Abstract
NKT cells have been shown to promote peripheral tolerance in a number of model systems, yet the processes by which they exert their regulatory effects remain poorly understood. Here, we show that soluble factors secreted by human NKT cells instruct human peripheral blood monocytes to differentiate into myeloid APCs that have suppressive properties. NKT-instructed monocytes acquired a cell surface phenotype resembling myeloid DCs. However, whereas control DCs that were generated by culturing monocytes with recombinant GM-CSF and IL-4 had a proinflammatory phenotype characterized by the production of IL-12 with little IL-10, NKT-instructed APCs showed the opposite cytokine production profile of high IL-10 with little or no IL-12. The control DCs efficiently stimulated peripheral blood T cell IFN-γ secretion and proliferation, whereas NKT-instructed APCs silenced these T cell responses. Exposure to NKT cell factors had a dominant effect on the functional properties of the DCs, since DCs differentiated by recombinant GM-CSF and IL-4 in the presence of NKT cell factors inhibited T cell responses. To confirm their noninflammatory effects, NKT-instructed APCs were tested in an in vivo assay that depends on the activation of antigen-specific human T cells. Control DCs promoted substantial tissue inflammation; however, despite a marked neutrophilic infiltrate, there was little edema in the presence of NKT-instructed APCs, suggesting the inflammatory cascade was held in check. These results point to a novel pathway initiated by NKT cells that can contribute to the regulation of human antigen-specific Th1 responses.
Keywords: dendritic cells, T cell activation, T cell suppression
Introduction
NKT cells are a unique subset of regulatory T lymphocytes. They are restricted by the nonclassical antigen-presenting molecule CD1d, which presents lipids and glycolipids for recognition at the cell surface [1]. Like other regulatory T cells, such as CD4+CD25+ Treg cells, NKT cells recognize specific self-antigens [2,3,4]. However, NKT cells also recognize certain microbial lipids [5,6,7], and they share a number of characteristics with innate lymphocytes, including being among the first responders during microbial infections [8]. Thus, NKT cells appear to occupy an unusual immunological niche that probably includes performing constitutive functions that result from self-antigen recognition, as well as functions that are induced by foreign antigenic stimulation.
In addition to their dualistic antigen recognition properties, NKT cells have been found to further both tolerogenic and proinflammatory immunological processes in vivo [9, 10]. They have been shown to protect against development of autoimmune diseases, including type I diabetes and experimental autoimmune encephalitis and are critical for the establishment of tolerance to antigens that are introduced into an immunologically privileged site [11,12,13,14,15,16,17]. Similarly, NKT cells can have suppressive effects on cell-mediated anti-tumor responses [18, 19]. On the other hand, NKT cells can promote resistance to pathogens and can enhance immunological memory in vaccination strategies [20,21,22,23,24,25]. Additionally, in some model systems NKT cells can promote tumor rejection [26,27,28]. Hence, understanding the basis for the contrasting immunological effects of NKT cells is an area of considerable interest.
A critical component appears to be the variable interactions of NKT cells with myeloid APCs. Myeloid cells, such as monocytes, macrophages, and DCs constitutively express CD1d molecules [29, 30]. Presentation by myeloid DCs of α-GalCer, a potent glycolipid antigen for NKT cells [31], can cause NKT cells to up-regulate costimulatory factors that promote proinflammatory DC functions, resulting in enhanced downstream activation of NK cells and MHC-restricted T cells [32,33,34]. Consistent with this, α-GalCer-pulsed myeloid DCs are highly potent activators of antitumor immune responses [35]. Moreover, activation of NKT cells by administration of α-GalCer has been shown to result in the rapid maturation in vivo of DCs that efficiently prime Th1-biased T cell responses to a coadministered protein antigen [36, 37]. However, in other cases, NKT cells have been found to promote DCs that have regulatory characteristics. For example, NKT cells that were exhausted by repeated exposure to α-GalCer-induced DCs that suppressed experimental autoimmune encephalomyelitis [38]. In NOD mice, activation of NKT cells by injection of α-GalCer was associated with the appearance of tolerogenic myeloid DCs that prevented the development of diabetes by suppressing pathogenic T cell responses [39, 40]. Thus, current data suggest that NKT cells can promote both proinflammatory and regulatory DC phenotypes.
Less is known about the immunological consequences of NKT cell interactions with other myeloid APC types, such as monocytes, macrophages, and myeloid-derived suppressor cells (MDSCs). Upon recognition of α-GalCer, NKT cells were found to stimulate IL-12p70 secretion by human monocytes as a result of NF-κB signaling induced by ligation of CD1d molecules on the monocytes [41]. Additionally, it was recently reported that NKT cells interact with MDSCs in a CD1d- and CD40-dependent manner and alter their functional properties such that their suppressive effects on antigen-specific T cell proliferation are relieved [42]. These results suggest NKT cells can enhance the proinflammatory or stimulatory properties of monocytes and MDSCs. It remains unclear whether NKT cells also mediate tolerogenic effects through interactions with these types of APCs.
Recently, we have shown that human NKT cells direct primary human peripheral blood monocytes to differentiate into cells resembling immature myeloid DCs [43]. NKT cell activation by recognition of CD1d expressed on monocytes resulted in secretion of GM-CSF and IL-13, and these cytokines promoted monocyte differentiation. The resulting myeloid cells showed up-regulation of DC-SIGN, little or no expression of CD14, and moderate expression levels of costimulatory markers such as CD40 and CD86 and thus had a cell surface phenotype consistent with that of immature myeloid DCs. Moreover, they showed localization of MHC class II molecules in LAMP-1+ intracellular vesicles as is characteristic of immature myeloid DCs. Upon exposure to LPS, they underwent changes associated with DC maturation, including up-regulation of CD83 and CCR7, and relocalization of MHC class II molecules to the cell surface [43]. Hence, the monocytes differentiated by this pathway had phenotypic characteristics similar to those of professional myeloid APCs. Here, we show that soluble factors secreted by NKT cells during monocyte differentiation dominantly instruct the resulting APCs to acquire a phenotype that suppresses downstream Th1-responses.
MATERIALS AND METHODS
Isolation of monocytes
PBMCs were purified from fresh blood obtained from healthy adult donors using Ficoll-Paque density gradient centrifugation (GE Health Sciences, New York, NY, USA). Monocytes were isolated by magnetic sorting using anti-CD14 beads (Miltenyi Biotech, Gladbach, Germany). The purity of the resulting monocytes, as assessed by flow cytometric analysis, was typically greater than 98%. Analyses involving human samples were approved by the University of Wisconsin Minimal Risk IRB, and written informed consent was obtained from all blood donors.
NKT cells
Human CD1d-restricted T cells used in this analysis included a polyclonal NKT cell line, and the following previously established NKT clones: J3N.1 (CD4-), J3N.4 (CD4+), J3N.5 (CD4+), J24N.22 (CD4+), J24L.10 (CD4+), J24L.17 (CD4+) [44, 45]. NKT cell clones were generated from peripheral blood of healthy human donors by single cell sorting T cells that stained positively with α-GalCer-loaded CD1d tetramers, followed by expansion in vitro using PHA (Sigma-Aldrich, St. Louis, MO, USA), recombinant IL-2 (Chiron, Emeryville, CA, USA), and irradiated total PBMC, as described previously [44, 45]. The NKT cell line was established by sorting a polyclonal population of CD1d/α-GalCer tetramer-stained T cells from human peripheral blood [43]. NKT cells were maintained by culturing them in RPMI 1640 medium supplemented with 2 mM l-glutamine, 100 μg/ml each of penicillin and streptomycin, 10% fetal bovine serum, and 5% bovine calf serum (Hyclone), 5% human AB serum (Atlanta Biologicals, Lawrenceville, GA, USA), 400 U/ml recombinant human IL-2, and were stimulated periodically by the addition of irradiated allogeneic PBMCs, and 250 ng/ml PHA.
