiers
Induction of apoptosis and arrest of the cell cycle by plant extracts has become a principal mechanism by which anticancer therapy is effective. Apoptosis triggered by the activation of the mitochondrial-dependent caspase pathway represents the main programmed cell death mechanism. Permeabilization of the outside mitochondrial membrane plays a vital role in cell apoptosis, during which loss of the mitochondrial membrane potential and release of cytochrome C into the cytosol, followed by caspase-9-dependent activation of caspase-3 occurs, resulting in apoptosis. Dysregulation of the cell cycle mechanism has also been shown to perform an important function in growth of various cancer cells, including HCC. The S phase is associated with DNA synthesis and plays a crucial role in cell cycle progression. One of the CDKIs, P21, can influence key functions in the control of the cell cycle by negatively regulating CDK4 activity, and plays an important role in regulation of the S phase of the cell cycle.
Innovations and breakthroughs
So far, there has been no evidence found to show that the mitochondrial pathway is involved in induction of apoptosis and cell cycle arrest by Chrysanthemum indicum extract (CIE) in human HCC cells. Therefore, the present study examined the anticancer activities of CIE and related mechanisms in MHCC97H cell lines. The data showed that CIE could induce apoptosis and arrest the cell cycle of MHCC97H cells. CIE exerted a significant apoptotic effect on MHCC97H cells through the mitochondrial-dependent caspase-3 pathway, and arrested the cell cycle in the S phase in cancer cells by upregulation of P21 and downregulation of CDK4.
Applications
This study suggests that Chrysanthemum indicum could be a promising plant with potential in the novel treatment of human cancer, particularly HCC.
Peer review
The manuscript written by Li ZF et al describes that Chrysanthemum indicum extract can induce apoptosis and cell cycle arrest in a hepatoma cell line. Many patients with HCC still die each year, and novel therapeutic strategies are needed. The data are encouraging and promising.
Footnotes
Supported by Grants From the National Natural Science Foundation of China, No. 30672766 and Science and Technology Developing Foundation of Shaanxi Province, China, No. 2006 K16-G4 (1)
Peer reviewer: Dr. Yukihiro Shimizu, Kyoto Katsura Hospital, 17 Yamada-Hirao, Nishikyo, Kyoto 615-8256, Japan
S- Editor Li LF L- Editor Cant MR E- Editor Ma WH
References
- 1.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, et al. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chau GY, Lui WY, Tsay SH, Chao Y, King KL, Wu CW. Postresectional adjuvant intraportal chemotherapy in patients with hepatocellular carcinoma: a case-control study. Ann Surg Oncol. 2006;13:1329–1337. doi: 10.1245/s10434-006-9004-1. [DOI] [PubMed] [Google Scholar]
- 3.Ono T, Yamanoi A, Nazmy El Assal O, Kohno H, Nagasue N. Adjuvant chemotherapy after resection of hepatocellular carcinoma causes deterioration of long-term prognosis in cirrhotic patients: metaanalysis of three randomized controlled trials. Cancer. 2001;91:2378–2385. [PubMed] [Google Scholar]
- 4.Dai ZJ, Wang XJ, Li ZF, Ji ZZ, Ren HT, Tang W, Liu XX, Kang HF, Guan HT, Song LQ. Scutellaria barbate extract induces apoptosis of hepatoma H22 cells via the mitochondrial pathway involving caspase-3. World J Gastroenterol. 2008;14:7321–7328. doi: 10.3748/wjg.14.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson IT. Phytochemicals and cancer. Proc Nutr Soc. 2007;66:207–215. doi: 10.1017/S0029665107005459. [DOI] [PubMed] [Google Scholar]
- 6.Greenwald P. Cancer chemoprevention. BMJ. 2002;324:714–718. doi: 10.1136/bmj.324.7339.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Wang LJ, Qiu GF, Yu JQ, Liang SC, Hu XM. Apoptosis of Hela cells induced by extract from Cremanthodium humile. Food Chem Toxicol. 2007;45:2040–2046. doi: 10.1016/j.fct.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Li WY, Chiu LC, Lam WS, Wong WY, Chan YT, Ho YP, Wong EY, Wong YS, Ooi VE. Ethyl acetate extract of Chinese medicinal herb Sarcandra glabra induces growth inhibition on human leukemic HL-60 cells, associated with cell cycle arrest and up-regulation of pro-apoptotic Bax/Bcl-2 ratio. Oncol Rep. 2007;17:425–431. [PubMed] [Google Scholar]
