Abstract
The Forkhead family of transcription factors modulates a wide variety of cellular functions in cardiovascular tissues. In this review article, we discuss recent advances in our understanding of regulation provided by the forkhead factors in cardiac myocytes and vascular cells.
Keywords: angiogenesis, endothelial cells, forkhead, transcription factors, vascular biology
Forkhead/winged helix proteins are characterized by a conserved 100-amino acid domain called the “forkhead box.”1 These proteins function as transcription factors, and they participate in diverse biological processes in development and disease.2,3 The forkhead family in humans consists of 39 distinct members, which have been divided into 19 subgroups (Forkhead box [FOX] A to S). The FoxO subgroup of transcription factors has received the most attention because of their recently discovered roles in controlling detoxification of reactive oxygen species (ROS),5,6 cell cycle,7 apoptosis,8,9 DNA repair,7 glucose metabolism,10-14 and the regulation of cell size.15-17a Thus, this review will primarily focus on the FoxO subgroup in cardiovascular cells. The reader should note that the role of forkhead factors in endothelial cells and angiogenesis has been reviewed recently.17b
FoxO Overview
The forkhead transcription factors of the O subgroup (FoxO) consist of 4 members, FoxO1 (FKHR), FoxO3 (FKHRL1), FoxO4 (AFX), and FoxO6. FoxO1, FoxO3, and FoxO4 are expressed in most tissues to varying degrees,18,19 FoxO1 is abundantly expressed in adipose tissues, FoxO3 is abundant in cardiac and neuronal tissues, and FoxO4 is abundant in skeletal and cardiac muscle. FoxO6 is predominantly expressed in a specific region of the brain.20 Interest in the FoxO subfamily developed when it was reported that alveolar rhabdomyosarcomas resulted from a genetic translocation forming a fusion gene composed of sequences from the “paired box” (Pax) and “forkhead in rhabdomyosarcoma” (FKHR) transcription factors.21 The fusion protein contained an intact DNA binding domain from Pax3 and part of the DNA binding and C-terminal domains of FKHR (later to be termed FoxO11). In further studies, another forkhead related factor termed AFX1, because of its location on the X chromosome (later to be termed FoxO4), was shown to participate in translocations that resulted in human malignancies involving fusion with the myeloid-lymphoid leukemia (MLL) gene.22 Further cloning studies based on sequence similarities to FKHR resulted in the isolation of the gene FKHRL1,23 later to be termed FoxO3. In more recent studies, the FoxO6 gene has been assigned to the FoxO group because it displays sequence similarity to the other members, although its regulatory properties have diverged from those of the other FoxO factors.24 For the purposes of this review, the generic term FoxO is used to describe properties identified in one or more of FoxO1, FoxO3, and FoxO4, unless otherwise specified. The functional diversification of the different FoxO isoforms was revealed by targeted gene disruption in mice (Table). FoxO1-null embryos die on embryonic day 10.5 as a consequence of incomplete vascular development.25,26 FoxO3-null mice survive to adulthood with FoxO3 females displaying diminished fertility.25,27 FoxO4-null mice display no apparent phenotype.25
Table. Cardiovascular Phenotypes of Mice Deficient in Different Forkhead Factors.