Generation of DCs
Monocyte differentiation was carried out in RPMI 1640 culture medium supplemented with 2 mM l-glutamine, 100 μg/ml penicillin, and streptomycin, and 10% fetal bovine serum. Monocytes alone were placed in the lower wells of transwell plates, and a 2:1 mixture of NKT cells and monocytes was placed in the transwell insert. Monocytes in the transwell inserts were either untreated, or pulsed for 2 h with 5 ng/ml α-GalCer (a kind gift from Dr. Gurdyal S. Besra) then washed, prior to addition of the NKT cells. After 8 h, the transwell insert was removed. The monocytes in the lower transwell were cultured for an additional 64 h to allow differentiation to occur. To generate the control DCs, monocytes were cultured in monocyte medium with the addition of 300 U/ml recombinant human GM-CSF (Berlex Labs, Montville, NJ, USA) and 200 U/ml recombinant human IL-4 (Peprotech, Rocky Hill, NJ). To test whether factors produced by NKT cells had a dominant effect on the DC phenotype, monocytes were cultured in medium containing 300 U/ml of GM-CSF and 200 U/ml of IL-4, with a transwell insert containing a 2:1 mixture of NKT cells and monocytes present for the first 8 h. To investigate the effect of IL-6 on DC phenotype, monocytes were cultured with 300 U/ml recombinant human GM-CSF and 2 U/ml recombinant human IL-13, in the presence or absence of 25-100 ng/ml recombinant human IL-6 (Peprotech).
Flow cytometric analysis of APC phenotype
Commercially available antibodies against the following markers were used for flow cytometric staining: CD13 (clone WM15), CD11b (ICRF-44), CD11c, (3.9), CD40 (HB14), CD33 (HIM3-4), CD80 (2D10), CD86 (IT2.2), CD83 (HB15e), and negative control murine IgG1 (MOPC-21), negative control murine IgG2b (MPC-11), (all from Biolegend, San Diego, CA, USA); CD34 (8G12, BD Biosciences, Franklin Lakes, NJ, USA), DC-LAMP (104.G4, Beckmann Coulter, Fullerton, CA, USA). Anti-HLA-DR (L243), Anti-CD3 (SPVT3b) mAbs and a negative control IgG2a mAb (UPC10) were conjugated to NHS activated Alexa 488 or Alexa 647 according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Staining and analysis were performed as described previously [46]. Samples were analyzed on a Becton Dickinson LSRII flow cytometer, and analysis was performed using Flowjo software (TreeStar Inc., Ashland, OR, USA).
APC cytokine production
NKT-instructed APCs or control DCs (1.0 × 105 cells) were incubated in medium containing 250 ng/ml LPS purified from Salmonella typhimurium (Sigma-Aldrich). Culture supernatants were collected at the specified time points, and tested in triplicate by ELISA for IL-12p70, IL-12p40, or IL-10 (Biolegend). The accumulated cytokine concentrations at each time point were determined by comparison to a standard curve of the relevant recombinant cytokine.
Stimulation of T cell IFN-γ secretion
Total T cells were purified from fresh peripheral blood by using a mixture of magnetic beads to deplete monocytes, B cells, and blood DCs, or by using a pan T cell isolation kit (Miltenyi). Memory T cells were isolated from the total T cell pool by positive selection of CD45RO+ cells (Miltenyi). NKT-instructed APCs and control DCs were matured by exposure to LPS for 8 h, then washed and cultured for 24 h at a 1:2 ratio with total or memory allogeneic T cells. Alternatively, where indicated, NKT-instructed APCs and control DCs were LPS-matured and pulsed with TSST-1 superantigen (Sigma–Aldrich) then cultured with total autologous T cells. Supernatants of the T cell and APC cocultures were tested after 24 h for IFN-γ using a standardized ELISA (BD Biosciences). To assess the responses of recently primed T cells, T cells were first incubated for 3 days at 5:1 ratio with the indicated allogeneic APCs, and then washed and restimulated by exposure to the indicated APCs at 2:1 ratio, and supernatants were collected after 24 h and tested for IFN-γ. APCs used in the first and second exposures were prepared from the same donor so that the allogeneic stimulation remained constant.
Stimulation of T cell proliferation
T cells were isolated from PBMCs as described above, and labeled with 2.5 μM 5-CFSE; Molecular Probes, Eugene, OR, USA). The T cells were cultured at a 50:1 or 100:1 ratio with allogeneic LPS-matured APCs. Alternatively, the T cells were cultured with autologous LPS-matured APCs that were pulsed with the indicated concentrations of TSST-1 superantigen, or with autologous APCs that were matured by exposure to LPS in the presence of 0.25 Lf/ml Tetanus toxoid (Massachusetts Biological Labs, North Worcester, MA, USA) or 1 μg/ml Epstein-Barr virus (EBV) antigen (Viral Antigens, Inc., Memphis, TN, USA). Proliferation was assessed on day 7 by flow cytometric analysis. The percentage of live T cells that had undergone cell division was determined by gating on DAPI-negative CD3+ cells, and assessing the fraction that showed diminished CFSE fluorescence intensity.
Stimulation of inflammatory responses in vivo
APCs were generated as described above and antigen pulsed with (0.25 Lf/ml Tetanus toxoid or 10 μg/ml EBV antigen), or treated with vehicle alone, in the presence of LPS for 8 h. The APCs (1×105 cells) were mixed with 10 × 106 purified autologous T cells and immediately injected subcutaneously into the footpads of CB17 SCID mice, as described previously [47, 48]. Control footpads were injected in parallel with PBS (no cells) or with T cells alone without APCs. Footpads were measured prior to injection using a dial thickness gauge, and remeasured 24 h after injection. The thickness of each footpad prior to injection was deducted from the postinjection value to obtain the net swelling, expressed in units of 10−3 mm.
Histological analysis
Immunohistochemistry was performed on mouse footpads that were taken for analysis 24 h after injection of human cells or PBS. Footpads were fixed in 10% neutral buffered formalin for 8–24 h, then decalcified using formic acid, and paraffin-embedded. Sections (5 μm thick) were deparaffinized and rehydrated, and antigen retrieval was performed by incubation with pepsin at 37°C for 5 min or with Rodent Decloaker solution (Biocare Medical, Concord, CA, USA) in an 80°C water bath for 3 h. Serial sections were stained with hematoxylin and eosin or blocked with Rodent M Block (Biocare Medical), and labeled with antibodies against human CD45 (Biocare Medical) or mouse Ly6G (eBioscience). Endogenous peroxidases were quenched using 3% hydrogen peroxide, and antibody labeling was detected using HRP conjugated second step reagents, then visualized with diaminobenzidine chromogen (DakoCytomation, Glostrup, Denmark).
RESULTS
In our previous studies, we found that exposure to soluble factors secreted by human NKT cells induced primary human monocytes to differentiate into cells that resembled immature myeloid DCs [43]. The DC differentiation appeared to be mainly due to GM-CSF and IL-13, cytokines that were produced by human NKT cells in response to recognition of CD1d on the monocytes [43]. However, many other factors were also present in the NKT cell-monocyte supernatants [43], and therefore, we reasoned that the biological mixture of factors produced by NKT cells might have different effects on the nature of the resulting DCs than the effects of recombinant cytokines alone. Hence, in this study, we set out to investigate the functional properties of “NKT-instructed” APCs that were differentiated by exposure to soluble factors from NKT cells, by comparing them to “control” DCs that were generated by culturing monocytes with recombinant GM-CSF and IL-4.