- 9.Norikura T, Kojima-Yuasa A, Shimizu M, Huang X, Xu S, Kametani S, Rho SN, Kennedy DO, Matsui-Yuasa I. Mechanism of growth inhibitory effect of Blumea balsamifera extract in hepatocellular carcinoma. Biosci Biotechnol Biochem. 2008;72:1183–1189. doi: 10.1271/bbb.70586. [DOI] [PubMed] [Google Scholar]
- 10.Shunying Z, Yang Y, Huaidong Y, Yue Y, Guolin Z. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J Ethnopharmacol. 2005;96:151–158. doi: 10.1016/j.jep.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Cheng W, Li J, You T, Hu C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linne. J Ethnopharmacol. 2005;101:334–337. doi: 10.1016/j.jep.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Chen XY, Li J, Cheng WM, Jiang H, Xie XF, Hu R. Effect of total flavonoids of Chrysanthemum indicum on the apoptosis of synoviocytes in joint of adjuvant arthritis rats. Am J Chin Med. 2008;36:695–704. doi: 10.1142/S0192415X08006168. [DOI] [PubMed] [Google Scholar]
- 13.Lee do Y, Choi G, Yoon T, Cheon MS, Choo BK, Kim HK. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. J Ethnopharmacol. 2009;123:149–154. doi: 10.1016/j.jep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Chun HS, Kim JM, Choi EH, Chang N. Neuroprotective effects of several korean medicinal plants traditionally used for stroke remedy. J Med Food. 2008;11:246–251. doi: 10.1089/jmf.2007.542. [DOI] [PubMed] [Google Scholar]
- 15.Cai HF. The research progression of Flos Chrysanthemum indicum on chemical constituent and medical application. Zhongguo Yiliao Qianyan. 2007;2:118–120. [Google Scholar]
- 16.Jin SR, Zhu BD, Qin XH. The effect of Chrysanthemum indicum on SMMC7721, PC3 and HL60 cell lines. Zhongyao Yaoli Yu Linchuang. 2005;21:39–41. [Google Scholar]
- 17.Wu DH, Yang LW, SuWW The research progression of Chrysanthemum indicum on chemical constituent and pharmacology. Zhongyaocai. 2004;27:142–144. [Google Scholar]
- 18.Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Go HY, Jin DH, Kim HP, Hong MH, Chung WY, Park JH, Jang JB, Jung H, Shin YC, et al. Inhibition of the PI3K-Akt/PKB survival pathway enhanced an ethanol extract of Rhus verniciflua Stokes-induced apoptosis via a mitochondrial pathway in AGS gastric cancer cell lines. Cancer Lett. 2008;265:197–205. doi: 10.1016/j.canlet.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 20.Choi EJ, Kim GH. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine. 2008;15:683–690. doi: 10.1016/j.phymed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Tsai SL, Suk FM, Wang CI, Liu DZ, Hou WC, Lin PJ, Hung LF, Liang YC. Anti-tumor potential of 15,16-dihydrotanshinone I against breast adenocarcinoma through inducing G1 arrest and apoptosis. Biochem Pharmacol. 2007;74:1575–1586. doi: 10.1016/j.bcp.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Sun J, Hai Liu R. Cranberry phytochemical extracts induce cell cycle arrest and apoptosis in human MCF-7 breast cancer cells. Cancer Lett. 2006;241:124–134. doi: 10.1016/j.canlet.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Mishra KP, Padwad YS, Dutta A, Ganju L, Sairam M, Banerjee PK, Sawhney RC. Aqueous extract of Rhodiola imbricata rhizome inhibits proliferation of an erythroleukemic cell line K-562 by inducing apoptosis and cell cycle arrest at G2/M phase. Immunobiology. 2008;213:125–131. doi: 10.1016/j.imbio.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Zhou J, Fan J, Tan CJ, Qiu SJ, Yu Y, Huang XW, Tang ZY. Sirolimus inhibits the growth and metastatic progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:715–722. doi: 10.1007/s00432-008-0506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan ZC, Chen D, Wu XZ, Xie GR, Ba Y, Yan Z. Effects of aqueous extracts of Aconitum carmichaeli, Rhizoma bolbostemmatis, Phytolacca acinosa, Panax notoginseng and Gekko swinhonis Guenther on Bel-7402 cells. World J Gastroenterol. 2007;13:2743–2746. doi: 10.3748/wjg.v13.i19.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao YF, Wu DD, Liu SX, Chen X, Ren LF. Effect of arsenic trioxide on vascular endothelial cell proliferation and expression of vascular endothelial growth factor receptors Flt-1 and KDR in gastric cancer in nude mice. World J Gastroenterol. 2007;13:6498–6505. doi: 10.3748/wjg.v13.i48.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen NY, Lai HH, Hsu TH, Lin FY, Chen JZ, Lo HC. Induction of apoptosis in human lung carcinoma A549 epithelial cells with an ethanol extract of Tremella mesenterica. Biosci Biotechnol Biochem. 2008;72:1283–1289. doi: 10.1271/bbb.70773. [DOI] [PubMed] [Google Scholar]
