Abstract
HIV1+ smokers develop emphysema at an earlier age and with a higher incidence than HIV1– smokers. Since human alveolar macrophages (AMs) are capable of producing proteases that degrade extracellular matrix components, we hypothesized that up-regulation of AM matrix metalloproteinases may be associated with the emphysema of HIV1+ smokers. Microarray analysis was used to screen which matrix metalloproteinases (MMPs) genes were expressed by AM of HIV1+ smokers with early emphysema. For each of the MMP genes expressed (MMP-1, -2, -7, -9, -10, -12 and -14), TaqMan PCR was used to quantify the relative expression in AM from four groups of individuals: HIV1– healthy nonsmokers, HIV1– healthy smokers, HIV1– smokers with early emphysema, and HIV1+ smokers with early emphysema. While AM gene expression of MMPs was higher in HIV1– individuals with emphysema in comparison with HIV1– healthy smokers, for the majority of the MMPs (-1, -7, -9, and -12), AM expression from HIV1+ smokers with early emphysema was significantly higher than in HIV1− smokers with early emphysema. HIV1+ individuals with early emphysema also had higher levels of epithelial lining fluid (ELF) MMPs (-2, -7, -9, and -12) than the 3 HIV1− groups. ELF MMP (-2,-7,-9, and -12) levels were similar in HIV1+ nonsmokers compared with HIV1− nonsmokers. Interestingly, the active forms of MMP-2, -9, and -12 were exclusively detected in ELF from HIV1+ individuals with early emphysema. Since the activities of the up-regulated AM MMPs include collagenases, gelatinases, matrilysins, and elastase, these data suggest that up-regulated AM MMP genes and activation of MMP proteins may contribute to the emphysema of HIV1+ individuals who smoke.
Keywords: lung, protease, lung disease, pathogenesis, human
Introduction
The advent of highly active antiretroviral therapy (HAART) has dramatically altered the survival of individuals infected with HIV1 [1,2,3]. This improved survival has led to the recognition of chronic conditions in clinically stable HIV1+ individuals in whom the HIV1 infection is controlled [4]. Among these, HIV1+ smokers develop emphysema at an earlier age and much higher prevalence in comparison with HIV1− smokers [5, 6].
AM play a central role in the pathogenesis of emphysema, due, in part, to their ability to synthesize and release enzymes into the extracellular space that are capable of degrading components of the alveolar wall extracellular matrix [7, 8]. A major class of these enzymes, the matrix metalloproteinases (MMPs), are important mediators of matrix degradation in emphysema [7,8,9,10,11]. Activated MMPs also exert significant control over inflammation, modulating the recruitment of leukocytes into the lung [7, 12]. Since HIV1+ smokers have a higher incidence of emphysema than HIV1− smokers and since alveolar macrophages are known to be activated in HIV1+ individuals [13,14,15,16,17,18,19,20] and in HIV1− smokers [8], we hypothesized that AM of HIV1+ smokers may up-regulate the expression of MMPs to a higher extent than HIV1– smokers.
To assess this hypothesis, we used microarrays to screen AM obtained from HIV1+ smokers for the levels of expression of MMPs. For those MMPs in which AM demonstrated expression, TaqMan real-time PCR was used for a quantitative comparison of the level of AM expression of these MMPs among 4 groups of individuals: HIV1− nonsmokers, HIV1− smokers without evidence of lung disease, HIV1− smokers with early emphysema, and HIV1+ smokers with early emphysema. For those MMPs demonstrating enhanced expression at the mRNA level in HIV1+ smokers, epithelial lining fluid (ELF) was analyzed for the status of the MMP protein. The data indicate that, for several MMPs, AM expression in HIV1+ smokers with emphysema is significantly up-regulated in excess of the increase that occurs in HIV1− smokers with emphysema, consistent with the concept that AM play a role in the accelerated emphysema observed in this population. Importantly, the observation of activated MMPs in the epithelial lining fluid of HIV1+ smokers with emphysema adds plausibility to their potential role in the accelerated development of emphysema.
MATERIALS AND METHODS
Human subjects
The study was approved by the Weill Cornell Medical College Institutional Review Board. Written informed consent was obtained from each individual prior to enrollment in the study. Individuals underwent an initial screening evaluation, which included history, complete physical exam, blood studies, urine analysis, chest X-ray, pulmonary function tests, and electrocardiogram. Individuals with any recreational drug use in the previous 6 months were excluded. The blood studies included a complete blood count, coagulation parameters, serum electrolytes, liver, and kidney function tests, serum evaluation for human immunodeficiency virus antibodies, HIV1 viral load, CD4 count, hepatitis profile (A, B, and C), anti-nuclear antibodies, sedimentation rate, and rheumatoid factor. Studies to verify current smoking status included urinary levels of nicotine and its derivative cotinine, and blood levels of carboxyhemoglobin. Pulmonary function tests were carried out according to American Thoracic Society guidelines [21]. Individuals who smoked and had a diffusing capacity <80% of what is predicted were further evaluated with noncontrast high-resolution computed tomography (CT) of the chest. The CT images of lung parenchyma were analyzed for the extent of emphysema by an automated software program [22, 23]. Emphysema was considered to be present if >5% of voxels analyzed had Houndsfield units (HU) more negative than –910 HU [22, 23]. The four groups evaluated were defined as follows (Table 1).
TABLE 1.
Study Population and Alveolar Macrophage Samples
| Parameter | Healthy HIV1− nonsmoker | Healthy HIV1− smoker | HIV1− smoker with early emphysema | HIV1+ smoker with early emphysemaa | HIV1+ nonsmokerb |
|---|---|---|---|---|---|
| n | 11 | 13 | 11 | 11 | 5 |
| Sex (male/female) | 9/2 | 10/3 | 8/3 | 9/2 | 4/1 |
| Age (yr) | 44 ± 2 | 46 ± 2 | 50 ± 2 | 43 ± 1 | 44 ± 5 |
| Race (AA/C/H)a | 4/5/2 | 6/7/0 | 7/3/1 | 9/0/2 | 1/2/2 |
| Smoking history (pack-yr) | 0 | 36 ± 6 | 37 ± 6 | 37 ± 6 | 0 |
| Urine nicotine(ng/ml)b | negative | 437 ± 158 | 1366 ± 322 | 802 ± 156 | negative |
| Urine cotinine (ng/ml)b | negative | 1336 ± 220 | 1397 ± 498 | 1514 ± 259 | negative |
| Blood carboxyhemoglobin (%)c | N.D.e | 3 ± 1 | 3 ± 0 | 3 ± 1 | 0.5 ± 0.4 |
| Pulmonary function parametersd | |||||
| FVC | 102 ± 5 | 109 ± 3 | 100 ± 3 | 93 ± 4 | 111 ± 5 |
| FEV1 | 104 ± 4 | 104 ± 3 | 94 ± 4 | 94 ± 5 | 110 ± 7 |
| FEV1/FVC | 81 ± 3 | 81 ± 3 | 77 ± 2 | 81 ± 1 | 81 ± 3 |
| TLC | 110 ± 7 | 99 ± 4 | 92 ± 4 | 86 ± 4 | 106 ± 4 |
| DLCO | 93 ± 5 | 94 ± 2 | 63 ± 3 | 63 ± 2 | 96 ± 3 |
| BAL cells7 | |||||
| Total number recovered (×10f) | 1.5 ± 0.3 | 2.9 ± 0.8 | 2.4 ± 1.1 | 2.6 ± 0.3 | 2.9 ± 0.6 |
| % viability | 98.9 ± 0.0 | 99 ± 0.3 | 98 ± 0.5 | 97 ± 0.4 | 98 ± 0.6 |
| % lymphocytes | 8.7 ± 2.8 | 2.7 ± 1.1 | 9.3 ± 1.4 | 4.1 ± 1.0 | 1.8 ± 0.4 |
| % alveolar macrophages | 88.8 ± 2.6 | 93.9 ± 1.2 | 89.0 ± 1.7 | 91.4 ± 1.4 | 96 ± 0.7 |
| % polymorphonuclear cells | 0 ± 0 | 0.7 ± 0.7 | 1.0 ± 0.3 | 1.3 ± 0.2 | 0.5 ± 0.1 |
| % epithelial cells | 2.4 ± 0.8 | 2.2 ± 1.0 | 1.0 ± 0.4 | 2.5 ± 0.5 | 1.4 ± 0.8 |
Demographic characteristics of subjects for whom data was obtained; bronchoalveolar lavage cell differentials at the bottom of the table represent the samples used to obtain the data; all data are mean ± se. a AA, African-American; C, Caucasian; H, Hispanic. b Urine nicotine and cotinine >200 = active smoker, 50–200 = passive smoker; <50 nonsmoker; data represent the mean of two determinations on the days of screening and bronchoscopy. c Blood carboxyhemoglobin is a secondary marker of current smoking status; nonsmokers <1.5%. d Pulmonary function parameters included: FVC, forced vital capacity (% predicted); FEV1, forced expiratory volume in 1 s FEV1/FVC (% observed); TLC, total capacity (% predicted); DLCO, diffusion capacity (% predicted). e N.D., not determined. f Cell differential prior to alveolar macrophage purification. g Individual plasma HIV1 viral loads: <50 (n=5); 600 (n=2); 3300; 23,000; 55,025; 308,000. h Individual plasma HIV1 viral loads: <50 (n=2); 693; 16,000; unknown (n=1).