To isolate the role of soluble factors produced by the NKT cells, we used a transwell system to generate NKT-instructed APCs without the presence contaminating NKT cells. Monocytes alone were placed in the lower transwells and exposed to inserts containing a mixture of NKT cells and monocytes. The inserts were removed after 8 h, and the monocytes in the lower well were further incubated for an additional 2 days. The lower-well cells were then harvested for use in the analyses described below. Because we have found that human NKT cells that are activated by recognition of auto-antigens mainly secrete GM-CSF and IL-13, whereas those that are stimulated by α-GalCer also secrete other cytokines (such as IFN-γ and IL-4) that may influence monocyte differentiation [49], we compared NKT-instructed APCs in which the NKT cells were stimulated by untreated monocytes (i.e., presenting auto-antigen), with those that differentiated in response to NKT cells that were stimulated by α-GalCer pulsed monocytes.
Phenotype of NKT-instructed APCs
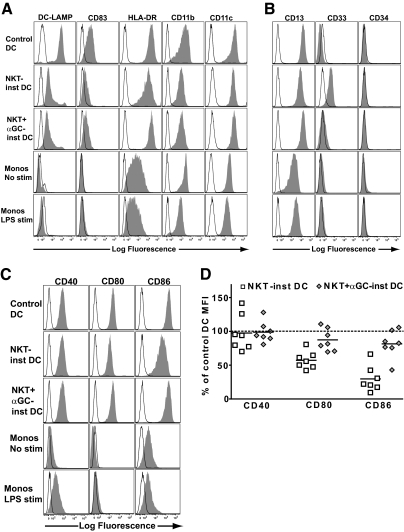
The NKT-instructed and control DCs were stimulated with LPS purified from Salmonella typhimurium and analyzed by flow cytometry for expression of markers associated with mature myeloid DCs. Control DCs showed high intracellular expression levels of DC-LAMP, and expressed CD83, HLA-DR, CD11b, and CD11c on the cell surface (Fig. 1A). The NKT-instructed APCs consistently had clearly detectable intracellular DC-LAMP, and generally stained positively for cell surface CD83, although the expression levels of these markers were lower than those of the control DCs (Fig. 1A). In contrast, neither unstimulated monocytes (cultured in medium alone for 2 days) nor monocytes stimulated by exposure to LPS for two days showed positive staining for DC-LAMP or CD83 (Fig. 1A). HLA-DR, CD11b, and CD11c were all positive on the cell surface of NKT-instructed APCs, and the expression levels of these markers appeared more similar to the control DCs than to the unstimulated or LPS-treated monocytes (Fig. 1A).
Figure 1.
Phenotype NKT-instructed APCs. Purified peripheral blood monocytes were cultured with recombinant GM-CSF and IL-4 (Control DC), or exposed to transwell inserts containing NKT cells with untreated or α-GalCer pulsed monocytes (NKT-inst APC or NKT+αGC-inst APC, respectively). After 3 days of differentiation, the antigen-presenting cells (APCs) were washed and then matured by exposure to LPS for an additional 48 h. As controls, monocytes cultured in medium alone for 2 days (Monos No stim), or monocytes treated with LPS for 2 days (Monos LPS stim), were also analyzed. (A) Flow cytometric analysis of expression of markers associated with mature myeloid dendritic cells (DCs). The APCs were stained with the indicated mAbs (solid histograms) or with isotype-matched negative control antibodies (open histograms). For analysis of DC-LAMP, the APCs were fixed and permeabilized prior to staining. One representative experiment out of 7 independent analyses is shown. (B) Analysis of expression of markers associated with myeloid-derived suppressor cells (MDSCs). Results are representative of 7 independent experiments. (C) Analysis of expression of costimulatory molecules. (D) Cell surface expression levels of the indicated costimulatory molecules on APCs that were exposed to NKT cells with unpulsed monocytes (white squares) or NKT cells with α-GalCer pulsed monocytes (gray diamonds), shown as a percentage of the corresponding marker on control DCs. Each symbol represents data from one independent experiment.
Since other myeloid APC types such as MDSCs can have a phenotype resembling myeloid DCs [50], we further investigated the NKT-instructed APCs for expression of markers found on these cells. The control and NKT-instructed myeloid cells appeared similar in their expression of CD13 (all brightly positive) and CD34 (all negative); however, the NKT-instructed APCs expressed higher levels of CD33 than the control DCs (Fig. 1B). Neither unstimulated nor LPS-treated monocytes showed expression of CD33 (Fig. 1B). Thus, the NKT-instructed APCs acquired a phenotype resembling myeloid DCs but also showed up-regulation of a marker that has been reported on MDSCs.
We next tested for expression of costimulatory ligands. The NKT-instructed APCs expressed cell surface levels of CD40, CD80, and CD86 that were clearly higher than those of unstimulated or LPS-treated monocytes (Fig. 1C). The cell surface expression levels of CD40 on NKT-instructed APCs were comparable to those of control DCs (Fig. 1D). APCs that were exposed to auto-antigen-activated NKT cells generally showed slightly lower expression levels of CD80 and CD86 than the control DCs, whereas those that were induced by α-GalCer-activated NKT cells showed CD80 and CD86 expression levels that were similar to those of the control DCs (Fig. 1D).
Cytokine production by NKT-instructed APCs
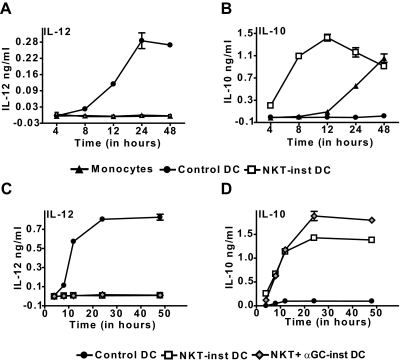
To further investigate the phenotype of the NKT-instructed APCs, we tested their cytokine secretion after exposure to LPS. Whereas LPS treatment resulted in robust IL-12 production by the control DCs, there was little or no detectable IL-12 secretion by the NKT-instructed APCs or by LPS-treated monocytes (Fig. 2A). In contrast, IL-10 secretion by the control DCs was minimal, while both NKT-instructed APCs and monocytes produced substantial amounts of this cytokine (Fig. 2B). Notably, we consistently found that the NKT-instructed APCs showed a rapid burst of IL-10 production, whereas LPS-stimulated monocytes showed slower kinetics of IL-10 secretion (Fig. 2B). Thus, the NKT-instructed APCs showed a pattern of cytokine production that was distinct from either control DCs or activated monocytes.
Figure 2.
APC cytokine production. Supernatants were withdrawn from cultures of control DCs, NKT-instructed APCs, or fresh monocytes at the indicated time points after the addition of LPS, and tested by standardized ELISAs for IL-12p70 (A) or IL-10 (B). (C and D) Comparison of APCs induced by auto-antigen or α-GalCer-activated NKT cells. Symbols and error bars show the means and standard deviations of 3 replicates (note that standard deviations are not always visible on the scales shown). Results are representative of 3-7 independent experiments. Similar results were obtained using ELISA reagents specific for IL-12p40 (data not shown).
We have previously found that NKT cells that are activated by recognition of auto-antigens secrete little or no IFNγ or IL-4, whereas those that are activated by the potent agonist α-GalCer robustly produce these cytokines [43, 49]. Since exposure to IFN-γ has been associated with enhancing IL-12p70 production by myeloid DCs, we investigated whether the route of NKT cell activation influences the pattern of cytokine production by the resulting APCs. Remarkably, APCs that differentiated in response to α-GalCer or auto-antigen activated NKT cells showed similar patterns of cytokine production upon LPS stimulation: no detectable IL-12p70 secretion, combined with rapid production of IL-10 (Fig. 2, C and D). Notably, the NKT-instructed APCs also did not produce detectable IL-12p40 within the first 48 h after LPS stimulation (data not shown). Together, these results suggested that the NKT-instructed APCs might differ from the control DCs in their effects on MHC-restricted T cells.
