Summary
Oligomeric assemblies of Amyloid-β (Aβ) are suggested to be central in the pathogenesis of Alzheimer’s disease, since levels of soluble Aβ much better correlate with the extent of cognitive dysfunctions than senile plaque counts do. Moreover, such Aβ species have been shown to be neurotoxic, to interfere with learned behavior and to inhibit maintenance of hippocampal long term potentiation. The tg-ArcSwe model, transgenic mice with the Arctic and Swedish Alzheimer mutations, expresses elevated levels of Aβ protofibrils in the brain, making tg-ArcSwe a highly suitable model to investigate the pathogenic role of these Aβ assemblies. In the present study, we estimated Aβ protofibril levels in the brain and cerebrospinal fluid of tg-ArcSwe mice, and also assessed their role with respect to cognitive functions. Protofibril levels, specifically measured with a sandwich ELISA, were found to be elevated in young tg-ArcSwe mice, as compared to several transgenic models lacking the Arctic mutation. In aged tg-ArcSwe mice with considerable plaque deposition, Aβ protofibrils were approximately 50 percent higher than in younger mice, whereas levels of total Aβ were exponentially increased. Young tg-ArcSwe mice showed deficits in spatial learning and individual performance in Morris water maze correlated inversely with levels of Aβ protofibrils, but not with total Aβ levels. We conclude that Aβ protofibrils accumulate in an age-dependent manner in tg-ArcSwe mice, although to a far less extent than total Aβ. Our findings suggest that increased levels of Aβ protofibrils could result in spatial learning impairment.
Keywords: Alzheimer’s disease, amyloid-β protofibrils, Arctic mutation, transgenic mice, spatial learning
Introduction
Alzheimer’s disease (AD), the most common form of dementia, is characterized by progressive neurodegeneration and presence of two major histopathological lesions in the brain; neurofibrillary tangles and senile plaques. Accumulation of amyloid-β (Aβ), the main constituent of senile plaques, is believed to be central in AD pathogenesis [1]. Even though the Aβ peptide was identified more than two decades ago, there is still a lack of understanding on how Aβ confers cognitive dysfunctions and neurodegeneration. Although the amount of senile plaques is critical to the neuropathological diagnosis it does not relate to the degree of dementia [2]. Instead, soluble Aβ correlates with the density of neurofibrillary tangles [3] and loss of synapses [4], i.e. with measures that are known to better reflect disease severity.
Many types of oligomeric assemblies of Aβ, with different structural characteristics, have been described and suggested to contribute to the pathogenesis of AD [5]. These include Aβ oligomers like dimers, trimers and dodecamers (Aβ*56), but also various large Aβ aggregates, such as Aβ protofibrils. The latter, which are intermediates in the assembly of Aβ fibrils, are neurotoxic and interfere with electrophysiological mechanisms associated with memory [6–8]. Smaller oligomeric species, like Aβ-derived diffusible ligands (ADDLs) have been demonstrated to induce synaptic dysfunction e.g. by binding to dendritic spines. Moreover, AD brain-derived Aβ dimers induce loss of synapses accompanied by reduced synaptic plasticity and disruption of cognitive functions in vivo [9–12]. The mechanism of synaptic dysfunctions might possibly be mediated through direct interaction on α7 nicotinic receptors or NMDA (N-methyl-D-aspartate), AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole) or other glutamate receptors [13–16]. Interestingly the Arctic APP mutation increases Aβ protofibril formation in vitro and leads to Alzheimer’s disease [17, 18], suggesting that these, and possibly also other soluble Aβ aggregates, are causative in AD pathogenesis. In an effort to examine the pathogenic role of Aβ protofibrils in vivo we have recently developed a sandwich ELISA specific for protofibrils [19] and a new transgenic mouse model, tg-ArcSwe [20, 21], with elevated levels of soluble Aβ aggregates early in life [19, 20, 22]. The aim of the present study was to compare Aβ protofibril levels with established biochemical and histological measures of Aβ accumulation over the life span of tg-ArcSwe mice. We also wanted to begin to assess the role of Aβ protofibrils with respect to cognitive functions by relating their abundance to measures of spatial learning and memory. Aβ protofibrils were present in animals before plaque onset, and the levels of protofibrils were stable with age in young tg-ArcSwe mice, but were approximately 50 percent higher in animals with substantial plaque deposition. In contrast, total Aβ in the brain increased exponentially with age. Behavioral deficits and elevated levels of Aβ protofibrils were apparent already at four months of age in tg-ArcSwe mice. Animals with high levels of protofibrils were less able to improve their performance in the Morris water maze i.e. the abundance of Aβ protofibrils related to spatial learning at an early age.