HIV1+ smokers with early emphysema
This group comprised 11 individuals that were HIV1+ and were current smokers. All HIV1+ individuals were taking HAART therapy. All HIV1+ smokers included had lung function studies with evidence of “early emphysema,” defined for the purposes of this study as normal forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC and total lung capacity (TLC), but with diffusing capacity <80% of what is predicted, together with evidence of emphysema on high-resolution CT [24]. HIV1+ smokers were excluded if they had active pulmonary symptoms or a history of lung infections. Also excluded were individuals with a history of drug use within the last 5 years or who had ever used crack cocaine or heroin.
HIV1– smokers with early emphysema
A comparable group of 11 HIV1− smokers with early emphysema were characterized by the same pulmonary function and radiographic criteria as for the HIV1+ smokers. Individuals were excluded for active pulmonary symptoms, history of lung infections, or history of chronic crack cocaine or heroin use.
HIV1– healthy smokers
This group of 13 HIV1− individuals were current smokers with a normal screening evaluation, normal pulmonary function tests, and chest X-ray and a positive screen for nicotine ingestion.
HIV1– healthy nonsmokers
These 11 HIV1− individuals were lifelong nonsmokers with a normal screening evaluation, normal pulmonary function tests and chest X-ray, and a negative screen for nicotine ingestion.
HIV1+ nonsmokers
These 5 HIV1+ individuals were lifelong nonsmokers with an otherwise normal screening evaluation, normal pulmonary function tests, and chest X-ray and a negative screen for nicotine ingestion.
Collection of alveolar macrophages
Fiberoptic bronchoscopy was performed to obtain alveolar macrophages using methods developed in our laboratory to ensure high-quality RNA for gene expression analysis [25, 26]. Before the procedure, intravenous meperidine (25 to 100 mg) and midazolam (1 to 4 mg) were administered for sedation. Atropine (0.6 mg) was administered by the intramuscular route. Lidocaine was used as a topical anesthetic on the vocal cords and airways. A fiberoptic bronchoscope (FB-15x, Pentax, Orangeburg, NY, USA) was advanced through a mouth piece and through the vocal cords to a distal airway and was gently wedged into a subsegmental bronchus. Sterile 0.9% saline solution (23°C) was infused in 20 ml aliquots, using a syringe. After each aliquot, the fluid was immediately recovered into a trap using suction (50 to 100 mmHg of negative pressure from a clinical suction apparatus). The total volume used per site was typically 100 ml. A maximum of three sites were evaluated, with a total volume not exceeding 300 ml. The right middle lobe and lingula were the usual sites for lavage. Of the saline used for lavage, 45–65% of the infused volume was recovered.
BAL fluid was filtered through 2 layers of gauze and centrifuged at 1200 rpm for 5 min, 4°C. Cells were washed twice in RPMI 1640 containing 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, and 2 mM glutamine (Invitrogen, Carlsbad, CA, USA). Cells were suspended in 10 ml of medium, and an aliquot of 0.5 ml was used for a differential cell count. Cell viability was estimated by Trypan blue exclusion and expressed as a percentage of the total cells recovered. Total cell number was determined by counting in a hemocytometer. Differential cell count was assessed on sedimented cells prepared by cytocentrifugation (Cytospin 11; Shandon Instruments, Pittsburgh, PA, USA) and stained with DiffQuik (Baxter Healthcare, Miami, FL, USA). The remainder was processed for RNA extraction. Cells recovered from BAL fluid were seeded in 6-well plastic culture dishes (2×106 per 2 ml/well) and alveolar macrophages were purified by 24-h adherence at 37°C in a 5% CO2 humidified incubator. Nonadherent cells were removed by washing with RPMI 1640, and total RNA was extracted using the TRIzol (Life Technologies, Gaithersburg, MD, USA) method followed by RNeasy (Qiagen, Valencia, CA, USA) to remove residual DNA, a procedure giving a yield of 2 to 4 μg from 106 cells.
Affymetrix microarrays
Analyses were performed using Affymetrix (Santa Clara, CA, USA) microarray HG-U133 Plus 2.0 and associated protocols. An aliquot of each RNA sample was run on an Agilent Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) to visualize and quantify the degree of RNA integrity. The concentration was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Three quality control criteria were used for an RNA sample to be accepted for further processing: 1) A260/A280 ratio between 1.7 and 2.3; 2) concentration within the range of 0.2 to 6 μg/ml; and 3) Agilent electropherogram displaying 2 distinct peaks corresponding to the 28S and 18S rRNA bands at a ratio of 28S/18S of >0.5 with minimal or no degradation. Double-stranded cDNA was synthesized from 3 μg of total RNA using the GeneChip One-Cycle cDNA Synthesis Kit, followed by cleanup with GeneChip Sample Cleanup Module, in vitro transcription (IVT) reaction using the GeneChip IVT Labeling Kit, and cleanup and quantification of the biotin-labeled cRNA yield by spectrophotometric analysis. All kits were from Affymetrix. Hybridizations to test chips and to microarrays were performed according to Affymetrix protocols, and microarrays were processed by the Affymetrix fluidics station and scanned with the Affymetrix GeneChip Scanner 3000 7G.
The overall microarray quality was verified by the criteria 1) 3′/5′ ratio for GAPDH < 3; 2) scaling factor range no more than ±2.5 SDs from the mean for all microarrays; and 3) expression level for all 100 housekeeping genes (as defined by Affymetrix, www.affymetrix.com) with coefficient of variation of <40%. After scanning, the data on each individual microarray were scaled to an arbitrary target intensity as recommended by Affymetrix, using the Microarray Suite version 5.0 software. To eliminate those genes not expressed in the alveolar macrophages, only the MMP genes with detectable expression in >65% of the AM samples from HIV1+ smokers with emphysema (Affymetrix Detection Call of Present in at least >65% samples) were chosen for further analysis.