Silencing of T cell IFN-γ secretion
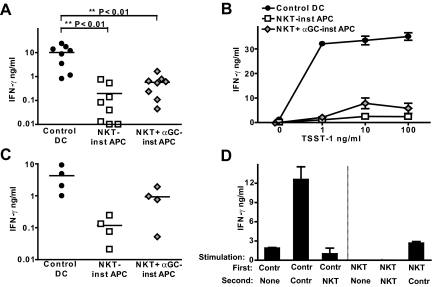
We tested the ability of the NKT-instructed APCs to stimulate IFN-γ secretion by freshly isolated peripheral blood T cells. Whereas control DCs readily stimulated IFN-γ production within 24 h by allogeneic T cells, the IFN-γ levels were ∼10 to 100-fold lower in response to NKT-instructed APCs (Fig. 3A). We had observed that the cell surface expression levels of MHC class II are typically similar on the NKT-instructed APCs and control DCs (Supplemental Fig. 1), suggesting that they should provide similar amounts of TCR stimulation in this alloreactive activation system. To further investigate whether the defect in promoting T cell IFN-γ secretion could be overcome by increased TCR stimulation, we used TSST-1 superantigen to directly ligate the TCRs of Vβ2+ T cells to MHC class II molecules on the DCs. There was only minimal IFN-γ secretion in response to NKT-instructed APCs that were coated even with high doses of superantigen (Fig. 3B), indicating that increasing the strength of the TCR stimulation was not sufficient to compensate for the defect. Notably, inclusion of a monoclonal antibody to block IL-10 did not result in enhanced T cell IFN-γ secretion in response to the NKT-instructed APCs (Supplemental Fig. 2), suggesting that lack of IFN-γ production was not simply due to inhibition by IL-10.
Figure 3.
Stimulation of T cell IFN-γ production. APCs were prepared as described in Figure 1 and activated by exposure to LPS for 8 h. (A) Control DCs or NKT-instructed APCs were incubated with allogeneic peripheral blood T cells (i.e., total T cells), and supernatants were withdrawn after 24 h and analyzed for IFN-γ by ELISA. Each symbol corresponds to the amount of cytokine (shown on a log10 scale) from an independent allogeneic T cell + DC pairing. (B) Control DCs or NKT-instructed APCs were treated with the indicated concentrations of TSST-1 superantigen and used to stimulate IFN-γ secretion by autologous T cells. Symbols and error bars show the means and standard deviations of 3 replicates. The results are from one representative experiment out of 3 independent analyses. (C) APCs were incubated with allogeneic memory T cells (i.e., the CD45RO+ subset of peripheral blood T cells). (D) Stimulation of IFN-γ secretion by preactivated T cells. Allogeneic T cells were incubated for 3 days with control DCs or NKT-instructed APCs as a first stimulus. The T cells were harvested and then stimulated with the indicated APCs, and supernatants were collected after 24 h and analyzed for IFN-γ by ELISA. The plot shows the means and standard deviations of 3 replicate samples. Similar results were obtained in 3 independent experiments.
It is known that T cell IFN-γ secretion can be enhanced by exposure to IL-12p70; however, the functional responses of memory or recently primed T cells are less dependent on such costimulatory factors than those of naive T cells. Therefore, we tested IFN-γ secretion by purified CD45RO+ T cells (the memory subset) in response to the NKT-instructed APCs. Surprisingly, memory T cells also showed markedly reduced IFN-γ secretion in response to the NKT-induced APCs (Fig. 3C). To investigate the responses of recently primed T cells, total T cells were first exposed to allogeneic control DCs for 3 days. The T cells were then harvested and washed and exposed again to either control DCs or NKT-instructed APCs, and IFN-γ secretion was measured after 24 h. T cells that were primed by control DCs and then stimulated again by exposure to control DCs showed significantly enhanced IFN-γ secretion compared with those that were stimulated by control DCs only once (Fig. 3D, left side). T cells that were primed by control DCs and then stimulated by exposure to NKT-instructed APCs showed only low levels of IFN-γ production (Fig. 3D, left side), suggesting that the NKT-instructed APCs failed to efficiently induce IFN-γ production by recently primed T cells. When the reverse experiment was performed and the T cells were exposed to NKT-instructed APCs first and then restimulated by exposure to control DCs, we observed levels of IFN-γ secretion that were similar to those of unprimed T cells, suggesting that the NKT-instructed APCs also failed to efficiently prime the T cells (Fig. 3D, right side). However, because these T cells did produce clearly detectable amounts of IFN-γ, it seems that an initial exposure to NKT-instructed APCs does not prevent subsequent responses to more highly stimulatory APCs.
Regulation of T cell proliferation
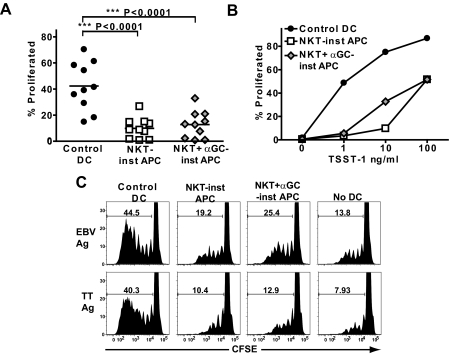
We next tested the effects of NKT-instructed APCs on T cell proliferation. We observed that compared with control DCs, APCs that differentiated in response to 6 different NKT cell clones or to a polyclonal NKT cell line all showed reduced abilities to stimulate proliferation by allogeneic human peripheral blood T cells (Supplemental Fig. 3). To further investigate, we compared APCs that differentiated in response to a single NKT cell clone (J24L.17) that was stimulated either by auto-antigen or by α-GalCer. Because costimulation by B7-family receptors on APCs, such as CD80 and CD86, is critical for stimulating T cell proliferation [51], it might be predicted based on their lower CD80 and CD86 expression levels (Fig. 1D), that APCs that differentiated in response to auto-antigen-activated NKT cells would be less potent stimulators of T cell proliferation than the control DCs, while those that differentiated in response to α-GalCer-activated NKT cells should be similar to the control DCs. Surprisingly, however, both types of NKT-instructed APCs stimulated significantly lower levels of allogeneic T cell proliferation than the control DCs (Fig. 4A). Approximately 100-fold higher concentrations of superantigen were required to stimulate similar levels of T cell proliferation by the NKT-instructed APCs compared with the control DCs (Fig. 4B). Additionally, we found that NKT-instructed APCs that were pulsed with recall antigens (Epstein-Barr virus protein or tetanus toxoid) promoted less T cell proliferation than similarly pulsed control DCs (Fig. 4C), indicating that the NKT-instructed APCs also impact the responses of antigen-specific memory T cells.
Figure 4.
Stimulation of T cell proliferation. (A) Allogeneic peripheral blood T cells were labeled with CFSE and cultured with the indicated APCs for 7 days. Each symbol shows the percentage of the live T cell population that underwent cell division (as assessed by reduction of CFSE fluorescence) from one allogeneic T cell + DC pairing. (B) Autologous peripheral blood T cells were stimulated by APCs that were treated with the indicated concentrations of TSST-1 superantigen. The results shown are from one representative experiment out of 3 independent analyses. (C) APCs were pulsed with purified Epstein-Barr virus protein (EBV Ag) or with Tetanus Toxoid (TT Ag) and used to stimulate autologous peripheral blood T cells. The plots show the CFSE fluorescence of DAPI−/CD3+ cells, with gate markers indicating the percentage of cells that show evidence of having proliferated. Similar results were obtained in 3 independent experiments.