Results
Age-dependent changes of Aβ protofibril levels in tg-ArcSwe mice
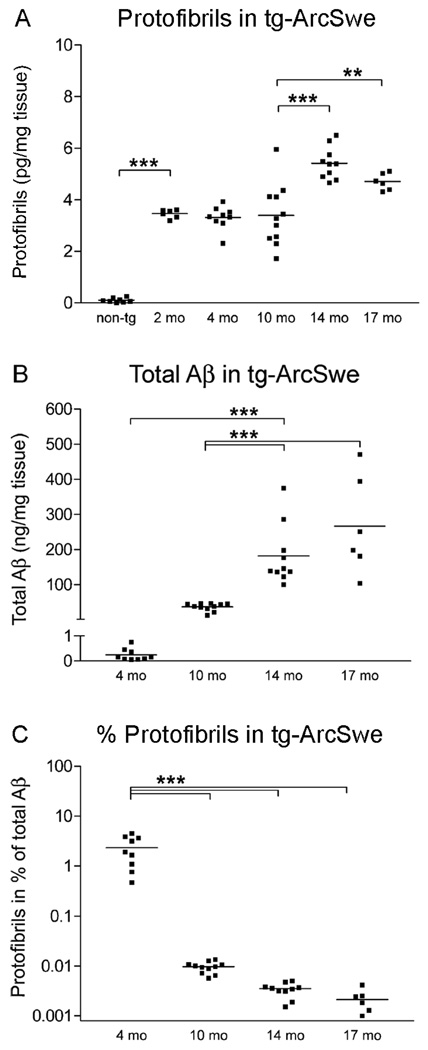
The development of amyloidosis and Alzheimer’s disease is highly age-dependent and a dramatic increase in Aβ accumulation occurs with aging. We therefore considered it of interest to quantify Aβ protofibril concentrations in tg-ArcSwe mice at different ages. Levels were elevated already in 2 months old tg-ArcSwe mice (3.5±0.1 pg/mg tissue) as compared to nontransgenic mice (0.1±0.01 pg/mg, P<0.001; Fig 1A). The levels did not differ significantly between 2, 4 and 10 months of age (mean concentration of 3.4±0.2 pg/mg tissue), but were increased at 14 (5.4±0.2 pg/mg tissue, P<0.001) and 17 (4.7±0.1 pg/mg tissue, P<0.01; Fig 1A) months of age. Total Aβ levels increased dramatically once plaque deposition began (Fig 1B), and Aβ protofibrils constituted only a small fraction of total Aβ in plaque-depositing tg-ArcSwe mice (Fig 1C). Thus, the absolute concentration of protofibrils increased ~50% when plaque deposition was present, while its relative proportion of the total Aβ pool markedly decreased. Aβ protofibrils were also present in cerebrospinal fluid (CSF) of tg-ArcSwe mice (230±34 pg/ml; n=3), but not in nontransgenic mice. This corresponds to approximately 7% of the Aβ protofibril concentration in brain tissue (~0.23 pg/mg), if one assumes that CSF has a density ~1.0 g/ml. The analysis of cerebrospinal fluid was limited to a few 12 months old mice.
Figure 1. Age-dependent changes in Aβ protofibril levels and total Aβ levels in tg-ArcSwe mice.
(A) Levels of protofibrils in nontransgenic (non-tg) and tg-ArcSwe mice at 2 (n=6), 4 (n=9), 10 (n=11), 14 (n=10) and 17 (n=6) months (mo) of age. Aβ protofibrils remained rather stable with age but increased approximately 50% from 10 to 14 months of age. (B) Total Aβ levels in the same set of mice increased dramatically after plaque onset, here represented by age groups of 10 months and older. (C) Aβ protofibrils as a fraction out of total Aβ (in %) was highest in young (4 mo) tg-ArcSwe mice and markedly decreased with age. (** P<0.01 and *** P<0.001, by one-way ANOVA and Tukey’s multiple comparison post hoc test).
Aβ protofibrils increase in early but not late stages of amyloid pathology
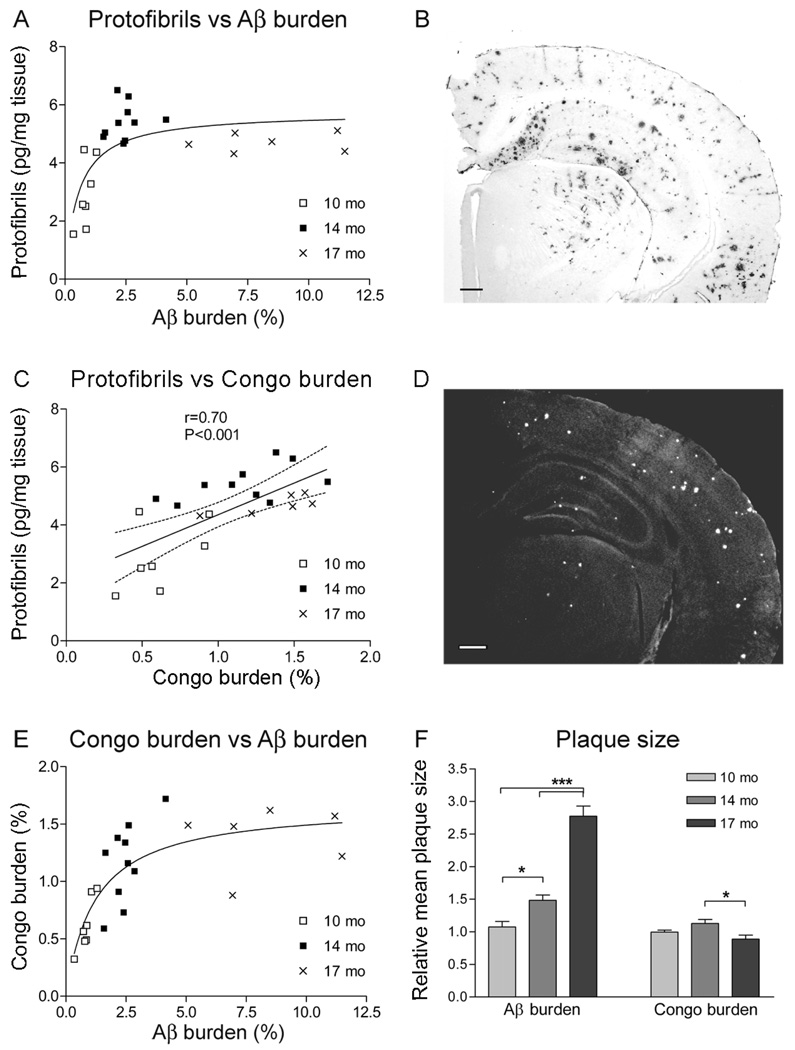
Progression of Aβ pathology in the brain is traditionally measured as Aβ burden by immunohistochemical staining with an Aβ antibody, or as amyloid burden by Congo red staining. Tissue sections of brains from tg-ArcSwe mice of different ages were analyzed for both Aβ and amyloid burden in order to compare these well established markers of Aβ deposition to the concentration of Aβ protofibrils. In this model, essentially all Aβ deposits have an amyloid core, and the presence of Aβ1–40 immunopositive diffuse deposits is negligible [20]. Since Aβ1–40 is the predominant Aβ peptide in the tg-ArcSwe model [21], and to avoid any possible cross-reactivity to APP, an Aβ40-specific polyclonal antibody was used to assess Aβ burden. However, the same immunostaining pattern of an N-terminal Aβ antibody (mAb1C3, described in [19]), indicates that all Aβ deposits were indeed detected with the Aβ40-specific antibody (Supplementary figure 1). In brain sections from 10 and 14 months old mice there was a close to linear relationship between Aβ protofibril levels and Aβ burden, but not at a more advanced age (17 months) when only a marked increase in Aβ burden was apparent (Fig 2A). In contrast, when Congo red positive deposition was compared with Aβ protofibril levels, there was a significant correlation among all animals (Fig 2C). This observation could be explained by a rapid increase in Aβ burden (from 2.5 to 8.4%) between 14 and 17 months of age, while Congo burden remained more stable (from 1.2 to 1.4%; Fig 2E). The data suggest that, apart from an increase in number, Aβ deposits were also increased in size with the accelerated plaque pathology. Indeed, when the mean size of immunostained Aβ plaques was estimated, there was an almost 2-fold increase between the 14 and 17 months old tg-ArcSwe mice, whereas the size of Congo red positive deposits did not increase (Fig 2F). Thus, with age and accelerated Aβ pathology, soluble and diffuse Aβ species are to a large extent added to existing congophilic cores resulting in bigger plaques. The density of the congophilic cores of these plaques more closely relates to Aβ protofibril levels.