TaqMan PCR
On the basis of the screening with the microarrays, the selected MMP genes were assessed by TaqMan real-time reverse transcriptase (RT) PCR analysis to quantify relative gene expression levels. First-strand cDNA was synthesized from 2 μg of total RNA in 2 × 50 μl reaction volume, using the TaqMan Reverse Transcriptase Reaction Kit (Applied Biosystems, Foster City, CA, USA), with random hexamers as primers. The primers specific for each mRNA used were from Applied Biosystems (unique ID #s were MMP-1: Hs00233958_m1; MMP-2: Hs00234422_m1, MMP-7: Hs00159163_m1, MMP-9: Hs00234579_m1, MMP-10: Hs00233987_m1, MMP-12: Hs00159178_m1, MMP-14: Hs00237119_m1). For each individual sample, two conditions were used: 1:10 and 1:100 dilution of the cDNA reaction, and each dilution was assayed in triplicate wells. The PCR reactions were run in an Applied Biosystems Sequence Detection System 7700. The threshold cycles (Cts) were calculated as an average of the triplicate reactions for each condition, and the ΔCt was calculated for each sample using the rRNA as an endogenous reference. ΔΔCt was calculated by subtracting the calibrator from the ΔCt in each individual sample using the algorithm provided by Applied Biosystems.
Assessment of MMPs in respiratory tract epithelial lining fluid
BAL fluid was concentrated 10-fold and quantified for total protein using a micro-bicinchoninic acid (BCA) assay according to the manufacturer’s directions (Pierce, Rockford, IL, USA). The concentrated fluid was assessed for enzymatic activity and Western blot analysis. A random subset of individuals from each demographic group was selected for each specific analysis.
MMP activity
For activity of MMP-2 and MMP-9, gelatin zymography was carried out by loading 10 μg total protein per sample onto 8% sodium dodecyl sulfate polyacrylamide gels impregnated with 0.1% gelatin and separated using nondenaturing electrophoresis. Recombinant MMP-2 and MMP-9 proteins in latent and active forms (Chemicon, Temecula, CA, USA) were used as positive controls.
Western blot analysis
To assess levels of MMP-1, -2, -7, -9, and -12, NuPAGE 4-12% Bis-Tris Gel (Invitrogen, Carlsbad, CA, USA) was used for electrophoresis, with 15 μg/lane protein using commercial recombinant human protein standards for MMP-1, -2, -7, and -9 or concentrated conditioned media for MMP-12 (Calbiochem, San Diego, CA, USA). Proteins were transferred to PVDF membrane (0.45-μm pore size; Invitrogen), washed and blocked with 5% nonfat milk for 1 h. The membrane was incubated with proMMP-1 mouse monoclonal or active MMP-2, proMMP-7, active MMP-9, or active MMP-12 rabbit polyclonal primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) 1:500–1:750 in 5% non-fat milk (16 h, 4°C). After washing and reblocking, we applied the secondary horseradish peroxidase-labeled goat anti-mouse or anti-rabbit antibody (Jackson ImmunoResearch Laboratory, West Grove, PA, USA) 1:8000-1:10000 (2 h, 23°C). Detection was performed with ECL Western analysis detection reagents (Amersham, Piscataway, NJ, USA) and used BioMax Light Film (Kodak, Rochester, NY, USA). After detection, the blot was stripped and reprobed to detect human albumin to confirm equal protein loading of lanes in cases in which there was no nonspecific band to demonstrate equal loading.
Statistics
Results were expressed as means ± SE. The significance of differences of mean values of demographic data between the 4 groups were analyzed by ANOVA. The significance of gene expression differences between two groups was determined by calculating the P value for expression levels using the Student’s t test, assuming a two-tailed distribution and unequal variances. Correlation coefficients were determined by calculating the r2 value.
Deposition of data
All microarray data have been deposited in the Gene Expression Omnibus (GEO) site, (http://www.ncbi.nlm.nih.gov/geo), which is curated by the National Center for Bioinformatics. The accession number is GSE10038.
RESULTS
Demographics
The majority of individuals studied were male (Table 1). The healthy HIV1− nonsmokers, the healthy HIV1− smokers, and the HIV1+ smokers with early emphysema were matched for age. As expected, the HIV1+ individuals with early emphysema were younger than their HIV1− counterparts (P<0.005). The HIV1− healthy nonsmokers and smokers were roughly equally divided between Caucasian and African-American, with the majority of HIV1− and HIV1+ individuals with early emphysema being African-American. All 3 groups of smokers were matched with respect to pack-yr, urine cotinine, and carboxyhemoglobin (P>0.1, all comparisons). The HIV1− healthy smokers and nonsmokers and HIV1+ nonsmokers had normal pulmonary function. Both the HIV1+ and HIV1− individuals with early emphysema had significantly lower DLCO than HIV1− healthy nonsmokers and smokers (P<0.001), without evidence of airway obstruction. Cell recovery from BAL fluid was higher in all of the smoking groups compared with healthy HIV1– nonsmokers (P<0.05). HIV1+ smokers were all taking HAART and had average CD4+ counts of 420 ± 190 and viral loads of 1.0 × 105 ± 1.8 × 105. All of the HIV1+ smokers with early emphysema had CD4 counts adequate to obviate the need for pneumocystis carinii prophylaxis.
Microarray screen
Microarray screening analysis of MMP gene expression of AM obtained from HIV1+ individuals with emphysema demonstrated AM expression of MMP-1, -2, -7, -9, -10, -12, and -14 (Table 2). We were unable to find any significant relationship between relative MMP expression and viral load, but the small sample size limited our power to definitively rule out an association (Supplemental Data, Table 1 and Supplemental Data, Fig. 1). On the basis of these data, these expressed MMPs were selected for further analysis. The MMPs are discussed in their order in the MEROPS peptidase database v.7.1 [27]. In the context that the human MMPs are traditionally divided into 6 major classes based on their structural similarities and substrate specificities [28], the individual MMPs within each class are discussed together.
TABLE 2.
Microarray Screen of Alveolar Macrophages from HIV1+ Smokers for Expression of Matrix Metalloproteinases
| MMP genea | Expressedb | MMP gene | Expressedb |
|---|---|---|---|
| Collagenases | Elastases | ||
| MMP1 (interstitial collagenase, M10.001) | Yes | MMP12 (macrophage elastase, M10.009) | Yes |
| MMP8 (neutrophil collagenase, M10.002) | No | Membrane type MMPs | |
| MMP13 (collagenase-3, M10.013) | No | MMP14 (membrane type-1, MMP M10.014) | Yes |
| Gelatinases | MMP15 - membrane type 2 MMP M10.015 | No | |
| MMP2 (gelatinase A, M10.003) | Yes | MMP16 (membrane type 3, MMP M10.016) | No |
| MMP9 (gelatinase B, M10.004) | Yes | MMP17 (membrane type 4, MMP M10.017) | No |
| Stromelysins | MMP24 (membrane type 5, MMP3) | No | |
| MMP3 (stromelysin-1, progelatinase M10.005) | No | MMP25 (membrane type 6, MMP3) | No |
| MMP10 (stromelysin-2, M10.006) | Yes | Others | |
| MMP11 (stromelysin-3, M10.007) | No | MMP19 (rheumatoid arthritis inflamed synovium-1, M10.021) | No |
| Matrilysins | MMP23 (type II transmembrane MMP, M10.022) | No | |
| MMP7 (matrilysin-1, M10.008) | Yes | MMP21 (matrix metallopeptidase-21, M10.026) | No |
| MMP26 (matrilysin-2 M10.029) | No | MMP27 (matrix metallopeptidase-27)c | No |
| MMP28 (epilysin, M10.030) | No |
M10.0xx designations per MEROPS peptidase database v7.1 (27).
Present in >65% of the HIV1+ AM RNA samples.
Not present in MEROPS peptidase database v7.1.