Dominant effect of NKT-instruction
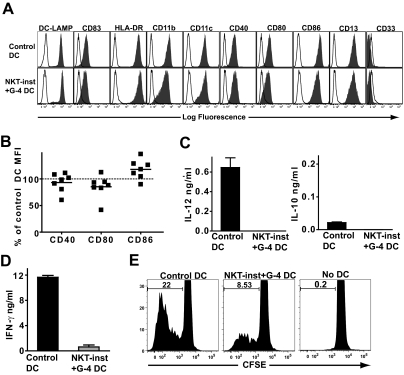
The failure of the NKT-instructed APCs to efficiently activate memory T cells might be due to incomplete DC maturation or “licensing”, perhaps because cytokine production by the NKT cells is not strong enough to stimulate full DC differentiation. To investigate this, we differentiated monocytes by culturing them in medium containing recombinant GM-CSF and IL-4 (i.e., the protocol used to generate the control DCs) and included exposure to a transwell insert containing NKT cells and monocytes (i.e., the source of the factors responsible for producing the NKT-instructed APCs). The resulting myeloid cells had a cell surface phenotype that appeared identical to that of the control DCs, and expressed very similar levels of costimulatory molecules (Fig. 5, A and B). After stimulation with LPS they produced little or no IL-12, but also did not produce elevated levels of IL-10 (Fig. 5C). Nevertheless, these DCs stimulated little INF-γ secretion by peripheral blood T cells and also promoted substantially less T cell proliferation than control DCs that had not been exposed to NKT cell factors (Fig. 5, D and E). Thus, exposure to factors produced by NKT cells has a dominant effect on the functional properties of the resulting APCs.
Figure 5.
Dominant effect of NKT cell instruction. (A) Monocytes were cultured in medium containing recombinant GM-CSF and IL-4 alone (Control DC) or with recombinant GM-CSF and IL-4 in the presence of a transwell insert containing NKT cells and monocytes (NKT-inst + G-4 DC), then matured with LPS. The resulting cells were stained with the indicated mAbs (solid histograms) or with isotype-matched negative control mAbs (open histograms). The results are from one representative experiment out of 7 independent analyses. (B) Cell surface expression levels of the indicated costimulatory molecules on DCs that were cultured with GM-CSF and IL-4 in the presence of NKT cell factors, shown as a percentage of the corresponding marker on control DCs. Each symbol represents data from one independent experiment. (C) DCs were stimulated with LPS, and after 24-h culture supernatants were tested by ELISA for IL-12p70 and IL-10. Results are representative of 4 independent experiments. Similar results were obtained using ELISA reagents specific for IL-12p40 (data not shown). (D) Stimulation of IFN-γ secretion by allogeneic T cells. Results are from one representative experiment out of 4 independent analyses. (E) Stimulation of allogeneic T cell proliferation. The histograms show the CFSE intensity of the live T cells (i.e., DAPI−/CD3+ cells). Results shown are from one representative experiment out of 3 independent analyses.
Role of IL-6
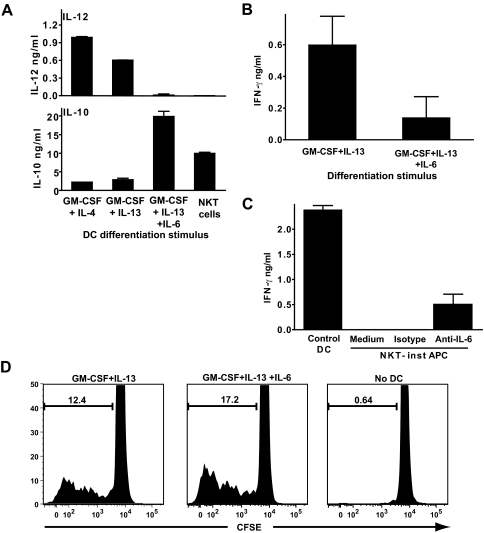
In our earlier studies, we found that NKT cell GM-CSF and IL-13 were the main factors responsible for inducing monocyte differentiation into DCs [43]. However, DCs that are differentiated by culturing monocytes with recombinant GM-CSF and IL-13 generally produce clearly detectable amounts of IL-12p70 and do not produce substantially elevated levels of IL-10 compared with control DCs (Fig. 6A). Therefore, we tested the effects of other factors that were detected at substantial levels in supernatants of NKT cells cocultured with monocytes, including IL-6 (present at ∼10-20 ng/ml), G-CSF (5-15 ng/ml), and IL-1β (1-3 ng/ml) [43]. Addition of these concentrations of recombinant G-CSF or IL-1β to monocytes cultured with GM-CSF and IL-13 did not have a significant impact on IL-12p70 and IL-10 production by the resulting DCs (data not shown). However, inclusion of similar levels of IL-6 resulted in DCs that resembled the NKT-induced DCs, showing little or no IL-12p70 and high levels of IL-10 (Fig. 6A). Including G-CSF and/or IL-1β along with the IL-6 did not significantly alter the DC response (data not shown). Hence, the presence of IL-6 during GM-CSF and IL-13 induced monocyte differentiation is sufficient to produce an IL-12 and IL-10 cytokine production pattern resembling that of the NKT-instructed APCs.
Figure 6.
Role of IL-6 in determining the functional properties of NKT-instructed APCs. (A) Monocytes were differentiated by culture with the indicated recombinant cytokines or by exposure to transwell inserts containing human NKT cells and unpulsed monocytes (NKT-inst APCs). The APCs were washed and stimulated with LPS, and after 24 h, culture supernatants were tested in triplicate by standardized ELISA for IL-12p70 or IL-10. Similar results were obtained in 5 independent experiments using concentrations of IL-6 ranging from 25 to 100 ng/ml. (B) Allogeneic peripheral blood T cells were incubated with DCs that were differentiated using recombinant GM-CSF and IL-13; or using GM-CSF, IL-13 and IL-6 (100 ng/ml). Supernatants were removed after 24 h and tested in triplicate by standardized ELISA for IFN-γ. (C) APCs were generated by culture with recombinant GM-CSF and IL-4 (Control DC); or by exposure to transwell inserts containing NKT cells and monocytes in the presence of an anti-IL-6 blocking mAb or an isotype-matched negative control mAb, or with no added mAb (Medium). Allogeneic peripheral blood T cells were incubated with the indicated APCs, and supernatants were withdrawn after 24 h and tested in triplicate for IFN-γ. (D) Allogeneic peripheral blood T cells were labeled with CFSE and cultured for 7 days alone (No DC) or with DCs that were generated using GM-CSF and IL-13; or using GM-CSF, IL-13, and IL-6 (100 ng/ml). The plots show the CFSE intensity of the live T cells (i.e., DAPI−/CD3+ cells).
We further investigated the role of IL-6 in determining the ability of the APCs to stimulate T cells. DCs that were generated by culturing monocytes with GM-CSF and IL-13 in the presence of IL-6 showed significantly reduced stimulation of T cell IFN-γ secretion compared with those that were cultured with GM-CSF and IL-13 alone (Fig. 6B). The addition of a blocking antibody against IL-6 to NKT cell supernatants resulted in monocyte differentiation into APCs that stimulated some T cell IFN-γ secretion, although they were not as potent as the control DCs (Fig. 6C). This supports the conclusion that IL-6 in NKT cell supernatants is sufficient to induce APCs that are poor stimulators of T cell IFN-γ secretion. Importantly, however, the presence of IL-6 during monocyte differentiation was not sufficient to reproduce the failure of the NKT-instructed APCs to efficiently stimulate T cell proliferation (Fig. 6D). Thus, we conclude that additional factors produced by NKT cells contribute to the functional properties of the NKT-instructed APCs.