Figure 2. Aβ protofibril levels in tg-ArcSwe mice and their relation to plaque pathology.
(A) Increased immunohistochemical Aβ burden was accompanied by raised Aβ protofibril levels in 10 (n=7) and 14 (n=10) months (mo) old animal. With a further increase in Aβ burden, at 17 mo (n=6), protofibril concentrations remained relatively stable. (C) When protofibril levels were compared to the extent of Congo red positive deposition, there was a significant correlation with linear regression when all animals were analyzed as a single group. (B, D) Representative pictures of immunohistochemical staining of Aβ burden and Congo red positive deposits converted to grayscale. The scale bars measure 200 µm. (E) Increased Congo red burden was paralleled by elevated Aβ burden at early stages of plaque accumulation (10 and 14 mo) but at the stage of advanced amyloid pathology (17 mo), the Congo red burden remained stable. (F) Relative mean plaque size of age groups 10, 14 and 17 mo was investigated. Plaque size at 10 months of age was set to 1. The average size of Aβ deposits increased drastically with age whereas the size of Congo red positive material remained relatively stable. (* P<0.05 and *** P<0.001, by one-way ANOVA and Tukey’s multiple comparison post hoc test).
Aβ protofibril formation occurs in several APP transgenic models and depends on human Aβ
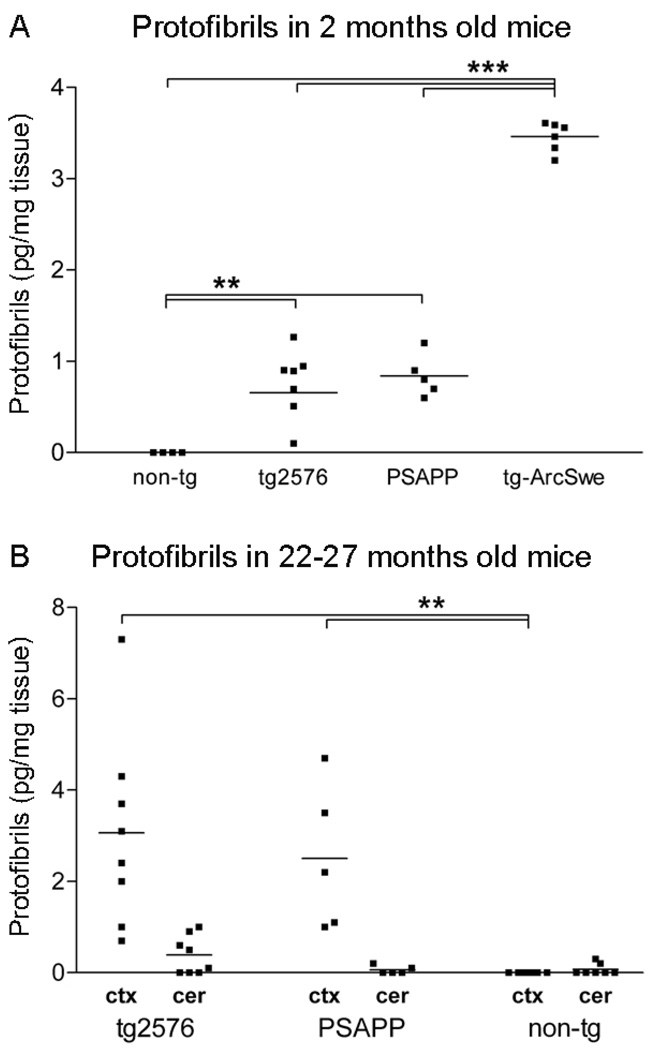
Formation of Aβ protofibrils in vivo is not a phenomenon exclusive for transgenic models carrying the protofibrillogenic Arctic mutation, since tg-Swe mice also form protofibrils [19]. Here we expanded upon these findings by analyzing Aβ protofibrils in brains of young (2 months old) and aged (22–27 months old) tg2576 and PSAPP mice with the mAb158 protofibril ELISA. Aβ protofibrils were found in cortical brain extracts both in young (Fig 3A) and aged (Fig 3B) mice, but the levels were lower than in tg-ArcSwe mice (Fig 3A). The PSAPP mice, which express human presenilin-1 (PS1) at a high level and show accelerated Aβ plaque pathology [23], did not have higher levels of Aβ protofibrils than tg2576 mice (Fig 3A, 3B). The presence of Aβ protofibrils in these two models was essentially restricted to brain regions later affected by amyloid pathology, with only very low levels in the cerebellum (Fig 3B). Aβ protofibrils could not be found in brain homogenates of nontransgenic mice or APP knockout mice (data not shown), suggesting that murine Aβ peptides were unable to form protofibrils, at least not at picomolar concentrations of Aβ.