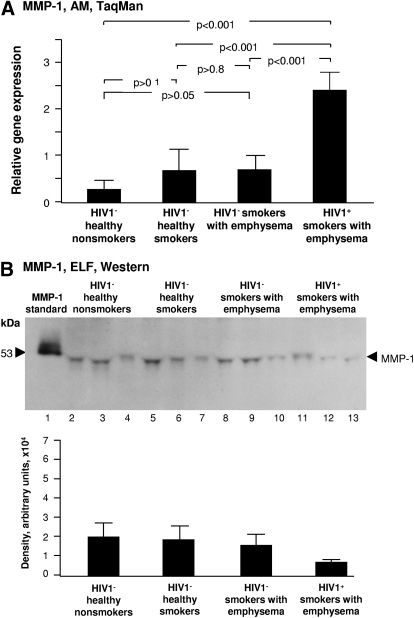
Figure 1.
MMP-1 expression. (A) Alveolar macrophage normalized gene expression levels of MMP-1 quantified by TaqMan PCR (mean ± se). Significantly higher levels of gene expression are present in HIV1+ smokers with emphysema compared with the other groups. (B) Western blot analysis of epithelial lining fluid MMP-1 was performed on n = 3 samples/each group. Lane 1, recombinant pro-MMP-1 standard; lanes 2–4, examples of HIV1− healthy nonsmokers; lanes 5–7, HIV1− healthy smokers; lanes 8–10, HIV1− smokers with early emphysema; lanes 11–13, HIV1+ smokers with early emphysema. MMP-1 protein was present in the ELF of all individuals without a discernable pattern of variation among the 4 groups. Variability of the apparent MW of the pro-MMP-1 band in the ELF samples was dependent upon the gel, based on rerunning the same samples.
MMP1 (collagenase)
MMP-1 has the ability to cleave interstitial collagen types I, II, and III [29]. Among the HIV1− groups, compared with HIV1− healthy nonsmokers, smoking did not increase AM MMP-1 gene expression in HIV1− healthy smokers (P>0.1) and HIV1− smokers with early emphysema (P>0.05), independent of whether emphysema is present or not (Fig. 1A). HIV1+ individuals with early emphysema had significantly increased expression of MMP-1 compared with all 3 groups of HIV1– individuals, including those with early emphysema (P<0.001; Fig. 1A). Western blot analysis showed that MMP-1 protein was detectable in concentrated BAL fluid of every individual (Fig. 1B). There were no obvious differences in ELF MMP-1 protein levels among the 4 groups (Fig. 1B).
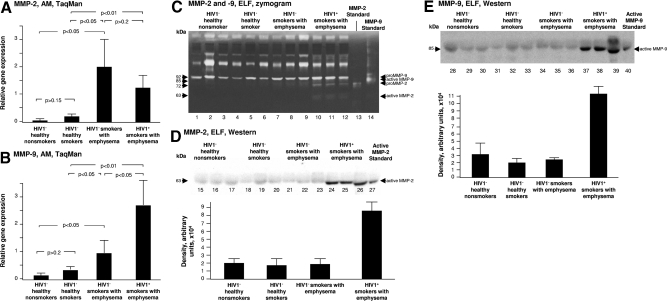
MMP-2 and MMP-9 (gelatinases)
MMP-2 and -9 degrade collagen types I, II, and III in newly synthesized extracellular matrix and many forms of the basement membrane collagen type IV [30, 31]. Relative expression of MMP-2 was significantly increased in both HIV1+ smokers with early emphysema and HIV1− smokers with early emphysema compared with both HIV1− healthy smokers and HIV1− healthy nonsmokers (P<0.01; Fig. 2A). AM MMP-9 relative expression was also up-regulated in HIV1− smokers with early emphysema compared with both HIV1− healthy smokers and HIV1− healthy nonsmokers (P<0.05; Fig. 2B). AM MMP-9 expression was significantly higher in the AM of HIV1+ smokers with early emphysema compared with HIV1− smokers with early emphysema (P<0.05; Fig. 2B). Gelatin zymography analysis of BAL fluid showed significant detectable pro MMP-2 only in ELF from HIV1+ smokers with early emphysema (Fig. 2C). Interestingly, the active form of MMP-2 was also detected in ELF of HIV1+ smokers with early emphysema (Fig. 2C). Gelatin zymography analysis of concentrated BAL fluid also showed pro-MMP-9 in ELF from all individuals, with a trend toward increased levels in the HIV1− smokers with early emphysema, and a trend toward further increase in the HIV1+ smokers with early emphysema (Fig. 2C). Western blot analysis confirmed the presence of active MMP-2 only in the ELF of HIV1+ smokers with early emphysema (Fig. 2D). The presence of the active form of MMP-9 in the ELF of HIV1+ individuals with early emphysema was confirmed by Western blot analysis (Fig. 2E).
Figure 2.
MMP-2 and -9 expression. (A) Alveolar macrophage normalized gene expression levels of MMP-2 quantified by TaqMan PCR (mean ± SE). Subjects with emphysema had significantly higher levels of MMP-2 gene expression than the other groups. (B) Alveolar macrophage normalized gene expression levels of MMP-9 quantified by TaqMan PCR (mean ± SE). The levels in HIV1+ smokers with early emphysema were significantly higher than in the HIV1− smokers with early emphysema. (C) Epithelial lining fluid gelatin zymography analysis of MMP-2 and -9 activity. Concentrated BAL fluid from each of the 4 groups was assessed by gelatin zymography on n = 3 samples/each group. Lanes 1–3, examples of HIV1− healthy nonsmokers; lanes 4–6, HIV1− healthy smokers; lanes 7–9, HIV1− smokers with early emphysema; lanes 10–12, HIV1+ smokers with early emphysema; lane 13, MMP-2 standard; lane 14, MMP-9 standard. The highest level of pro-MMP-9 and active MMP-9 was observed in ELF of HIV1+ smokers with early emphysema, pro-MMP-2, and active MMP-2 were uniquely present in the ELF of HIV1+ smokers with early emphysema. D) Western blot analysis of active MMP-2 in ELF was performed on n = 3 samples/each group. Lanes 15–17, examples of HIV1− healthy nonsmokers; lanes 18–20, HIV1− healthy smokers; lanes 21-23 - HIV1− smokers with early emphysema; lanes 24-26 - HIV1+ smokers with early emphysema; lane 27, active MMP-2 standard. Immunoreactive active MMP-2 was confirmed in the ELF of HIV1+ smokers with early emphysema. (E) Western blot analysis of active MMP-9 in ELF was performed on n=3 samples/each group. Lanes 28-30, examples of HIV1− healthy nonsmokers; lanes 31–33, HIV1− healthy smokers; lanes 34–36, HIV1− smokers with early emphysema; lanes 37–39, HIV1+ smokers with early emphysema; lane 40, active MMP-2 standard. Immunoreactive active MMP-9 was confirmed in the ELF of HIV1+ smokers with emphysema.
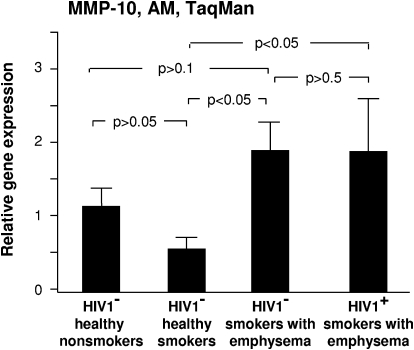
MMP-10 (stromelysin)
Stromelysins are enzymatically active against proteoglycans, procollagenase, and fibronectin [32]. AM stromelysin 2/MMP-10 expression was highest in the HIV1− and HIV1+ smokers with early emphysema, where it was significantly higher than in HIV1− healthy smokers (P<0.05) (Fig. 3). The increase in AM MMP-10 expression in comparison with HIV1− healthy nonsmokers and HIV1− smokers with early emphysema was not significant (P>0.5; Fig. 3).
Figure 3.