Noninflammatory phenotype in vivo
To further investigate the effects of the NKT-instructed APCs on T cell-mediated responses, we used an in vivo assay that assesses inflammatory tissue swelling that is initiated by human T cells. Human T cells and antigen-pulsed APCs are injected into the footpads of SCID mice. Antigen-dependent activation of the T cells leads to a localized inflammatory response that is characterized by edema and a murine neutrophilic infiltrate [47]. Swelling responses can be prevented by blocking either the human IL-17 or IFN-γ pathways, depending on the nature of the human cells responsible for initiating the inflammatory cascade [52]. Interestingly, swelling responses in this assay system can be prevented by human regulatory cells [53], and thus, this method provides a way of assessing the overall outcome of the proinflammatory and inflammation-dampening mechanisms occurring during interactions between human lymphocytes and APCs.
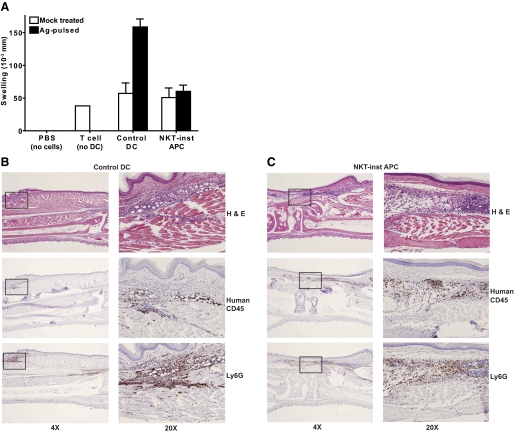
The NKT-instructed APCs and control DCs were pulsed with recall antigens (EBV protein or tetanus toxoid) or mock treated, then matured by exposure to LPS, and washed and injected with purified autologous peripheral blood T cells into the footpads of SCID mice. Footpads that were injected with buffer alone (i.e., no human cells) showed no detectable swelling after 24 h, whereas those that received T cells but no APCs, or T cells mixed with mock-treated APCs, showed modest amounts of swelling (Fig. 7A). Robust swelling responses were observed when antigen-pulsed control DCs were coinjected with the T cells, but there was little antigen-dependent swelling when NKT-instructed APCs were used (Fig. 7A). The inability to promote a swelling response did not appear to be due to a failure of the NKT-instructed DCs to take up and process protein antigens, since NKT-instructed DCs were able to present tetanus toxoid to antigen-specific human T cell clones in vitro (data not shown).
Figure 7.
Noninflammatory phenotype of NKT-instructed APCs. (A) Control DCs or NKT-instructed APCs were pulsed with recall antigens (solid bars) or mock-treated (open bars) in the presence of LPS for 8 h, then mixed with purified autologous T cells and injected into the footpads of CB17 SCID mice, and the footpad swelling was measured after 24 h. Control footpads were injected with sterile PBS buffer (no cells) or with human T cells that were not mixed with APCs (no DC). The plot shows the means and standard deviations of the net swelling from 6-10 independent experiments. (B and C) Histological analysis of murine footpads that were injected with human T cells and antigen-pulsed control DCs (B) or human T cells and antigen-pulsed NKT-instructed APCs (C). Serial sections were stained with hematoxylin and eosin (top panels), or immunolabeled for human CD45 (middle panels), or for the murine neutrophil marker Ly6G (bottom panels). Panels on the left show longitudinal serial sections of footpad at ×4 magnification. The boxed areas of the left panels are shown at ×20 magnification on the right.
To further investigate the basis for the swelling response in this assay system, we performed histological analysis of footpads that were injected with T cells and antigen-pulsed control DCs or NKT-instructed APCs. Staining with anti-human CD45 to detect human leukocytes revealed that human cells were present in dermal and subdermal areas of the footpads in both cases (Fig. 7, B and C, middle panels). Staining with Ly-6G, a marker of murine neutrophils, revealed that in both cases, substantial numbers of murine neutrophils were colocalized with the human cells (Fig. 7, B and C, bottom panels). Thus, in both cases murine neutrophils were recruited to tissue sites infiltrated by human cells. However, there appeared to be more infiltration of surrounding muscle tissues by human leukocytes and murine neutrophils in the presence of the control DCs than the NKT-instructed APCs and also evidence of more widespread edema (Fig. 7, B and C, and data not shown). Hence, in the presence of the NKT-instructed APCs, the recruitment of neutrophils was not averted, but there appeared to be a block in the inflammatory cascade such that neutrophilic infiltration of surrounding tissues and widespread edema were prevented.
DISCUSSION
The results presented here show for the first time that factors secreted by human NKT cells cause monocyte-derived APCs to acquire a phenotype that suppresses Th1 responses. Previous studies have shown that innate lymphocytes, including NK cells, γδ T cells, NKT cells, and other CD1-restricted T cells can stimulate immature human myeloid DCs to mature into IL-12p70-producing cells by providing costimulatory signals, such as CD40L and IFN-γ [33, 54,55,56]. Observations of this type have led to the hypothesis that innate lymphocytes play a critical role in enhancing the initiation of adaptive immune responses during microbial challenge by amplifying the licensing of DCs [57]. This hypothesis is consistent with data showing that mice that are deficient in NKT cells are more susceptible to certain bacterial, fungal, viral, or parasitic infections [58]. However, this model does not address the role of NKT cells in promoting peripheral tolerance, a role that has been well established by studies of autoimmune disease model systems such as type I diabetes in NOD mice, but that is not yet well understood mechanistically [59]. Thus, the data presented here add to the picture of how NKT cells impact immune function by showing that they can also instruct the differentiation of APCs that suppress adaptive T cell responses.
In a previous analysis, the effects of human NKT cells on DC function appeared to depend on the nature of the antigenic stimulus received by the NKT cells [33]. In that study immature myeloid DCs were generated by culturing peripheral blood monocytes with GM-CSF and IL-4 and were then exposed to NKT cells. In the presence of α-GalCer, the NKT cells promoted the maturation of proinflammatory DCs by a CD40L-dependent mechanism, whereas in the absence of α-GalCer they induced mature DCs that produced high levels of IL-10 and promoted T cell proliferation but failed to induce IFN-γ secretion, and this effect appeared to be mainly due to NKT cell secretion of TNF-α [33]. In contrast, in the pathway characterized here, the strength of the antigenic stimulus (i.e., α-GalCer or auto-antigen) had little effect on the functional properties of the resulting APCs. Additionally, the APCs derived in the current analysis had more profound regulatory effects, since they not only silenced T cell IFN-γ secretion but also severely dampened T cell proliferative responses.
A major difference in the system used here is that NKT cell instruction is mediated during monocyte differentiation rather than after the monocytes have already been differentiated by exposure to GM-CSF and IL-4. Thus, perhaps NKT cells have a more potent effect during monocyte differentiation than during the change from immature to mature DCs. Our results indicate that soluble factors present in the supernatants of NKT cells cultured with monocytes have a dominant effect on the properties of monocyte-derived APCs, since monocytes that were differentiated by exposure to a combination of NKT cell factors along with GM-CSF and IL-4 resembled the NKT-instructed APCs and not the control DCs. Thus, we conclude that auto-antigenic activation is sufficient to induce NKT cell secretion of the factors responsible for the properties of the NKT-instructed APCs and that although our previous studies have shown that α-GalCer-mediated activation of NKT cells stimulates them to secrete additional cytokines [49], these do not overcome the effects of the suppressive-phenotype factors.
