Abstract
AIM: To describe a condition that we define as early graft dysfunction (EGD) which can be identified preoperatively.
METHODS: Small-for-size graft dysfunction following living-related liver transplantation (LRLT) is characterized by EGD when the graft-to-recipient body weight ratio (GRBWR) is below 0.8%. However, patients transplanted with GRBWR above 0.8% can develop dysfunction of the graft. In 73 recipients of LRLT (GRBWR > 0.8%), we identified 10 patients who developed EGD. The main measures of outcomes analyzed were overall mortality, number of re-transplants and length of stay in days (LOS). Furthermore we analyzed other clinical pre-transplant variables, intra-operative parameters and post transplant data.
RESULTS: A trend in favor of the non-EGD group (3-mo actuarial survival 98% vs 88%, P = 0.09; 3-mo graft mortality 4.7% vs 20%, P = 0.07) was observed as well as shorter LOS (13 d vs 41.5 d; P = 0.001) and smaller requirement of peri-operative Units of Plasma (4 vs 14; P = 0.036). Univariate analysis of pre-transplant variables identified platelet count, serum bilirubin, INR and Meld-Na score as predictors of EGD. In the multivariate analysis transplant Meld-Na score (P = 0.025, OR: 1.175) and pre-transplant platelet count (P = 0.043, OR: 0.956) were independently associated with EGD.
CONCLUSION: EGD can be identified preoperatively and is associated with increased morbidity after LRLT. A prompt recognition of EGD can trigger a timely treatment.
Keywords: Small-for-size graft dysfunction, Living-related liver transplantation, Graft-to-recipient body weight ratio, Partial liver transplantation, Allograft dysfunction
INTRODUCTION
Small-for-size graft dysfunction (SFSGD) is one of the greatest limiting factors for the expansion of segmental liver transplantation from living donors[1], and is characterized by: (1) onset within 2 wk after living-related liver transplantation (LRLT); (2) a graft-to-recipient body weight ratio (GRBWR) below 0.8%; (3) total bilirubin higher than 5 mg/dL, and/or output of ascites through abdominal drainages of more than 1 L/d; and (4) exclusion of technical (e.g. arterial or portal occlusion, outflow congestion, bile leak), infective (e.g. sepsis) and immunological (e.g. acute cellular rejection) complications.
By definition, SFSGD can be diagnosed only in the presence of a GRBWR of less than 0.8%, or a ratio of graft volume (GV) relative to the standard liver volume (SLV) of the recipient (GV/SLV) of less then 30%[1-4]. However, despite a GRBWR above 0.8%, some recipients of LRLT may have a worse clinical course.
The aim of this study was to analyze a group of LRLT recipients in order to identify those who developed a clinical picture of SFSGD in the absence of a GBWR of < 0.8% and with a GV/SLV ratio highest than 30%. Those patients were defined as affected by early graft dysfunction (EGD).
MATERIALS AND METHODS
We evaluated the rate of EGD in 73 consecutive recipients of adult-to-adult LRLT performed at our institute between July 2004 and September 2008, and whose GRBWR was > 0.8% and with a GV/SLV ratio higher than 30%. Follow-up in months was 27.34 ± 13.77.
There were 43 males and 30 females, with a median age of 57 years (range 18-68 years). The etiology of the liver disease was related to hepatitis C virus infection in 47 cases, to hepatitis B virus infection in nine patients, to both B and C virus infection in three patients, and to non-viral causes in 14 patients. Twenty-two patients had hepatocellular carcinoma (HCC). Donor liver resection resulted in 73 right hepatectomies (liver segments 5-8). Graft implantation was performed with the piggy back technique and, in all cases, with the use of veno-venous bypass. Details of surgical procedures are reported elsewhere[5,6]. Volumetric computed tomography (CT) scan was used to calculate liver and spleen volumes.
The main measures of outcomes analyzed were overall mortality, number of re-transplants and length of stay in days (LOS).
In order to identify predictors of EGD, epidemiologic pre-transplant variables such as age of the recipient and donor, sex of the recipient and donor, recently reported as markers of graft function[7], were evaluated (Table 1).
Table 1.