MMP-10 expression. Alveolar macrophage normalized gene expression levels of MMP-10 quantified by TaqMan PCR (mean ± se). There was significantly higher relative expression of MMP-10 in the HIV1− and HIV1+ smokers with emphysema in comparison with HIV1− healthy smokers.
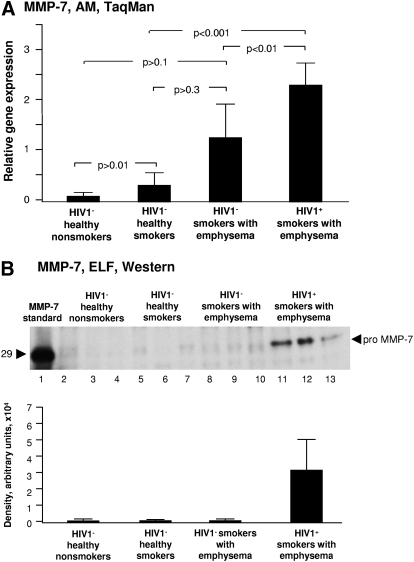
MMP-7 (matrilysin)
Matrilysins proteolyze a number of extracellular matrix/basement membrane components, including type IV collagens and proteoglycans and appear to be important for neutrophil migration into the lung in animal models of lung inflammation [12, 33, 34]. HIV1− smokers with early emphysema had a trend toward increased relative AM matrilysin/MMP-7 gene expression compared with HIV1− healthy smokers and HIV1− healthy nonsmokers (P>0.1; Fig. 4A). HIV1+ smokers with early emphysema had significantly higher relative expression of AM MMP-7 than both HIV1− healthy smokers and HIV1− healthy nonsmokers (P<0.001). HIV1+ smokers with early emphysema had increased expression over and above the increase in HIV1− smokers with early emphysema (P<0.05; Fig. 4A). ELF proMMP-7 protein was not detectable by Western blot analysis in any of the 3 HIV1− groups, including HIV1– smokers with early emphysema (Fig. 4B). In contrast, ELF pro-MMP-7 was readily detectable in the ELF of HIV1+ smokers with early emphysema (Fig. 4B).
Figure 4.
MMP-7 expression. (A) AM relative normalized gene expression levels of MMP-7 quantified by TaqMan PCR (mean ± se). There was significantly higher relative expression of MMP-7 in the HIV1+ smokers with early emphysema in comparison with HIV1− smokers with early emphysema. (B) Western blot analysis of MMP-7 in epithelial lining fluid was performed on n = 3 samples/each group. Lane 1, recombinant pro-MMP-7 standard; lanes 2–4, examples of HIV1− healthy nonsmokers; lanes 5–7, HIV1− healthy smokers; lanes 8–10, HIV1− smokers with early emphysema; lanes 11–13, HIV1+ smokers with early emphysema. MMP-7 protein was uniquely identified in the ELF of HIV1+ individuals with early emphysema.
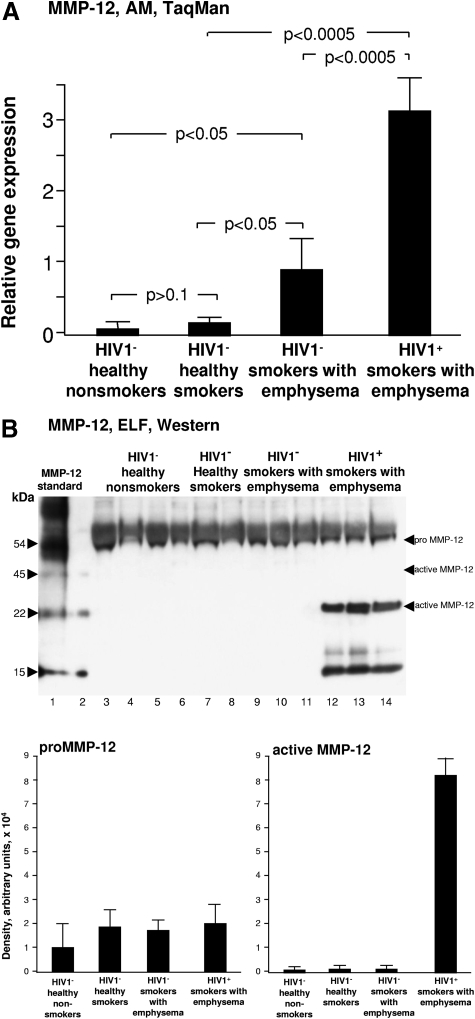
MMP-12 (elastase)
Elastin is a key structural component of the lung extracellular matrix, responsible for important elastic recoil properties of the lung, and degradation of elastin is central to the pathogenesis of emphysema [35]. There was a trend toward increased expression of AM macrophage MMP-12 in HIV1− healthy smokers vs. HIV1− healthy nonsmokers (P>0.05). MMP-12 expression was further significantly increased in HIV1– smokers with early emphysema in comparison with both HIV1− healthy nonsmokers and HIV1− healthy smokers (P<0.05; Fig. 5A). Relative gene expression of AM MMP-12 was further increased in HIV1+ smokers with early emphysema over and above the increase in HIV1- smokers with early emphysema (P<0.0005; Fig. 5A). ELF pro-MMP-12 protein was detectable in all samples in all 4 groups of individuals by Western blot analysis (Fig. 5B). The 22-kDa active MMP-12 protein was readily detectable only in the ELF of the HIV1+ smokers with early emphysema, but not in the ELF of any of the 3 groups of HIV1− individuals, including HIV1− smokers with early emphysema (Fig. 5B). A 15-kDa immunoreactive band was observed only in the ELF of HIV1+ smokers with early emphysema, as well as in the positive control, suggesting it was an enzymatic product of proMMP-12 (Fig. 5B).
Figure 5.
MMP-12 expression. (A) AM normalized gene expression levels of MMP-12 quantified by TaqMan PCR (mean±SE). MMP-12 expression was significantly increased in HIV1+ smokers with early emphysema compared with all other groups. (B) Western blot analysis of MMP-12 in epithelial lining fluid was performed on n = 3 samples/each group. Lane 1, MMP-12 standard; lane 2, blank control; lanes 3–5, examples of HIV1− healthy nonsmokers; lanes 6–8, HIV1− healthy smokers; lanes 9–11, HIV1− smokers with early emphysema; lanes 12–14, HIV1+ smokers with early emphysema. While pro-MMP-12 was detected in ELF of all individuals, and active MMP-12 (45 kDa) was not observed in any samples, 22-kDa active MMP-12 was uniquely observed in the ELF of HIV1+ smokers with early emphysema. The 15-kDa immunoreactive band in the ELF of HIV1+ smokers with early emphysema may represent a proteolytic fragment of pro-MMP12.
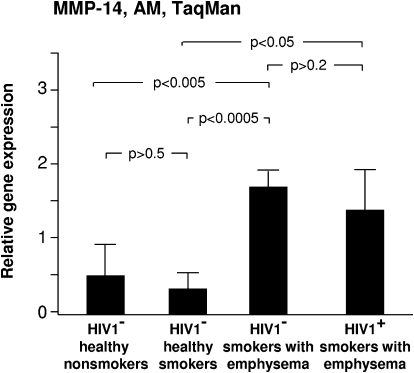
MMP-14 (membrane-type MMP)
Membrane-type MMPs are membrane spanning binding sites that play an important role in the cell surface activation of MMPs such as MMP-2 [36]. HIV1− smokers with early emphysema had significantly higher relative expression of MMP-14 in comparison with HIV1− healthy smokers and HIV1− healthy nonsmokers (P<0.05; Fig. 6). HIV1+ smokers with early emphysema also had significantly higher relative expression of AM MMP-14 in comparison with HIV1− normal smokers and HIV1− normal nonsmokers (P<0.05; Fig. 6). There was no difference between the relative expression of AM MMP-14 between the HIV1− smokers with early emphysema and the HIV1+ smokers with early emphysema (P>0.2; Fig. 6).