- 28.Vinodhkumar R, Song YS, Devaki T. Romidepsin (depsipeptide) induced cell cycle arrest, apoptosis and histone hyperacetylation in lung carcinoma cells (A549) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression. Biomed Pharmacother. 2008;62:85–93. doi: 10.1016/j.biopha.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Tang W, Liu JW, Zhao WM, Wei DZ, Zhong JJ. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci. 2006;80:205–211. doi: 10.1016/j.lfs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Lu Q, Shen Y, Hu X. Schisandrin B enhances doxorubicin-induced apoptosis of cancer cells but not normal cells. Biochem Pharmacol. 2006;71:584–595. doi: 10.1016/j.bcp.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Wang LJ, Qiu GF, Yu JQ, Liang SC, Hu XM. Apoptosis of Hela cells induced by extract from Cremanthodium humile. Food Chem Toxicol. 2007;45:2040–2046. doi: 10.1016/j.fct.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Hsu YL, Kuo PL, Cho CY, Ni WC, Tzeng TF, Ng LT, Kuo YH, Lin CC. Antrodia cinnamomea fruiting bodies extract suppresses the invasive potential of human liver cancer cell line PLC/PRF/5 through inhibition of nuclear factor kappaB pathway. Food Chem Toxicol. 2007;45:1249–1257. doi: 10.1016/j.fct.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Won HJ, Han CH, Kim YH, Kwon HJ, Kim BW, Choi JS, Kim KH. Induction of apoptosis in human acute leukemia Jurkat T cells by Albizzia julibrissin extract is mediated via mitochondria-dependent caspase-3 activation. J Ethnopharmacol. 2006;106:383–389. doi: 10.1016/j.jep.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 35.Cheng AC, Jian CB, Huang YT, Lai CS, Hsu PC, Pan MH. Induction of apoptosis by Uncaria tomentosa through reactive oxygen species production, cytochrome C release, and caspases activation in human leukemia cells. Food Chem Toxicol. 2007;45:2206–2218. doi: 10.1016/j.fct.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Sa F, Gao JL, Fung KP, Zheng Y, Lee SM, Wang YT. Anti-proliferative and pro-apoptotic effect of Smilax glabra Roxb. extract on hepatoma cell lines. Chem Biol Interact. 2008;171:1–14. doi: 10.1016/j.cbi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Lee HZ, Leung HW, Lai MY, Wu CH. Baicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cells. Anticancer Res. 2005;25:959–964. [PubMed] [Google Scholar]
- 38.Han YH, Kim SH, Kim SZ, Park WH. Antimycin A as a mitochondria damage agent induces an S phase arrest of the cell cycle in HeLa cells. Life Sci. 2008;83:346–355. doi: 10.1016/j.lfs.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Masaki T, Shiratori Y, Rengifo W, Igarashi K, Yamagata M, Kurokohchi K, Uchida N, Miyauchi Y, Yoshiji H, Watanabe S, et al. Cyclins and cyclin-dependent kinases: comparative study of hepatocellular carcinoma versus cirrhosis. Hepatology. 2003;37:534–543. doi: 10.1053/jhep.2003.50112. [DOI] [PubMed] [Google Scholar]
- 40.Shishodia S, Sethi G, Ahn KS, Aggarwal BB. Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem Pharmacol. 2007;74:118–130. doi: 10.1016/j.bcp.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song G, Chen GG, Chau DK, Miao J, Lai PB. Bid exhibits S phase checkpoint activation and plays a pro-apoptotic role in response to etoposide-induced DNA damage in hepatocellular carcinoma cells. Apoptosis. 2008;13:693–701. doi: 10.1007/s10495-008-0195-8. [DOI] [PubMed] [Google Scholar]
- 42.Xiang LP, Ouyang H, Xiao YL. The clinical observation of Juzao pill antitumor postoperative breast cancer in recurrence and metastasis. Zhongguo Linchuang Yaoli Yu Zhiliaoxue. 2002;7:63–64. [Google Scholar]
- 43.Bi X, Song XL, Zhang JZ. Analysis of xiaoliu formula treatment of esophageal carcinoma patients with advanced stage. Zhongchengyao. 2008;30:1266–1268. [Google Scholar]