Univariate analysis of epidemiologic data in the group with EGD vs the group without EGD, median (range)
| With EGD (10 pts) | Without EGD (63 pts) | P value | |
| Age recipient | 52.72 (38-61) | 57.6 (18-68) | NS |
| Age donors | 29.5 (26-54) | 30 (18-53) | NS |
| Sex recipient (M/F) | 5/5 | 38/25 | NS |
| Sex donors (M/F) | 5/5 | 39/24 | NS |
EGD: Early graft dysfunction.
Furthermore, we analyzed other clinical pre-transplant variables such as: serum bilirubin, serum albumin, serum sodium, INR, platelets count, WBC count, Child-Pugh score, MELD score, Meld-NA score, recently described[8-10], percentage of donor liver steatosis, liver volume and spleen volume evaluated using CT, spleen/liver volume ratio (S/LVR), GBWR and GV/SLV (Table 2).
Table 2.
Univariate analysis of pre-transplant clinical data in the two groups: EGD vs non-EGD
| EGD (10 pts) | Non-EGD (63 pts) | P value | |
| Pre-transplant serum bilirubin (mg/dL) | 8.71 (1.27-29.21) | 2.01 (0.28-24.82) | 0.013 |
| 1Pre-transplant serum albumin (g/dL) | 2.6 (2.2-3.3) | 2.8 (1.31-4) | NS |
| Pre-transplant serum sodium (mEq/L) | 133 (122-145) | 138 (126-144) | NS |
| Pre-transplant INR | 1.38 (1.27-2.55) | 1.22 (0.81-2.55) | 0.001 |
| Pre-transplant platelets (mmc) | 48 000 (22 000-60 000) | 71 000 (24-400) | 0.007 |
| Pre-transplant WBC (mmc) | 4575 (1700-7200) | 4200 (1500-15 500) | NS |
| Child-Pugh score, points | 10.00 (8-12) | 8.0 (5-12) | NS |
| MELD score | 20.50 (12-40) | 15.0 (6-28) | NS |
| Meld-Na score | 24.25 ± 7.9 | 18.13 ± 5.8 | 0.004 |
| Steatosis (No/Macro 1%-2%/Macro 10%-20%/Macro 25%-30%) | (6/2/2/0) | (35/15/9/4) | NS |
| Liver volume (mL) | 780 (590-1186) | 1016 (557-1482) | NS |
| Spleen volume (mL) | 983 (648-1382) | 709 (161-2711) | NS |
| S/LVR | 0.96 (0.55-2.34) | 0.78 (0.13-2.95) | NS |
| GRDWR | 1.26 (0.79-1.59) | 1.48 (0.81-2.96) | NS |
| GS/SLV | 59.52 (37.34-70.19) | 68.5 (38.7-132.6) | NS |
Neither group of patients received albumin supplementation before transplant. S/LVR: Spleen/liver volume ratio; GRDWR: Graft-to-recipient body weight ratio; GS/SLV: Graft-to-recipient standard liver volume. Data are expressed as mean (range), or mean ± SD.
Then we observed the following intra-operative parameters: mean arterial pressure, systemic vascular resistance, cardiac output, cardiac index, units of transfused packed red blood cells, units of transfused platelets, and units of transfused fresh frozen plasma (Table 3).
Table 3.
Univariate analysis of intraoperative parameters in the two groups: EGD vs non-EGD
| EGD (10 pts) | Non-EGD (63 pts) | P value | |
| MAP, mmHg | 73.8 ± 13 | 76.7 ± 12 | NS |
| SVR dyn·s·cm-5 | 676 (350-1429) | 704 (308-1249) | NS |
| C/O, L/min | 6.9 (3.7-12) | 9.4 (6-11.8) | NS |
| C/I, L/min per meter2 | 4.5 (3.6-7.5) | 4.2 (2.2-6.4) | NS |
| PRBC | 12 (0-47) | 3 (0-34) | NS |
| Units plasma transfused | 14 (0-47) | 4 (0-34) | 0.036 |
MAP: Mean arterial pressure; SVR: Systemic vascular resistance; C/O: Cardiac output; C/I: Cardiac index; PRBC: Units of transfused packed red blood cells. Data are expressed as mean (range), or mean ± SD.