Figure 6.
MMP-14 expression. Alveolar macrophage normalized gene expression levels of MMP-14 quantified by TaqMan PCR (mean ± se). Increased AM expression of MMP-14 was observed in both HIV1+ and HIV1− smokers with early emphysema.
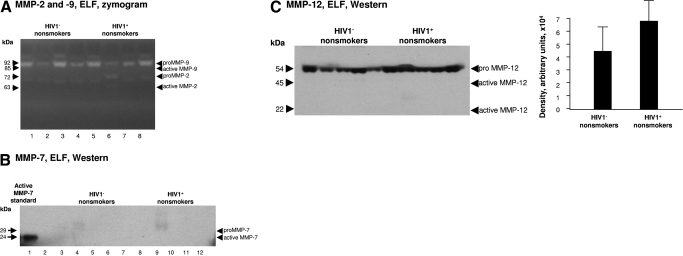
Comparison of MMPs in HIV1– nonsmokers with HIV1+ nonsmokers
To determine whether MMPs are up-regulated by HIV1 in the absence of cigarette smoke exposure, MMP-2 and MMP-9 activity in ELF in HIV1− nonsmokers were compared with HIV1+ nonsmokers. In contrast to the increased active MMP-9, proMMP-2 and active MMP-2 observed in HIV1+ smokers with early emphysema, none of these were observed in HIV1+ nonsmokers (Fig. 7A). These results were confirmed with Western blot analysis (not shown). Neither was MMP-7 detectable in HIV1+ nonsmokers (Fig. 7B). ProMMP-12 activity was detected in all individuals, with a nonsignificant trend toward increased levels in the HIV1+ nonsmokers, but without any detectable active MMP-12 (Fig. 7C).
Figure 7.
MMP-2, -7, -9, and -12 expression. (A) Epithelial lining fluid gelatin zymography analysis of MMP-2 and -9 activity. Concentrated BAL fluid from each of the 2 nonsmoking groups was assessed by gelatin zymography that was performed on n = 4 samples/each group . Lanes 1–4, examples of HIV1− healthy nonsmokers; lanes 5–8, HIV1− nonsmokers. (B) Western blot analysis of MMP-7 in epithelial lining fluid of nonsmokers was performed on n=5 samples/each group. Lane 1, recombinant active MMP-7 standard; lane 2, blank; lanes 3–7, examples of HIV1− healthy nonsmokers; lanes 8–12, HIV1+ nonsmokers. MMP-7 protein was not identified in the ELF of nonsmokers. (C) Western blot analysis of MMP-12 in epithelial lining fluid was performed on n=5 samples/each group. Lanes 1–5, examples of HIV1− healthy nonsmokers; lanes 6–10, HIV1+ nonsmokers. While pro-MMP-12 was detected in the ELF of all individuals, active MMP-12 (45 kDa and 22 kDa) was not observed in any samples.
DISCUSSION
Alveolar macrophages are targets of HIV1 infection and support active viral replication in untreated HIV1+ individuals [37,38,39,40]. AM also play a central role in the pathogenesis of emphysema via pivotal roles in both control of inflammation and proteolytic degradation of the extracellular matrix [7,8,9,10, 12]. Since HIV1+ individuals who smoke develop emphysema at an earlier age of onset and with a higher prevalence than in HIV1− smokers [5, 6, 41], it is likely that AM may play a key role in this process. Since MMPs are active in both proteolysis and inflammation, up-regulation of AM MMP gene expression would likely contribute to the emphysema that develops in HIV1+ smokers. The data in the present study indicate that gene expression of AM MMPs in HIV1+ smokers with early emphysema is not only up-regulated above that of HIV1− nonsmokers and healthy smokers, but is also up-regulated over and above the relative expression of HIV1− smokers with early emphysema. The correlation between the increase in observed AM MMP gene expression levels and the protein levels in epithelial lining fluid in HIV1+ smokers with emphysema suggests that the gene expression changes are biologically relevant.
Matrix metalloproteinases and emphysema
The up-regulated MMPs have a broad spectrum of target extracellular matrix and basement membrane substrates, suggesting that they have the capability to degrade all of the major classes of matrix components [8, 10, 11]. It is reasonable to infer that they may contribute to the accelerated emphysema that occurs in HIV1+ individuals who smoke by virtue of their ability to degrade virtually all of the structural components of the alveolar wall [9, 10]. In addition, several of the MMPs have the ability to control the influx of inflammatory cells into the lung [10,11,12]. Because lung inflammation is also a central paradigm in the pathogenesis of emphysema, up-regulation of AM expression of MMPs could be augmenting this aspect of emphysema pathophysiology as well.
Relative expression of selected AM matrix metalloproteinases, such as MMP-12, are known to be up-regulated by cigarette smoke in humans [26]. This increased expression has been linked to emphysema in humans, as well as in animal models, where MMP-12-induced TNF-α release mediates lung neutrophil infiltration [34, 42, 43]. Increased AM and ELF MMP-1 and MMP-9 expression are also linked to emphysema in humans and in animal models [8, 44,45,46]. Yearsley et al. [41] were the first to observe increased lung tissue expression of MMP-9 in autopsies of HIV1+ individuals with emphysema who died of AIDS. Cultured AM from smokers with emphysema have increased MMP-1 and -9 expression [47], and MMP-2 expression has been shown to correlate with COPD progression [8, 48]. Overexpression of IL-13 in the murine lung is associated with emphysema and increased lung MMP-2, -9, -12, -13, and -14 expression and is prevented with a synthetic broad spectrum pharmacologic inhibitor of MMP-1, -2, -3, and -9 [49].
Up-regulation of MMP expression by HIV1+ AM
What potential explanation is there for the augmentation of AM MMP expression in HIV1+ smokers with emphysema above that of HIV1− smokers with emphysema? While a direct effect of HIV1 replication in the AM and/or insertion of the HIV1 genome into the genome of AM cannot be ruled out, this seems unlikely given the fact that AM from individuals under treatment with HAART do not evidence spontaneous HIV1 replication in vitro [20, 50, 51]. As an alternative explanation, several previous studies have demonstrated increased cytokine and chemokine expression by AM from HIV1+ individuals [14,15,16, 19, 52]. Moreover, there is evidence of activation of the immune system within the lung early in HIV1 infection, even if the immune system is ineffective in controlling the viral infection [13, 17, 19, 53]. Prior to antiretroviral therapy, there are high levels of proinflammatory cytokines in the pulmonary ELF [54]. While cytokine levels decrease during antiretroviral therapy, several of these cytokines remain elevated above basal levels [54]. This induction of AM MMP expression by cytokines provides another potential explanation for the exaggerated increase in MMP gene expression and the levels and activation of MMPs observed in the ELF of HIV1+ smokers with early emphysema, in that proinflammatory cytokines are known to induce macrophage MMP expression and activation [55,56,57].
MMPs are exported from the cell as zymogens that must be proteolytically activated to have biological activity [11, 28]. Our data indicate that ELF from HIV1+ smokers with emphysema contains readily detectable amounts of the active forms of MMP-2, -9, and -12. The fact that these active forms of the enzymes are not readily detectable in HIV1– smokers with emphysema, lends support to the notion that AM MMPs may contribute to the accelerated emphysema in HIV1+ smokers.
Our data on HIV1+ nonsmokers indicate that HIV1 infection by itself appears to be insufficient to cause ELF MMP activation in the absence of cigarette smoke exposure. HIV1+ individuals rather appear to be more susceptible to MMP activation induced by cigarette smoke in comparison with HIV1− individuals.