The identity of the NKT cell factor(s) responsible for the functional phenotype of the NKT-instructed APCs remains unclear. Our data suggest that the presence of IL-6 in cocultures of NKT cell and monocytes is sufficient to produce APCs that fail to promote strong Th1 responses, but that other factor(s) are required for the lack of efficient T cell proliferation in response to the NKT-instructed APCs. Additionally, the mechanisms by which NKT-instructed APCs inhibit T cell responses require further analysis. The failure of NKT-instructed APCs to activate T cell responses does not appear to be due to insufficient expression of costimulatory molecules, since APCs that were differentiated by GM-CSF, IL-4, and NKT cell factors had a cell surface phenotype that appeared essentially identical to that of proinflammatory DCs, yet nevertheless showed suppressive effects (Fig. 5). IL-10 secretion also does not seem to be responsible, since DCs that were generated by exposure to GM-CSF and IL-4 in the presence of NKT cell factors did not secrete IL-10 yet nevertheless showed poor stimulation of T cell IFN-γ production and proliferation (Fig. 5, C–E). Moreover, the addition of anti-IL-10 blocking antibodies did not enhance T cell responses (Supplemental Fig. 2). Hence, further studies will be required to elucidate how the effects of the NKT-instructed APCs on T cells are mediated.
The potency of the regulatory effects of the NKT-instructed APCs is illustrated by their almost completely noninflammatory phenotype in an assay that measures the ability of human leukocytes to initiate an inflammatory response in vivo. In this assay, antigen-dependent activation of human T cells is transmitted to murine inflammatory effector pathways, resulting in measurable tissue swelling. Depending on the kinds of human cells and antigens used to initiate the response, swelling can be blocked by antibodies against human IFN-γ or human IL-17 [52], suggesting that the murine inflammatory response is sensitive to signals arising from different types of human T cells. The human leukocyte-derived signals responsible for activating the murine inflammatory response in this system have not yet been fully characterized; however, we have observed that this is a highly reproducible system (for example, note that the data from NKT-instructed APC and control DC-injected footpads shown in Fig. 7A represent the means of 10 independent experiments, yet the standard deviations are quite low) and that it is a sensitive means of detecting antigen-specific T cell activation. Interestingly, the histology results suggest that the block in the inflammatory cascade is not at the level of neutrophil recruitment, but at a later stage. Although murine neutrophils are reproducibly recruited to tissue sites infiltrated by human leukocytes, we have observed in many experiments that when there is little or no footpad swelling, the neutrophils seem to localize near the human cells but are not well integrated among them, whereas when swelling is observed, the human cells and neutrophils appear fully intermixed (data not shown). Additionally, increased neutrophil and leukocyte infiltration of surrounding muscle tissues appears to be associated with increased edema. We conclude from this analysis that there is a relatively late block in the inflammatory cascade in the presence of NKT-instructed APCs, but further studies will be required to characterize the mechanisms involved.
Another very interesting area for further investigation is the location and nature of the interactions between NKT cells and monocytes in vivo. Monocytes do not express high cell surface levels of costimulatory molecules and do not efficiently process and present peptide antigens and are therefore not usually effective stimulators of MHC-restricted T cell responses. However, they do constitutively express CD1d and can serve as efficient APCs for CD1d-restricted NKT cells, probably because NKT cells do not require the same types of costimulation as classical adaptive T cells [29, 30, 43]. Hence, NKT cells may be uniquely oriented toward interactions with monocytes as APCs. Moreover, monocytes circulate throughout the blood, lymphoid tissues, and bone marrow, which are all sites that have been found to contain NKT cells [46, 60, 61], and monocytes and NKT cells share expression of the chemokine receptors CCR5, CCR2, and CX3CR1 [62, 63]. Thus, NKT cells and monocytes are likely to colocalize in vivo under both normal and inflammatory conditions. It is therefore conceivable that monocytes and NKT cells could interact in vivo in a way that reflects the “instruction” effect that we report here, although this remains to be investigated.
However, in addition to their in vivo functions, monocytes are a precursor cell population of choice for human immunotherapeutic applications because of their ready availability and developmental plasticity. Thus, monocyte-derived APCs are of great interest for their clinical potential. Understanding the functions of different types of myeloid APCs, such as the NKT-instructed APCs described here, is likely to be of direct benefit for developing therapeutic approaches that use these cells and may also shed new light on processes that contribute to immunopathology in a variety of conditions.
Supplementary Material
Acknowledgments
Major funding was from the Pew Scholars in the Biomedical Sciences Program (J. E. G.), and National Institutes of Health grant R01 AI060777 to J.E.G. The work was also supported by R21DK077354 and R01AI066219-04 to W. J. B. The authors would like to thank Jason Keaton for providing technical help and Dr. Gurdyal S. Besra for providing α-GalCer.
Footnotes
Abbreviations: α-GalCer=α-galactosylceramide, APC=antigen-presenting cell, CFSE=carboxyfluorescein diacetate succinimidyl ester; DCs=dendritic cells, GM-CSF=granulocyte-macrophage CSF, NKT=natural killer T cell, MDSCs=myeloid-derived suppressor cells, MHC=major histocompatability complex, PBMC=peripheral blood mononuclear cells
References
- Brigl M, Brenner M B. CD1: Antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Gumperz J E, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli S A, Cardell S, Brenner M B. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- Rauch J, Gumperz J, Robinson C, Sköld M, Roy C, Young D C, Lafleur M, Moody D B, Brenner M B, Costello C E, Behar S M. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J Biol Chem. 2003;48:47508–47515. doi: 10.1074/jbc.M308089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Mattner J, Cantu C, III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu Y P, Yamashita T. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Wu D, Kim G, Xing G W, Poles M A, Ho D D, Tsuji M, Kawahara K, Wong C H, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- Mattner J, Debord K L, Ismail N, Goff R D, Cantu C, III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia M R, Zajonc D M, Ben-Menachem G, Ainge G D, Painter G F. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Joyce S. Innate immunity: NKT cells in the spotlight. Curr Biol. 2005;15:R429–R431. doi: 10.1016/j.cub.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Yu K O, Porcelli S A. The diverse functions of CD1d-restricted NKT cells and their potential for immunotherapy. Immunol Lett. 2005;100:42–55. doi: 10.1016/j.imlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach J F, Monteiro R C. Overexpression of natural killer T cells protects Valpha14- Jalpha281 transgenic nonobese diabetic mice against diabetes. J Exp Med. 1998;188:1831–1839. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond K J, Poulton L D, Palmisano L J, Silveira P A, Godfrey D I, Baxter A G. alpha/beta-T cell receptor (TCR)+CD4-CD8- (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187:1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F D, Flodstrom M, Balasa B, Kim S H, Van Gunst K, Strominger J L, Wilson S B, Sarvetnick N. Germ line deletion of the CD1 locus exacerbates diabetes in the NOD mouse. Proc Natl Acad Sci USA. 2001;98:6777–6782. doi: 10.1073/pnas.121169698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Facciotti F, Ghidoli N, Monti P, Olivieri S, Zaccagnino L, Bonifacio E, Casorati G, Sanvito F, Sarvetnick N. Up-regulation of CD1d expression restores the immunoregulatory function of NKT cells and prevents autoimmune diabetes in nonobese diabetic mice. J Immunol. 2004;172:5908–5916. doi: 10.4049/jimmunol.172.10.5908. [DOI] [PubMed] [Google Scholar]