Figure 3. Aβ protofibril levels in different APP transgenic models.
Aβ protofibrils in TBS-soluble cortical extracts from several APP transgenic mouse lines were measured with mAb158 protofibril ELISA. (A) Aβ protofibril levels were elevated in 2 months old tg2576 (n=7) and PSAPP (n=5) mice as compared to nontransgenic (non-tg) littermates (n=4), but were significantly lower than in age-matched tg-ArcSwe (n=6) mice. Aβ protofibril levels did not differ between young tg2576 and PSAPP mice. (B) Aβ protofibril levels were increased by more than 3-fold in cortical extracts (ctx) of 22–27 months old tg2576 (n=8) and PSAPP (n=5) mice. Cerebellar extracts (cer) from the same set of transgenic mice were essentially devoid of Aβ protofibrils, although a few tg2576 mice had measurable levels in the cerebellum. (** P<0.01 and *** P<0.001, by one-way ANOVA and Tukey’s multiple comparison post hoc test).
Spatial learning performance inversely correlates to Aβ protofibril levels in young tg-ArcSwe mice
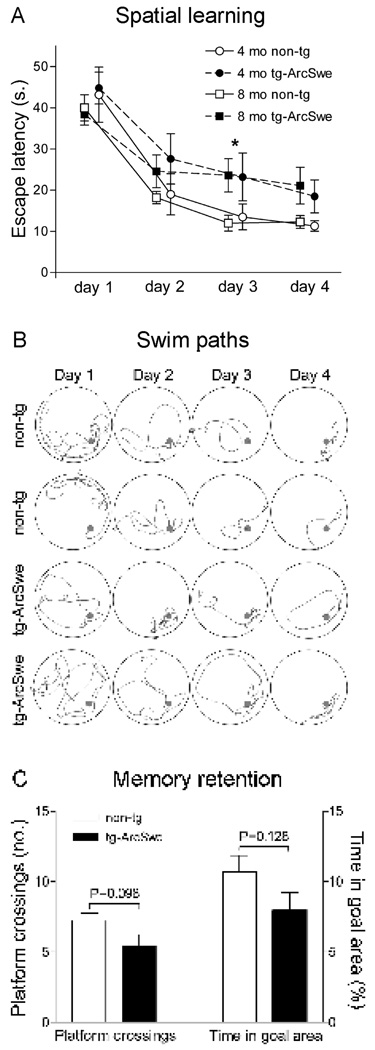
To investigate how Aβ protofibril concentration in the brain relates to spatial learning and memory, 4 and 8 months old tg-ArcSwe mice were tested in Morris water maze. Tg-ArcSwe mice demonstrated longer escape latencies as compared to nontransgenic littermates (Fig 4A), suggesting impaired acquisition of spatial learning. Initial single variance analysis showed no effect of age (F1,146=0.107, P=0.74). When pooling the 4 and 8 months groups and analyzing with two-way factorial ANOVA with time and genotype as categorical variables, there was a significant effect of both genotype (F1,140=6.45, P<0.05) and time (F3,140=32.2, P<0.01). Subsequent analyses with Fischer LSD post-hoc revealed a significant increased escape latency of tg-ArcSwe mice (23.4±3.3, n=20) at day 3 as compared to nontransgenic littermates (14.3±2.3, n=17, P<0.05). Swim speed did not differ between the nontransgenic (20.6±1.0 cm/s, n=20) and transgenic (21.3±0.8, n=17) animals, as there was no effect of genotype with a three-way ANOVA (F1,132=3.58, P=0.061). This implies that the inferior learning performance of tg-ArcSwe mice was not due to sensorimotor dysfunctions or motivational shortage. In addition, at day 3 of learning, distances to reach the platform of nontransgenic mice were shorter as compared to tg-ArcSwe mice (data not shown), consistent with the observed difference in escape latency. Representative swim paths of nontransgenic and transgenic mice are presented in Figure 4B. Nontransgenic mice clearly developed a spatial bias to the target area, since the time spent in goal area (10.5±1.1%; n=17) by far exceeded chance performance (2.5%, see materials and methods). They spent more time in the goal area and crossed the platform area more often in the probe trial than tg-ArcSwe mice did (Fig 4C), but the differences did not reach significance. Both these measures inversely correlated to escape latency at the last day of training (Supplementary figure 2), suggesting that spatial search strategies were used by most of the animals. Four individuals (two 4 months old nontransgenic mice, one 8 months old transgenic mouse and one 8 months old nontransgenic mouse) were excluded from the study due to floating, thigmotaxis (wallhugging) or circling behaviors.
Figure 4. Spatial learning and memory in tg-ArcSwe mice.
Transgenic (tg-ArcSwe) and nontransgenic (non-tg) mice were tested in the Morris water maze at 4 months (4 mo) and 8 months (8 mo) of age, n=17 (non-tg) and 20 (tg-ArcSwe) in total. (A) Escape latency in seconds (s.) was used as a measure of spatial learning. Each point in the figure represents average performance at each day ± standard error of the mean. Different age groups (4 and 8 mo) were offset in the figure for the sake of clarity. Learning was modestly impaired in tg-ArcSwe mice as compared to non-tg littermates with a significant effect of both genotype and time in a two-way factorial ANOVA. Fischer LSD pos-hoc showed longer escape latencies of tg-ArcSwe mice at day 3 (* P<0.05). There was no evidence that age affected performance in an initial single variance analysis (P=0.74). (B) Representative swim paths of two non-tg mice (upper panels) and two tg-ArcSwe mice (lower panels). Arrowheads (▷) illustrate the start position of each mouse. (C) In the probe trial, 72 h after last training session, non-tg mice crossed the platform more often than tg-ArcSwe mice and also spent more time in the goal area, but these differences did not reach significance.