In addition to the MMPs previously associated with emphysema (MMP-1, -2, -9, -12, and -14) [9, 10], we observed increased expression in the HIV1+ smokers with emphysema of MMP-7, a MMP not previously so associated with emphysema [33], although increased levels of MMP-7 have been detected in interstitial lung disease [58]. Further studies will be necessary to determine whether MMP-7 is up-regulated at lower levels in HIV1− individuals with early emphysema compared with healthy HIV1− smokers or whether the increased expression in HIV1+ individuals with early emphysema is unique to this group of individuals. One limitation of our study is that few women participated, so it is uncertain as to whether our observations of increased MMP activation in HIV1+ smokers apply to females as well as males.
In summary, the data indicate that up-regulation of AM MMP gene expression and concomitant increased protein levels and MMP activation in ELF may contribute to the increased incidence and early development of emphysema observed in HIV1+ individuals who smoke. Pharmacologic blockade of MMPs should be seriously considered as a strategy to minimize the development of emphysema in HIV1+ individuals who smoke. In the context that monocytes and macrophages at sites other than lung may also be activated to release MMPs in HIV1+ individuals [59], such pharmacologic intervention could have systemic therapeutic effects as well.
Supplementary Material
Acknowledgments
These studies were supported, in part, by P50 HL084936; CTSA UL1-RR024996; the Will Rogers Memorial Fund (Los Angeles, CA, USA); and Four Friends Foundation (Los Angeles, CA, USA). We thank N. Mohamed for help in preparing this manuscript.
Footnotes
Abbreviations: AM=alveolar macrophage(s), BAL=bronchoalveolar lavage, DLCO=diffusing capcity for carbon monoxide, ELF=epithelial lung fluid, FEV1=forced expiratory volume in 1 s, FVC=forced vital capacity, HAART=highly active antiretroviral therapy, IVT=in vitro transcription, MMP=matrix metalloproteinase, TLC=total lung capacity
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
References
- Egger M, Hirschel B, Francioli P, Sudre P, Wirz M, Flepp M, Rickenbach M, Malinverni R, Vernazza P, Battegay M. Impact of new antiretroviral combination therapies in HIV-infected patients in Switzerland: prospective multicentre study. Swiss HIV Cohort Study. BMJ. 1997;315:1194–1199. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detels R, Munoz A, McFarlane G, Kingsley L A, Margolick J B, Giorgi J, Schrager L K, Phair J P. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA. 1998;280:1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- Palella F J, Jr, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Carr A, Samaras K, Chisholm D J, Cooper D A. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- Diaz P T, King M A, Pacht E R, Wewers M D, Gadek J E, Neal D, Nagaraja H N, Drake J, Clanton T L. The pathophysiology of pulmonary diffusion impairment in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999;160:272–277. doi: 10.1164/ajrccm.160.1.9812089. [DOI] [PubMed] [Google Scholar]
- Diaz P T, King M A, Pacht E R, Wewers M D, Gadek J E, Nagaraja H N, Drake J, Clanton T L. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132:369–372. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- Shapiro S D. The macrophage in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S29–S32. doi: 10.1164/ajrccm.160.supplement_1.9. [DOI] [PubMed] [Google Scholar]
- Barnes P J. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- Parks W C, Shapiro S D. Matrix metalloproteinases in lung biology. Respir Res. 2001;2:10–19. doi: 10.1186/rr33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churg A, Wright J L. Proteases and emphysema. Curr Opin Pulm Med. 2005;11:153–159. doi: 10.1097/01.mcp.0000149592.51761.e3. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald A J, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Park P W, Wilson C L, Parks W C. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- Agostini C, Poletti V, Zambello R, Trentin L, Siviero F, Spiga L, Gritti F, Semenzato G. Phenotypical and functional analysis of bronchoalveolar lavage lymphocytes in patients with HIV infection. Am Rev Respir Dis. 1988;138:1609–1615. doi: 10.1164/ajrccm/138.6.1609. [DOI] [PubMed] [Google Scholar]
- Agostini C, Zambello R, Trentin L, Garbisa S, Di Celle P F, Bulian P, Onisto M, Poletti V, Spiga L, Raise E. Alveolar macrophages from patients with AIDS and AIDS-related complex constitutively synthesize and release tumor necrosis factor-α. Am Rev Respir Dis. 1991;144:195–201. doi: 10.1164/ajrccm/144.1.195. [DOI] [PubMed] [Google Scholar]
- Agostini C, Trentin L, Zambello R, Bulian P, Caenazzo C, Cipriani A, Cadrobbi P, Garbisa S, Semenzato G. Release of granulocyte-macrophage colony-stimulating factor by alveolar macrophages in the lung of HIV-1-infected patients. A mechanism accounting for macrophage and neutrophil accumulation. J Immunol. 1992;149:3379–3385. [PubMed] [Google Scholar]
- Twigg H L, III, Iwamoto G K, Soliman D M. Role of cytokines in alveolar macrophage accessory cell function in HIV-infected individuals. J Immunol. 1992;149:1462–1469. [PubMed] [Google Scholar]
- Buhl R, Jaffe H A, Holroyd K J, Borok Z, Roum J H, Mastrangeli A, Wells F B, Kirby M, Saltini C, Crystal R G. Activation of alveolar macrophages in asymptomatic HIV-infected individuals. J Immunol. 1993;150:1019–1028. [PubMed] [Google Scholar]
- Agostini C, Zambello R, Trentin L, Semenzato G. HIV and pulmonary immune responses. Immunol Today. 1996;17:359–364. doi: 10.1016/0167-5699(96)30022-4. [DOI] [PubMed] [Google Scholar]
- Agostini C, Trentin L, Sancetta R, Facco M, Tassinari C, Cerutti A, Bortolin M, Milani A, Siviero M, Zambello R, Semenzato G. Interleukin-15 triggers activation and growth of the CD8 T-cell pool in extravascular tissues of patients with acquired immunodeficiency syndrome. Blood. 1997;90:1115–1123. [PubMed] [Google Scholar]
- White N C, Agostini C, Israel-Biet D, Semenzato G, Clarke J R. The growth and the control of human immunodeficiency virus in the lung: implications for highly active antiretroviral therapy. Eur J Clin Invest. 1999;29:964–972. doi: 10.1046/j.1365-2362.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- Miller M R, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten C P, Gustafsson P, Jensen R, Johnson D C, MacIntyre N, McKay R, Navajas D, Pedersen O F, Pellegrino R, Viegi G, Wanger J, ATS/ERS Task Force General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- Kinsella M, Muller N L, Abboud R T, Morrison N J, DyBuncio A. Quantitation of emphysema by computed tomography using a “density mask” program and correlation with pulmonary function tests. Chest. 1990;97:315–321. doi: 10.1378/chest.97.2.315. [DOI] [PubMed] [Google Scholar]
- Coxson H O, Rogers R M. New concepts in the radiological assessment of COPD. Semin Respir Crit Care Med. 2005;26:211–220. doi: 10.1055/s-2005-869540. [DOI] [PubMed] [Google Scholar]
- Coxson H O, Chan I H, Mayo J R, Hlynsky J, Nakano Y, Birmingham C L. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med. 2004;170:748–752. doi: 10.1164/rccm.200405-651OC. [DOI] [PubMed] [Google Scholar]
- Russi T J, Crystal R G. Bronchoalveolar lavage. Crystal R G, West J B, Weibel E R, Barnes P J, editors. Philadelphia, PA, USA: Lippencott-Raven; The LungScientific Foundations. 1997:371–382. [Google Scholar]