- Jahng A W, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars L T, Laloux V, Goude K, Desbois S, Saoudi A, Van Kaer L, Lassmann H, Herbelin A, Lehuen A, Liblau R S. Cutting edge: V alpha 14-J alpha 281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J Immunol. 2002;168:6007–6011. doi: 10.4049/jimmunol.168.12.6007. [DOI] [PubMed] [Google Scholar]
- Sonoda K H, Exley M, Snapper S, Balk S P, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson D D, Carbone D P, Paul W E, Berzofsky J A. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- Moodycliffe A M, Nghiem D, Clydesdale G, Ullrich S E. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–525. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Yamamoto N, Kinjo Y, Miyagi K, Nakasone C, Uezu K, Kinjo T, Nakayama T, Taniguchi M, Saito A. Critical role of Valpha14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003;33:3322–3330. doi: 10.1002/eji.200324254. [DOI] [PubMed] [Google Scholar]
- Grubor-Bauk B, Simmons A, Mayrhofer G, Speck P G. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J Immunol. 2003;170:1430–1434. doi: 10.4049/jimmunol.170.3.1430. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann C C, Wilson J M, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, Tsuji M. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J D, Hermans I F, Gileadi U, Chong T W, Shepherd D, Salio M, Mathew B, Schmidt R R, Lunt S J, Williams K J. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114:1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Qin H, Kang C Y, Kim S, Kwak L W, Dong C. An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent antilymphoma immunity. Blood. 2007;110:2013–2019. doi: 10.1182/blood-2006-12-061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Smyth M J, Thia K Y, Street S E, Cretney E, Trapani J A, Taniguchi M, Kawano T, Pelikan S B, Crowe N Y, Godfrey D I. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe N Y, Smyth M J, Godfrey D I. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada F M, Borriello F, Sugita M, Watts G F, Koezuka Y, Porcelli S A. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur J Immunol. 2000;30:3468–3477. doi: 10.1002/1521-4141(2000012)30:12<3468::AID-IMMU3468>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Exley M, Garcia J, Wilson S B, Spada F, Gerdes D, Tahir S M A, Patton K T, Blumberg R S, Porcelli S, Chott A. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 2000;100:37–47. doi: 10.1046/j.1365-2567.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M S, Leslie D S, Gumperz J E, Xiong X, Grant E P, Brenner M B. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3:1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- Hermans I F, Silk J D, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris A L, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Kronenberg M, Steinman R M. Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman R M. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang W S, Kim S, Kang C Y. NKT cell ligand α-galactosylceramide blocks the induction of oral tolerance by triggering dendritic cell maturation. Eur J Immunol. 2004;34:2471–2479. doi: 10.1002/eji.200425027. [DOI] [PubMed] [Google Scholar]
- Kojo S, Seino K, Harada M, Watarai H, Wakao H, Uchida T, Nakayama T, Taniguchi M. Induction of regulatory properties in dendritic cells by Vα14 NKT cells. J Immunol. 2005;175:3648–3655. doi: 10.4049/jimmunol.175.6.3648. [DOI] [PubMed] [Google Scholar]
- Naumov Y N, Bahjat K S, Gausling R, Abraham R, Exley M A, Koezuka Y, Balk S B, Strominger J L, Clare-Salzer M, Wilson S B. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc Natl Acad Sci USA. 2001;98:13838–13843. doi: 10.1073/pnas.251531798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y G, Choisy-Rossi C M, Holl T M, Chapman H D, Besra G S, Porcelli S A, Shaffer D J, Roopenian D, Wilson S B, Serreze D V. Activated NKT cells inhibit autoimmune diabetes through tolerogenic recruitment of dendritic cells to pancreatic lymph nodes. J Immunol. 2005;174:1196–1204. doi: 10.4049/jimmunol.174.3.1196. [DOI] [PubMed] [Google Scholar]
- Yue S C, Shaulov A, Wang R, Balk S P, Exley M A. CD1d ligation on human monocytes directly signals rapid NF-κB activation and production of bioactive IL-12. Proc Natl Acad Sci USA. 2005;102:11811–11816. doi: 10.1073/pnas.0503366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santo C, Salio M, Masri S H, Lee L Y, Dong T, Speak A O, Porubsky S, Booth S, Veerapen N, Besra G S. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S, Chen X, Keaton J M, Reddington F, Besra G S, Gumperz J E. NKT cells direct monocytes into a DC differentiation pathway. J Leukoc Biol. 2007;81:1224–1235. doi: 10.1189/jlb.1206718. [DOI] [PubMed] [Google Scholar]
- Brigl M, Bry L, Kent S C, Gumperz J E, Brenner M B. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- Brigl M, van den Elzen P, Chen X, Meyers J H, Wu D, Wong C H, Reddington F, Illarianov P A, Besra G S, Brenner M B. Conserved and heterogeneous lipid antigen specificities of CD1d-restricted NKT cell receptors. J Immunol. 2006;176:3625–3634. doi: 10.4049/jimmunol.176.6.3625. [DOI] [PubMed] [Google Scholar]
- Gumperz J E, Miyake S, Yamamura T, Brenner M B. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrodeguas L, Orosz C G, Waldman W J, Sedmak D D, Adams P W, VanBuskirk A M. Trans vivo analysis of human delayed-type hypersensitivity reactivity. Hum Immunol. 1999;60:640–651. doi: 10.1016/s0198-8859(99)00002-6. [DOI] [PubMed] [Google Scholar]
- Burlingham W J, Jankowska-Gan E. Mouse strain and injection site are crucial for detecting linked suppression in transplant recipients by trans-vivo DTH assay. Am J Transplant. 2007;7:466–470. doi: 10.1111/j.1600-6143.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen X, Rodenkirch L, Simonson W, Wernimont S, Ndonye R M, Veerapen N, Gibson D, Howell A R, Besra G S. Natural killer T-cell autoreactivity leads to a specialized activation state. Blood. 2008;112:4128–4138. doi: 10.1182/blood-2008-05-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S, Gabrilovich D I. Myeloid-derived suppressor cells. Adv Exp Med Biol. 2007;601:213–223. doi: 10.1007/978-0-387-72005-0_22. [DOI] [PubMed] [Google Scholar]
- Greenfield E A, Nguyen K A, Kuchroo V K. CD28/B7 costimulation: a review. Crit Rev Immunol. 1998;18:389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- Burlingham W J, Love R B, Jankowska-Gan E, Haynes L D, Xu Q, Bobadilla J L, Meyer K C, Hayney M S, Braun R K, Greenspan D S. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks R A, Jankowska-Gan E, Xu Q, Burlingham W J. Dendritic cell type determines the mechanism of bystander suppression by adaptive T regulatory cells specific for the minor antigen HA-1. J Immunol. 2007;179:3443–3451. doi: 10.4049/jimmunol.179.6.3443. [DOI] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioli D, Sbrana S, Melandri E, Valiante N M. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie D S, Vincent M S, Spada F M, Das H, Sugita M, Morita C T, Brenner M B. CD1-mediated gamma/delta T cell maturation of dendritic cells. J Exp Med. 2002;196:1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz C, Steinman R M, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- Cardell S L. The natural killer T lymphocyte: a player in the complex regulation of autoimmune diabetes in non-obese diabetic mice. Clin Exp Immunol. 2006;143:194–202. doi: 10.1111/j.1365-2249.2005.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J L, Naidenko O V, Gapin L, Nakayama T, Taniguchi M, Wang C R, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S Y, Hou R, Boyson J E, Means T K, Hess C, Olson D P, Strominger J L, Brenner M B, Gumperz J E, Wilson S B. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J Immunol. 2003;171:2571–2580. doi: 10.4049/jimmunol.171.5.2571. [DOI] [PubMed] [Google Scholar]
- Kim C H, Johnston B, Butcher E C. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V α 24(+)V β 11(+) NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11–16. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.