Finally as post transplant data we looked at the LOS.
Statistical analysis
Survival analysis was performed using the Kaplan-Meier analysis with SPSS (SPSS Inc., Chicago, Ill, United States), and a descriptive analysis was used for the outcome. Normality was tested with the Wilk-Shapiro test. Differences between the two groups were tested using the unpaired Student’s t-test, Mann-Whitney test, χ2 test; P < 0.05 were considered significant. Multivariate analysis was performed to identify independent determinants for occurrence of EGD (logistic regression stepwise backward procedure).
RESULTS
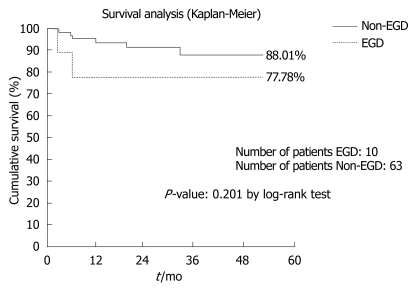
Ten out of 73 patients (13.7%) fit our criteria for EGD. No statistically significant differences were found between EGD and non-EGD recipients in terms of 3-mo patient and graft mortality [one patient out of ten (10%) vs one patient out of 63 (1.6%), P = 0.13; two patients out of ten (20%) vs three patients out of 63 (4.7%), P = 0.07], number of re-transplants during the first 3 mo after LRLT [one patient out of ten (10%) vs two patients out of 63 (3.2%), P = 0.33] and 3-mo and 1-year actuarial patient survival (88% vs 98%: P = 0.09 by the log-rank test; 80% vs 94%, P = 0.12 by the log-rank test).
The 4-year actuarial patient survival was 77.78% vs 88.01%, (P = 0.201 by the log-rank test) (Figure 1). Although the statistical analysis doesn’t indicate any statistical significance, probably due to the small size of the sample examined, the survival analysis points out a lower survival rate (77.78%) on the EGD patient vs non-EGD patient (88.01%); this is clinically relevant.
Figure 1.
Survival analysis.
In the EGD patients, we observed two deaths: one because of sepsis and the second one due to multiorgan failure. In the non-EGD group, we observed six deaths: three because of neoplastic recurrence of HCC and three due to multiorgan failure. HCC recurrence could be explained by the advanced stage of the tumor at the pathologic examination, although the patients were classified within Milan criteria.
We did observe a significant difference between the two groups in terms of LOS, with the EGD group having a longer median LOS (13 d vs 41 d, P = 0.001) and greater median number of units of plasma transfused during surgery (4 vs 14, P = 0.036).
At univariate analysis of the variables collected, INR, platelet count, serum bilirubin and Meld-Na score, were identified as predictors of EGD (Table 3).
In the multivariate analysis (logistic regression, backward stepwise procedure), we analyzed INR, platelet count, serum bilirubin and Meld-Na score. Meld-Na score (P = 0.025, OR: 1.175) and pre-transplant platelet count (P = 0.043, OR: 0.956) were the variables independently associated with occurrence of EGD (Table 4).
Table 4.
Multivariate analysis of pre-transplant epidemiologic and clinical data in the two groups: EGD vs non-EGD (logistic regression, backward stepwise procedure)
| P value | OR | 95% CI per OR | |
| Meld-Na score | 0.025 | 1.175 | 1.021; 1.352 |
| Pre-transplant platelets, mmc | 0.043 | 0.956 | 0.915; 0.999 |
In conclusion, the main clinical outcomes of the two groups were not statistically significant in terms of both early and late patient survival, probably because of the small size of the sample. In fact, as the survival rate was 77.78% vs 88.01% for EGD and non-EGD patients, we can hypothesize that survival rate acquires a statistically significant difference by enrolling a larger number of patients.
DISCUSSION
A GRBWR below 0.8% is considered mandatory for the diagnosis of SFSGD. Despite these findings in the literature, there are few patients who fully develop SFSGD by classic definition.
On the other hand, there are many patients who do not do well immediately after LRLT. We observed a clinical picture similar to that of SFSGD in patients who received partial livers that could not be described as small (GRBWR > 0.8).