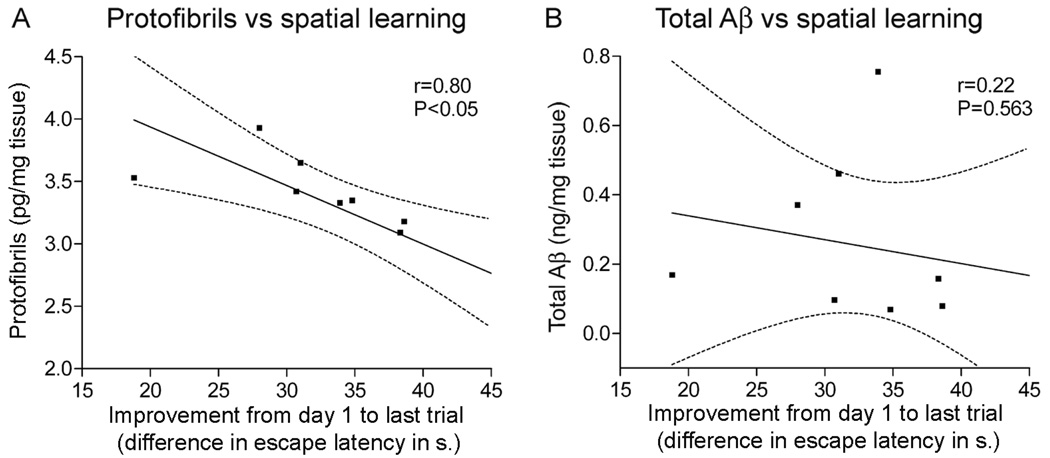
Brains from 4 months old tg-ArcSwe mice, devoid of senile plaques, were harvested shortly after cognitive testing and Aβ protofibrils were measured with the mAb158 protofibril specific ELISA. Aβ protofibrils correlated inversely with spatial learning, measured as improvement in escape latency (Fig 5A). The escape latency at the last learning trial was subtracted from the mean escape latency at the first training session. Little ability to improve was then associated with high levels of protofibrils. In contrast, total Aβ levels in formic acid-extracts from the same set of mice did not correlate to improved escape latency (Fig 5B).
Figure 5. Aβ levels in tg-ArcSwe mice without plaque pathology and their relation to spatial learning.
Aβ protofibril levels in TBS-extracts and total Aβ levels in formic acid extracted brains of 4 months old tg-ArcSwe mice were investigated and related to spatial learning (n=9). (A) Aβ protofibrils inversely correlated with improvement in escape latency, measured as performance in the last trial subtracted from performance at the first acquisition session. (B) Levels of total Aβ, in the same set of mice, were not associated with improved escape latency and spatial learning.
Discussion
Here, Aβ protofibril levels in young and aged animals were assessed in three different AD mouse models. Aβ protofibrils were present in both brain and cerebrospinal fluid of tg-ArcSwe mice, and levels in the brain were stable in young animals but higher in aged animals with elevated Aβ burden. In a microdialysis study it was reported that soluble Aβ levels, analyzed in the interstitial fluid of PDAPP mice, did not differ between 3 months and 12–15 months of age [24]. Moreover, soluble Aβ*56 levels in tg2576 mice remained stable after 6 months of age, while total Aβ levels increased [25]. Thus Aβ protofibrils appear, like other soluble Aβ species, not to accumulate until advanced age and significant senile plaque deposition. Steady state levels of Aβ protofibrils in brains of young animals were increased by the Arctic mutation, but not by mutant presenilin-1, most likely because Arctic Aβ is more prone to form Aβ protofibrils than wild type Aβ [17, 18]. Absolute concentrations of Aβ protofibrils in tg-ArcSwe mice increased modestly (~50%) in association with accelerated Aβ fibrillization and senile plaque formation (14 months old mice), but remained stable thereafter. It is well-known that Aβ burden rapidly increases once plaque deposition has begun, and that the raise in total Aβ is even more marked with biochemical analysis compared to histological analysis [26, 27]. In accordance, the proportion of Aβ protofibrils of total Aβ was markedly reduced with age in tg-ArcSwe mice. Soluble Aβ also seemed to be rapidly added to existing congophilic cores resulting in bigger plaques, as the mice grew old. We therefore speculate that protofibrils are not predominantly formed by detachment from the surface of Aβ deposits, since one would then expect to find greatly elevated Aβ protofibril concentrations in aged animals with very high Aβ burden. Detection of Aβ protofibrils in cerebrospinal fluid of transgenic mice, but not nontransgenic controls, further suggests that the mAb158 protofibril ELISA indeed measures biological metabolites.