- Heguy A, O'Connor T P, Luettich K, Worgall S, Cieciuch A, Harvey B G, Hackett N R, Crystal R G. Gene expression profiling of human alveolar macrophages of phenotypically normal smokers and nonsmokers reveals a previously unrecognized subset of genes modulated by cigarette smoking. J Mol Med. 2006;84:318–328. doi: 10.1007/s00109-005-0008-2. [DOI] [PubMed] [Google Scholar]
- Rawlings N D, Morton F R, Barrett A J. MEROPS: the peptidase database. Nucleic Acids Res. 2006;34:D270–D272. doi: 10.1093/nar/gkj089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Billinghurst R C, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole A R. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimes R T, Quigley J P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific. 1995;3/4- and 1/4-length fragments. J. Biol. Chem. 270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Patel K D. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res. 2005;31:599–621. doi: 10.1080/019021490944232. [DOI] [PubMed] [Google Scholar]
- Barksby H E, Milner J M, Patterson A M, Peake N J, Hui W, Robson T, Lakey R, Middleton J, Cawston T E, Richards C D, Rowan A D. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: implications for cartilage degradation in arthritis. Arthritis Rheum. 2006;54:3244–3253. doi: 10.1002/art.22167. [DOI] [PubMed] [Google Scholar]
- Filippov S, Caras I, Murray R, Matrisian L M, Chapman H A, Jr, Shapiro S, Weiss S J. Matrilysin-dependent elastolysis by human macrophages. J Exp Med. 2003;198:925–935. doi: 10.1084/jem.20030626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W C, Wilson C L, Lopez-Boado Y S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Shapiro S D, Endicott S K, Province M A, Pierce J A, Campbell E J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurschat P, Zigrino P, Nischt R, Breitkopf K, Steurer P, Klein C E, Krieg T, Mauch C. Tissue inhibitor of matrix metalloproteinase-2 regulates matrix metalloproteinase-2 activation by modulation of membrane-type 1 matrix metalloproteinase activity in high and low invasive melanoma cell lines. J Biol Chem. 1999;274:21056–21062. doi: 10.1074/jbc.274.30.21056. [DOI] [PubMed] [Google Scholar]
- Salahuddin S Z, Rose R M, Groopman J E, Markham P D, Gallo R C. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986;68:281–284. [PubMed] [Google Scholar]
- Hammer S M, Gillis J M, Pinkston P, Rose R M. Effect of zidovudine and granulocyte-macrophage colony-stimulating factor on human immunodeficiency virus replication in alveolar macrophages. Blood. 1990;75:1215–1219. [PubMed] [Google Scholar]
- Plata F, Garcia-Pons F, Ryter A, Lebargy F, Goodenow M M, Dat M H, Autran B, Mayaud C. HIV-1 infection of lung alveolar fibroblasts and macrophages in humans. AIDS Res Hum Retroviruses. 1990;6:979–986. doi: 10.1089/aid.1990.6.979. [DOI] [PubMed] [Google Scholar]
- Semenzato G, Agostini C, Ometto L, Zambello R, Trentin L, Chieco-Bianchi L, De R A. CD8+ T lymphocytes in the lung of acquired immunodeficiency syndrome patients harbor human immunodeficiency virus type 1. Blood. 1995;85:2308–2314. [PubMed] [Google Scholar]
- Yearsley M M, Diaz P T, Knoell D, Nuovo G J. Correlation of HIV-1 detection and histology in AIDS-associated emphysema. Diagn Mol Pathol. 2005;14:48–52. doi: 10.1097/01.pas.0000142168.72253.11. [DOI] [PubMed] [Google Scholar]
- Hautamaki R D, Kobayashi D K, Senior R M, Shapiro S D. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- Demedts I K, Morel-Montero A, Lebecque S, Pacheco Y, Cataldo D, Joos G F, Pauwels R A, Brusselle G G. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax. 2006;61:196–201. doi: 10.1136/thx.2005.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Armiento J, Dalal S S, Okada Y, Berg R A, Chada K. Collagenase expression in the lungs of transgenic mice causes pulmonary emphysema. Cell. 1992;71:955–961. doi: 10.1016/0092-8674(92)90391-o. [DOI] [PubMed] [Google Scholar]
- Imai K, Dalal S S, Chen E S, Downey R, Schulman L L, Ginsburg M, D'Armiento J. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir Crit Care Med. 2001;163:786–791. doi: 10.1164/ajrccm.163.3.2001073. [DOI] [PubMed] [Google Scholar]
- Foronjy R F, Okada Y, Cole R, D'Armiento J. Progressive adult-onset emphysema in transgenic mice expressing human MMP-1 in the lung. Am J Physiol Lung Cell Mol Physiol. 2003;284:L727–L737. doi: 10.1152/ajplung.00349.2002. [DOI] [PubMed] [Google Scholar]
- Finlay G A, O'Driscoll L R, Russell K J, D'Arcy E M, Masterson J B, Fitzgerald M X, O'Connor C M. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med. 1997;156:240–247. doi: 10.1164/ajrccm.156.1.9612018. [DOI] [PubMed] [Google Scholar]
- Baraldo S, Bazzan E, Zanin M E, Turato G, Garbisa S, Maestrelli P, Papi A, Miniati M, Fabbri L M, Zuin R, Saetta M. Matrix metalloproteinase-2 protein in lung periphery is related to COPD progression. Chest. 2007;132:1733–1740. doi: 10.1378/chest.06-2819. [DOI] [PubMed] [Google Scholar]
- Zheng T, Zhu Z, Wang Z, Homer R J, Ma B, Riese R J, Jr, Chapman H A, Jr, Shapiro S D, Elias J A. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106:1081–1093. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Weiden M, Harkin T, Ho D, Rom W N. Low copy number and limited variability of proviral DNA in alveolar macrophages from HIV-1-infected patients: evidence for genetic differences in HIV-1 between lung and blood macrophage populations. Mol Med. 1995;1:744–757. [PMC free article] [PubMed] [Google Scholar]
- Kazanjian P, Adams D, Tate S, Newman G. HIV production from purified monocytes isolated from antiretroviral-naive and protease inhibitor-treated HIV-1-infected patients. HIV Clin Trials. 2002;3:469–474. doi: 10.1310/EF26-V3PC-AG5X-FCF4. [DOI] [PubMed] [Google Scholar]
- Denis M, Ghadirian E. Alveolar macrophages from subjects infected with HIV-1 express macrophage inflammatory protein-1 α (MIP-1 α): contribution to the CD8+ alveolitis. Clin Exp Immunol. 1994;96:187–192. doi: 10.1111/j.1365-2249.1994.tb06540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J M, Barbers R G, Oishi J S, Prince H. Cellular and T-lymphocyte subpopulation profiles in bronchoalveolar lavage fluid from patients with acquired immunodeficiency syndrome and pneumonitis. Am Rev Respir Dis. 1984;130:786–790. doi: 10.1164/arrd.1984.130.5.786. [DOI] [PubMed] [Google Scholar]
- Twigg H L, III, Day R B, Smith P A, Knox K S. Highly active antiretroviral therapy (HAART) markedly decreases bronchoalveolar lavage (BAL) chemokine concentrations. Proc Am Thorac Soc. 2007;175:A248. [Google Scholar]
- Wang Z, Zheng T, Zhu Z, Homer R J, Riese R J, Chapman H A, Jr, Shapiro S D, Elias J A. Interferon γ induction of pulmonary emphysema in the adult murine lung. J Exp Med. 2000;192:1587–1600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Kang M J, Lee C G, Chapoval S, Liu W, Chen Q, Coyle A J, Lora J M, Picarella D, Homer R J, Elias J A. Role of CCR5 in IFN-γ-induced and cigarette smoke-induced emphysema. J Clin Invest. 2005;115:3460–3472. doi: 10.1172/JCI24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee K J, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, Sheppard D, Pardo A, Selman M, Heller R A. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA. 2002;99:6292–6297. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster N L, Crowe S M. Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. J Leukoc Biol. 2006;80:1052–1066. doi: 10.1189/jlb.0306152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.