In this study, the relevant clinical impact of EGD is suggested by the reduced 3-mo and 1-year patient survival and the increased graft-loss rate in the group of patients with this condition, even though there was no statistically significant difference, which is probably due to an insufficient sample size (and a small number of events). The increased LOS in the EGD group reflects the increased time of recovery. Those patients who developed EGD were in fact those with worse INR, platelet count and total bilirubin. In addition, as previously reported by Yoshizumi et al[11], we noted that patients with a higher MELD score, higher Child Pugh score and hyponatremia, tended to have a worse outcome.
In fact, in the EGD group (Table 3), these parameters were higher than in the non-EGD patient.
Our data, although not significant in accordance to others[10], are clinically relevant especially at the time of selection of donors and recipient.
Our study was also aimed at finding objective criteria for identifying those patients who had a worse clinical course in the 2 wk after LRLT, and with a GRBWR above 0.8%. Our data support the hypothesis that SFSGD and EGD have a multi-factorial genesis in which the combination of the donor’s factors (GV and quality of the graft) and the recipient’s factors (portal hypertension and stage of liver disease) lead to allograft dysfunction after partial liver transplantation[3,9,10,12].
The clinical variables identified at the univariate analysis as predictors of EGD confirmed the relevant roles of liver disease and portal hypertension in graft dysfunction.
Serum bilirubin, INR, and Meld-Na score are markers of liver function and platelet count is a marker of portal hypertension. However, at the multivariate analysis, the only variables independently associated with occurrence of EGD were Meld-Na score and pre-transplant platelet count.
The transplant community is now focused on the possibility of detecting predictive factors based on simple biochemical and imaging assessments which could allow physicians to treat those patients at risk of EGD immediately after surgery.
It has been demonstrated that in patients with cirrhosis and severe portal hypertension, the occlusion of the splenic artery causes a significant reduction in portal pressure, which is directly related to the spleen volume and indirectly related to the liver volume[13]. This concept is at the center of our strategy for performing early splenic artery embolization for the treatment of SFSGD following LRLT[14].
EGD can be identified preoperatively and is associated with increased morbidity after LRLT. Obviously, a prompt recognition of EGD can trigger a timely and appropriate treatment.
COMMENTS
Background
Small-for-size graft dysfunction (SFSGD) following living-related liver transplantation (LRLT) is characterized by early graft dysfunction (EGD) when the graft-to-recipient body weight ratio (GRBWR) is below 0.8%. However, patients transplanted with GRBWR above 0.8% can develop dysfunction of the graft.
Research frontiers
The study was aimed at finding objective criteria for identifying those patients who had a worse clinical course in the 2 wk after LRLT and had a GRBWR above 0.8%. They describe a condition that they define as EGD which can be identified preoperatively and seems to be associated with increased morbidity after LRLT.
Innovations and breakthroughs
A GRBWR below 0.8% is considered mandatory for the diagnosis of SFSGD. Despite the findings in the literature, there are few patients who fully develop SFSGD by classic definition. The authors observed a clinical picture similar to that of SFSGD in patients who received partial livers that could not be described as small (GRBWR > 0.8).
Applications
A prompt recognition of EGD can trigger a timely and appropriate treatment.
Terminology
The authors describe a condition that they define as EGD which can be identified preoperatively and seems to be associated with increased morbidity after LRLT.
Peer review
This is an important study which impacts on the field. Gruttadauria and colleagues report herein the parameters which allow preoperative identification of a condition defined as EGD i.e. the transplant Meld-Na score and pre-transplant platelet count. The study is original, well designed and performed.