Tg-ArcSwe mice show prominent early pathology with enhanced formation of Aβ protofibrils and intraneuronal Aβ accumulation beginning months before plaque onset [19, 20]. Given this we found it of particular interest and relevance to examine cognitive functions before plaques emerged. We showed that young tg-ArcSwe mice devoid of plaque deposition, but with substantial amounts of Aβ protofibrils, displayed learning deficits. A Morris water maze setting was chosen to measure spatial learning and memory, since it depends upon hippocampal functions [28], and hippocampus is a brain region severely affected early on in AD pathogenesis [29, 30]. Tg-ArcSwe mice were poor learners, as compared to nontransgenic mice, and needed more time to find the hidden platform. In contrast, memory impairment could not be demonstrated in the probe trial. It is possible that the lack of significance in memory retention was due to large variability within the experimental groups, and that larger cohorts of animals would have revealed a subtle memory retention deficit in young tg-ArcSwe mice. Here memory retention was investigated at 72 hours post training, instead of 24 hours which is more commonly used. The genotype differences might have been more pronounced with a probe trial at 24 hours. Interestingly, high levels of protofibrils, but not total Aβ, were associated with inferior spatial learning, at least in young mice. This implies that Aβ protofibril levels in the brain of tg-ArcSwe mice could better reflect cognitive dysfunctions than the total pool of Aβ. Escape latencies of 4 and 8 months old tg-ArcSwe mice were similar and one might therefore hypothesize that these relate to the stable levels of Aβ protofibrils observed between 4 and 10 months of age. If spatial learning is affected by protofibril levels, as our results in young tg-ArcSwe mice suggest, it would be of great interest to examine aged mice (14 months or older) with enhanced Aβ protofibril levels in the Morris water maze. Conclusions from such future studies, in aged tg-ArcSwe mice, would of course not be straight forward since Aβ burden and insoluble Aβ are also increased. The present studies only suggest that Aβ protofibrils have an impact on spatial learning in tg-ArcSwe mice. If Aβ protofibril levels are of general importance to cognitive deficits and also help explain functional deficits in other models, like PSAPP and tg2576 mice is however unclear. In fact, since lower Aβ protofibril levels were found in these models than in tg-ArcSwe mice, one would predict to observe lesser deficits in spatial learning. Cognition in young PSAPP is only modestly impaired, but aged PSAPP mice do exhibit robust and progressive deficits in learning and memory tests [31, 32], and some data imply a correlation of deposited Aβ and memory dysfunction [33], whereas other data in younger mice do not [34]. Smaller aggregates/oligomers of soluble Aβ, which are probably not efficiently detected by the mAb158 protofibril ELISA, are likely also present in tg-ArcSwe mice and could of course contribute to the spatial learning deficits observed. For example in tg2576 mice, Aβ56* has been shown to impair memory functions before the appearance of plaque pathology [25]. Aβ derived diffusible ligands (ADDLs) cause synaptic dysfunction by down-regulation of memory-related receptors such as NMDA [10] and Aβ dimers were recently extracted from AD brain and demonstrated to inhibit LTP [12].
In spite of similarities and obvious differences between animal models and human disease, it is still interesting and important to reflect upon these observations and to try to find explanations. A comparison between APP transgenic models and the human disease is of course complicated by the lack of key features of AD neuropathology, such as neurofibrillary tangles, neuronal loss and macroscopic atrophy, in the animal models. However, in AD brains, plaque pathology is widespread and well developed when symptoms begin to appear, but disease severity does not relate to the density of Aβ deposits. On the contrary, in transgenic mice functional deficits and loss of synapses are, at least in some models, observed in young transgenic animals devoid of plaque deposits suggesting that soluble Aβ species are deleterious [34–36]. In aged APP transgenic mice learning deficits often correlate to Aβ burden, suggesting that insoluble Aβ also is detrimental [33, 37, 38]. However, with active vaccination the development of age-related memory deficits can be significantly prevented although the accumulation of amyloid deposits is only partly inhibited [39]. An interpretation of these findings is that soluble Aβ aggregates, cleared by the treatment, contribute to cognitive dysfunctions in aged animals. In contrast, the human brain remains cognitively functional until late in life, perhaps due to additional reserve capacity associated with its more evolved structure. Thus, much longer time would be needed for soluble Aβ to exert enough neuronal damage to cause symptoms in humans. Another difference is the artificially high APP expression in APP transgenic mice, as compared to sporadic Alzheimer’s disease. The high expression of APP is needed to enable onset of amyloid pathology within the lifespan of a mouse, but it might also result in a higher turnover of deleterious soluble Aβ species than in human brain.
Here we show that the levels of soluble Aβ protofibrils accumulate in an age-dependent manner in tg-ArcSwe mice, although to a far less extent than levels of total Aβ. As Morris water maze performances of young tg-ArcSwe mice correlated inversely with Aβ protofibril levels, we suggest that Aβ protofibrils could affect spatial learning. If so, stimulating the clearance or inhibiting the formation of this Aβ species could be feasible and efficient ways to mitigate cognitive dysfunctions of patients with Alzheimer’s disease.
Materials and methods
Transgenic mice
APP transgenic mice with the Swedish (K670N, M671L) and Arctic (E693G) mutations (tg-ArcSwe mice) [20], nontransgenic littermates and APP knockout mice (APP-KO) (#004133, Jackson laboratory) were kept at the animal facility at Uppsala University. Tg2576 mice [40] and PSAPP mice [23] were obtained from University of South Florida, where PSAPP mice had been generated by breeding tg2576 mice with line 5.1 M146L PS1 [41]. All mice in this study, regardless of animal facility, were housed under standard conditions with a 12 h light/dark cycle and provided food and water ad libitum. The experiments were approved by ethical committees and performed in compliance with national and local animal care and use guidelines (protocols #C258/6 and #C242/5 at Uppsala University and #M2804 and #M2814 at University of South Florida).
Morris water maze
Mice were transported to the animal facility at Uppsala University Hospital, allowed to habituate for one week, handled daily for yet another week and then tested in a water maze (1.4 m in diameter) located in a laboratory exclusively used for behavioural studies. Water was filled and drained daily and maintained at 22 ± 1°C. The platform (11 cm in diameter) was submerged 1 ± 0.5 cm beneath the surface and located at a fixed position, while the starting positions were randomized and counterbalanced. Mice were allowed to swim for up to 60 s to find the platform, where they were let to remain for 15 s. Animals unable to locate the platform were guided to it and the maximal 60 s time was recorded. Tg-ArcSwe and nontransgenic littermates, either 4 months old (n=9 and 8) or 8 months old (n=12 and 12), were trained five trials per day during four consecutive days. 72 h after the last learning trial, as previously described [42], mice were tested for memory retention in a probe trial without the platform. The mice were monitored using a video camera and an automated tracking system (HVS Image, Hampton, England). Parameters recorded were escape latency (time to find platform), swim path (distance to find platform), swim speed, time spent in goal area (defined as a circle area with 2× the diameter of the platform representing 2.5% of the total pool area) and platform crossings. The escape latency at the last trial of training was subtracted from mean escape latency at the first day of training, to measure improvement in spatial learning. Thigmotaxis, circling and floating activities, as defined by the HSV Image software, were recorded and animals (n=4) displaying such behaviours were excluded.
Histology and image analyses
Mice were anesthetized with 0.4 ml Avertin (25 mg/ml) and intracardially perfused with 0.9% saline solution. Their brains were divided in two hemispheres; cerebellum was prepared and treated separately. One brain half was frozen on dry ice for biochemical analysis (described below), and the other half was immersed for 24 h in 4% paraformaldehyde and used for histology. Fixed brains were cryoprotected through sequential immersion in 10, 20 and 30% (w/v) sucrose for 24 h. Coronal sections of 25 µm thickness were collected with a sledge microtome and stored at +4°C in PBS with 10 mM NaN3. Five sections per individual, approximately 500 µm apart (Bregma −1.0 mm to −3.0 mm), were selected for each immunostaining and mounted on slides. Sections were treated for antigen retrieval and immunostained for Aβ, as previously described [20]. An Aβ40-specific antibody (gift from Dr. J. Näslund) was used to visualize Aβ burden (1 µg/ml). Sections were also stained with Congo red (86,095-6; Sigma-Aldrich) to detect amyloid deposits. Aβ burden in the cerebral cortex and hippocampus was measured in two image fields of each section at 20× magnification. Images were captured in bright field at a defined setting with a Nikon microscope (DXM1200F; Nikon Instruments Inc., Melville, NY, USA) equipped with a digital camera, converted to greyscale and processed with an auto threshold command (Image Pro-Plus, Cybernetics, Silver Spring, MD, USA). Custom-made macros were used to measure the stained area of interest as percentage of total tissue area.
Biochemical Aβ analyses
Cortical and cerebellar brain tissues from perfused animals were extracted at a ratio of 1:10 (tissue weight : extraction volume) in TBS (tris buffered saline, 20 mM tris, 137 mM NaCl, pH 7.6) with Complete protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany) using a tissue grinder with teflon pestle (2×10 strokes on ice). The homogenates were centrifuged at 100 000×g at +4°C for 60 min, and the supernatants were used to obtain a preparation of TBS-soluble extracellular and cytosolic proteins. To measure total Aβ, brain tissue was directly extracted in 70% formic acid at a ratio of 1:10, sonicated for 30 s at a defined setting and thereafter centrifuged at 100 000×g at +4°C for 60 min. All supernatants were stored in aliquots at −80°C prior to analyses. Cerebrospinal fluid (CSF) was isolated from the cisterna magna of mice based on the method of DeMattos et al. 2002 [43]. The animals were anesthetized with 100 mg/kg ketamin and 10 mg/kg xylazine i.p. and placed in a stereotaxic frame under an operating microscope and the meninges overlying the cisterna magna were surgically exposed. The arachnoid membrane was punctured with a thin needle connected to a rubber tube and CSF was withdrawn using a syringe connected to the tube. The CSF was placed on dry ice and stored at −80°C until the time of analysis. On average 10 µl CSF was collected from each mouse with minimal blood contamination.
mAb158 protofibril ELISA
The assay was performed as previously described [19]. In short, 96-well plates were coated at +4°C over night with 200 ng/well of mAb158 before being blocked with 1% BSA in PBS. To assure that all samples were devoid of insoluble Aβ fibrils they were centrifuged at 17 900×g for 5 min at 16°C immediately before analysis. Samples were then added to the plate in duplicates and incubated for 2 h at room temperature (RT). Biotinylated mAb158 (1 µg/ml) was added and incubated for 1 h at RT, followed by streptavidin-coupled HRP (Mabtech AB, Nacka Strand, Sweden) for 1 h at RT. K-blue enhanced (ANL-Produkter AB, Älvsjö, Sweden), was used as HRP-substrate and the reaction was stopped with 1 M H2SO4. Wells were washed three times between each step after blocking the plates and antibodies and samples were diluted in ELISA incubation buffer (PBS with 0.1% BSA, 0.05% Tween-20).
Total Aβ ELISA
A 96-well plate was coated at +4°C over night with 100 ng/well of the N-terminal Aβ antibody 82E1 (IBL-Hamburg, Hamburg, Germany) in PBS, and then blocked with 1% BSA in PBS. Formic acid-extracted mouse brains were neutralized in 1 M Tris (pH 10) and diluted in ELISA incubation buffer (PBS with 0.1% BSA, 0.05% Tween-20). Samples and a standard series of Aβ monomers were then added to the plate in duplicates and incubated for 2 h at RT. Biotinylated mAb27 (1 µg/ml), with an epitope in the mid-domain of the Arctic Aβ peptide (generated at our laboratory, for specificity see Supplementary figure 3), was added and incubated for 1 h at RT. Subsequent steps were performed in the same way as for the mAb158 protofibril ELISA.
Statistical analyses
The Morris water maze data were analyzed by three-way factorial ANOVA with genotype, age and time as categorical factors. After analyzing the effect of age on escape latencies with single variance analysis, age groups were pooled and escape latencies were analyzed with two-way ANOVA and later with post hoc Fisher LSD test (STATISTICA, OK, USA). Biochemical data were analyzed with one-way ANOVA and Tukey’s multiple comparison post hoc test (GraphPad Software, CA, USA) and presented as scattergrams with lines representing the mean. Significance were reported as * P<0.05, ** P<0.01 and *** P<0.001. Correlations were examined by linear regression analysis (GraphPad Software, CA, USA) with P and r values and 95% confidence intervals included in the graphs. P values less than 0.05 were regarded as statistically significant.