Footnotes
Peer reviewer: Pietro Invernizzi, MD, PhD, Division of Internal Medicine and Hepatobiliary Immunopathology Unit, IRCCS Istituto Clinico Humanitas, via A. Manzoni 113, 20089 Rozzano, Milan, Italy
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM
References
- 1.Soejima Y, Shimada M, Suehiro T, Hiroshige S, Ninomiya M, Shiotani S, Harada N, Hideki I, Yonemura Y, Maehara Y. Outcome analysis in adult-to-adult living donor liver transplantation using the left lobe. Liver Transpl. 2003;9:581–586. doi: 10.1053/jlts.2003.50114. [DOI] [PubMed] [Google Scholar]
- 2.Man K, Fan ST, Lo CM, Liu CL, Fung PC, Liang TB, Lee TK, Tsui SH, Ng IO, Zhang ZW, et al. Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg. 2003;237:256–264. doi: 10.1097/01.SLA.0000048976.11824.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiuchi T, Tanaka K, Ito T, Oike F, Ogura Y, Fujimoto Y, Ogawa K. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9:S29–S35. doi: 10.1053/jlts.2003.50198. [DOI] [PubMed] [Google Scholar]
- 4.Ito T, Kiuchi T, Yamamoto H, Oike F, Ogura Y, Fujimoto Y, Hirohashi K, Tanaka AK. Changes in portal venous pressure in the early phase after living donor liver transplantation: pathogenesis and clinical implications. Transplantation. 2003;75:1313–1317. doi: 10.1097/01.TP.0000063707.90525.10. [DOI] [PubMed] [Google Scholar]
- 5.Gruttadauria S, Marsh JW, Cintorino D, Biondo D, Luca A, Arcadipane A, Vizzini G, Volpes R, Marcos A, Gridelli B. Adult to adult living-related liver transplant: report on an initial experience in Italy. Dig Liver Dis. 2007;39:342–350. doi: 10.1016/j.dld.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Gruttadauria S, Marsh JW, Vizzini GB, di Francesco F, Luca A, Volpes R, Marcos A, Gridelli B. Analysis of surgical and perioperative complications in seventy-five right hepatectomies for living donor liver transplantation. World J Gastroenterol. 2008;14:3159–3164. doi: 10.3748/wjg.14.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsuragawa H, Yamamoto M, Katagiri S, Yoshitoshi K, Ariizumi S, Kotera Y, Takahashi Y, Takasaki K. Graft size and donor age are independent factors for graft loss in adult-to-adult living-donor liver transplantation using the left liver. J Hepatobiliary Pancreat Surg. 2009;16:178–183. doi: 10.1007/s00534-008-0026-x. [DOI] [PubMed] [Google Scholar]
- 8.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Haim M, Emre S, Fishbein TM, Sheiner PA, Bodian CA, Kim-Schluger L, Schwartz ME, Miller CM. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient's disease. Liver Transpl. 2001;7:948–953. doi: 10.1053/jlts.2001.29033. [DOI] [PubMed] [Google Scholar]
- 10.Yoshizumi T, Taketomi A, Uchiyama H, Harada N, Kayashima H, Yamashita Y, Soejima Y, Shimada M, Maehara Y. Graft size, donor age, and patient status are the indicators of early graft function after living donor liver transplantation. Liver Transpl. 2008;14:1007–1013. doi: 10.1002/lt.21462. [DOI] [PubMed] [Google Scholar]
- 11.Yoshizumi T, Taketomi A, Soejima Y, Uchiyama H, Ikegami T, Harada N, Kayashima H, Yamashita Y, Shimada M, Maehara Y. Impact of donor age and recipient status on left-lobe graft for living donor adult liver transplantation. Transpl Int. 2008;21:81–88. doi: 10.1111/j.1432-2277.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 12.Marcos A, Olzinski AT, Ham JM, Fisher RA, Posner MP. The interrelationship between portal and arterial blood flow after adult to adult living donor liver transplantation. Transplantation. 2000;70:1697–1703. doi: 10.1097/00007890-200012270-00006. [DOI] [PubMed] [Google Scholar]
- 13.Luca A, Miraglia R, Caruso S, Milazzo M, Gidelli B, Bosch J. Effects of splenic artery occlusion on portal pressure in patients with cirrhosis and portal hypertension. Liver Transpl. 2006;12:1237–1243. doi: 10.1002/lt.20762. [DOI] [PubMed] [Google Scholar]
- 14.Gruttadauria S, Mandala' L, Miraglia R, Caruso S, Minervini MI, Biondo D, Volpes R, Vizzini G, Marsh JW, Luca A, et al. Successful treatment of small-for-size syndrome in adult-to-adult living-related liver transplantation: single center series. Clin Transplant. 2007;21:761–766. doi: 10.1111/j.1399-0012.2007.00735.x. [DOI] [PubMed] [Google Scholar]