Supplementary Material
Acknowledgements
This work was supported by grants from Hjärnfonden (FC, LL) and Bertil Hållstens forskningsstiftelse (LL), Alzheimerfonden (HE, LL), The Swedish Research Council (2006–2822, LL, 2006–2818, LNGN, 2007–3254 LH), Stiftelsen Gamla Tjänarinnor (AL, HE, FEP, LNGN), Stohnes stiftelse (AL, HE, FEP, LNGN), Frimurarstiftelsen (LNGN), Åhlensstiftelsen (FEP, LNGN, LH), Uppsala University Hospital (LH), Lars Hiertas Minne and Lundströms minne (LNGN). Work at USF was supported by AG15490, AG 18478, AG04418, AG 25509 and AG 25711 from the NIH (USA) and research support from the Johnnie B. Byrd Alzheimer’s Center and Research Institute. We thank Paul O’Callaghan for help with figures and linguistic advice.
Abbreviations
- Aβ
Amyloid-β
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- ELISA
enzyme-linked immunosorbent assay
Footnotes
Supporting information
The following supplementary material is available:
Fig. S1. Anti-Aβ immunostaining in tg-ArcSwe mouse brain.
Fig. S2. Probe trial measurements.
Fig. S3. mAb27 characterization.
References
- 1.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 2.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 3.McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 6.Harper JD, Wong SS, Lieber CM, Lansbury PT. Observation of metastable Abeta amyloid protofibrils by atomic force microscopy. Chem Biol. 1997;4:119–125. doi: 10.1016/s1074-5521(97)90255-6. [DOI] [PubMed] [Google Scholar]
- 7.Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- 9.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 10.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 12.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 15.Wang HY, Lee DH, D'Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. J Biol Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 16.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson AS, Berglind-Dehlin F, Karlsson G, Edwards K, Gellerfors P, Lannfelt L. Physiochemical characterization of the Alzheimer's disease-related peptides A beta 1–42Arctic and A beta 1–42wt. Febs J. 2006;273:2618–2630. doi: 10.1111/j.1742-4658.2006.05263.x. [DOI] [PubMed] [Google Scholar]
- 18.Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, Naslund J, Lannfelt L. The 'Arctic' APP mutation (E693G) causes Alzheimer's disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001;4:887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 19.Englund H, Sehlin D, Johansson AS, Nilsson LN, Gellerfors P, Paulie S, Lannfelt L, Pettersson FE. Sensitive ELISA detection of amyloid-beta protofibrils in biological samples. J Neurochem. 2007;103:334–345. doi: 10.1111/j.1471-4159.2007.04759.x. [DOI] [PubMed] [Google Scholar]
- 20.Lord A, Kalimo H, Eckman C, Zhang XQ, Lannfelt L, Nilsson LN. The Arctic Alzheimer mutation facilitates early intraneuronal Abeta aggregation and senile plaque formation in transgenic mice. Neurobiol Aging. 2006;27:67–77. doi: 10.1016/j.neurobiolaging.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Philipson O, Hammarstrom P, Nilsson KP, Portelius E, Olofsson T, Ingelsson M, Hyman BT, Blennow K, Lannfelt L, Kalimo H, Nilsson LN. A highly insoluble state of Abeta similar to that of Alzheimer's disease brain is found in Arctic APP transgenic mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.11.022. In Press. [DOI] [PubMed] [Google Scholar]
- 22.Stenh C, Englund H, Lord A, Johansson AS, Almeida CG, Gellerfors P, Greengard P, Gouras GK, Lannfelt L, Nilsson LN. Amyloid-beta oligomers are inefficiently measured by enzyme-linked immunosorbent assay. Ann Neurol. 2005;58:147–150. doi: 10.1002/ana.20524. [DOI] [PubMed] [Google Scholar]
- 23.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 24.Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 26.Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, Lieberburg I, Schenk D, Seubert P, McConlogue L. Amyloid precursor protein processing and A beta42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci U S A. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clausen F, Lewen A, Marklund N, Olsson Y, McArthur DL, Hillered L. Correlation of hippocampal morphological changes and morris water maze performance after cortical contusion injury in rats. Neurosurgery. 2005;57:154–163. doi: 10.1227/01.neu.0000163412.07546.57. discussion 154–163. [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 30.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 31.Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 32.Gordon MN, Holcomb LA, Jantzen PT, DiCarlo G, Wilcock D, Boyett KW, Connor K, Melachrino J, O'Callaghan JP, Morgan D. Time course of the development of Alzheimer-like pathology in the doubly transgenic PS1+APP mouse. Exp Neurol. 2002;173:183–195. doi: 10.1006/exnr.2001.7754. [DOI] [PubMed] [Google Scholar]
- 33.Gordon MN, King DL, Diamond DM, Jantzen PT, Boyett KV, Hope CE, Hatcher JM, DiCarlo G, Gottschall WP, Morgan D, Arendash GW. Correlation between cognitive deficits and Abeta deposits in transgenic APP+PS1 mice. Neurobiol Aging. 2001;22:377–385. doi: 10.1016/s0197-4580(00)00249-9. [DOI] [PubMed] [Google Scholar]
- 34.Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D. Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav Genet. 1999;29:177–185. doi: 10.1023/a:1021691918517. [DOI] [PubMed] [Google Scholar]
- 35.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson LN, Arendash GW, Leighty RE, Costa DA, Low MA, Garcia MF, Cracciolo JR, Rojiani A, Wu X, Bales KR, Paul SM, Potter H. Cognitive impairment in PDAPP mice depends on ApoE and ACT-catalyzed amyloid formation. Neurobiol Aging. 2004;25:1153–1167. doi: 10.1016/j.neurobiolaging.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 40.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 41.Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 42.Marklund N, Bareyre FM, Royo NC, Thompson HJ, Mir AK, Grady MS, Schwab ME, McIntosh TK. Cognitive outcome following brain injury and treatment with an inhibitor of Nogo-A in association with an attenuated downregulation of hippocampal growth-associated protein-43 expression. J Neurosurg. 2007;107:844–853. doi: 10.3171/JNS-07/10/0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeMattos RB, Bales KR, Parsadanian M, O'Dell MA, Foss EM, Paul SM, Holtzman DM. Plaque-associated disruption of CSF and plasma amyloid-beta (Abeta) equilibrium in a mouse model of Alzheimer's disease. J Neurochem. 2002;81:229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.