| Forkhead Knock-Out Mouse | Cardiovascular Phenotype | References |
|---|---|---|
| FoxC1 | Congenital hydrocephalous and perinatal lethality. Aortic arch interruption, valve dysplasias, ventricular septal defects. | 155 |
| FoxC2 | Embryonic and perinatal lethality. Aortic arch interruption, septal defects, aberrant recruitment of mural cells to lymphatic vessels, absence of lymphatic valves. | 122,140 |
| Compound FoxC1/FoxC2 | Embryos die around 9.0 days postcoitum. Disorganized mesodermal patterning, abnormal remodeling of vascular plexi, deficient arterial specification, absence of outflow tract, incomplete cardiac morphogenesis. | 121,124 |
| FoxF1 | Embryos die around 8.5 days post coitum. Absence of vascularization in the yolk sac and allantois. Ectopic expression of endothelial and smooth muscle cell-lineage markers in the amnion and other extra embryonic tissues. | 127 |
| FoxH1 | Embryos lack midline structures and the absence of anterior heart field results in malformations in the outflow tract and right ventricle. | 152,156 |
| FoxM1 | Aberrant cardiomyocyte polyploidy apparent as early as embryonic day 13 (E13) attributable to irregular re-replication. Arteriolar smooth muscle cells appear hypertrophied. Defective formation and maintenance of lung microvasculature. | 132,134,153,157 |
| FoxO1 | Impaired angiogenesis evident around E9.5 with defects found in dorsal aorta, branches of carotid artery and intersomitic and yolk sac vessels. Delayed cardiac looping accompanied by distended pericardium. Deletion of FoxO1 alleles in the adult results in mild hemangiomas present mainly in uteri of female mice. | 25,26,74 |
| FoxO3 | Homozygous null mice are viable and grossly indistinguishable from wild type littermates. Enhanced formation and maturation of vessels following hind limb ischemia. Enhanced hypertrophic growth of the heart at baseline. | 25,74,87,147 |
| FoxO4 | Homozygous null mice are viable, fertile and grossly indistinguishable from wild type littermates. Following carotid artery ligation mice exhibit reduced intimal hyperplasia. | 25,74,120 |
| Compound FoxO1/FoxO3/ FoxO4 | Deletion of all six alleles in the adult results in the development of hemangiomas in tissues such as the liver, skeletal muscle, bone marrow, abdominal wall and uterus. Tumors do not form in lung or kidney. | 74 |
| FoxP1 | Embryos die at E14.5 attributable to aberrant cardiac development. Defects include reduced thickness of myocardium, valve dysplasias and insufficient outflow tract septation. | 151 |
Regulation of FoxO Proteins
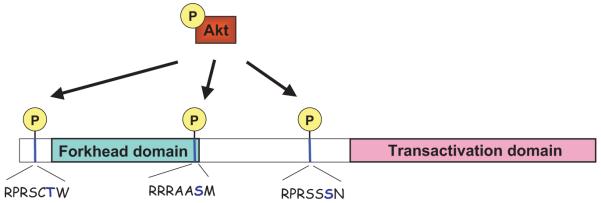
In simple organisms such as the nematode Caenorhabditis elegans, the FoxO homolog Daf-16 (abnormal Dauer formation-16) has been shown to prolong lifespan,28-30 and the longevity phenotype resembles that observed in worms with mutations in the Insulin/insulin-like growth factor-1 receptor homolog Daf2.31 Moreover, flies overexpressing the FoxO homolog forkhead in their fat bodies also display extended lifespan.32 Therefore, genetic evidence suggests that FoxO participates in an evolutionarily conserved pathway which integrates hormonal inputs to regulate lifespan. A major downstream mediator of Insulin/insulin-like growth factor-1 signaling, the serine/threonine protein kinase Akt has been demonstrated by a number of laboratories to directly phosphorylate FoxO factors and regulate their transcriptional activity.9,33,34 Akt is activated by various extracellular stimuli in a phosphatidylinositol-3 kinase (PI3K)-dependent manner and regulates multiple aspects of cellular function in cardiovascular tissues.35,36 As shown in Figure 1, FoxO factors have 3 Akt phosphorylation sites which are conserved from C. elegans to humans, although the third site does not occur in FoxO6.24 When FoxO factors are phosphorylated at those conserved sites by Akt they are exported from the nucleus, whereas unphosphorylated FoxO factors reside in the nucleus.9,34 Therefore, transcriptional activity of FoxO factors is negatively regulated by Akt. Conditions that inhibit Akt signaling, such as serum-deprivation, result in the accumulation of the dephosphorylated FoxO factors in the nucleus where they activate or repress gene promoters. The FoxO factors are also regulated through phosphorylation by serum- and glucocorticoid-inducible kinase-1 (SGK1)37 and IkappaB kinase (IKK).38 SGK1 is a serine/threonine kinase that is present in the heart.39 SGK1 has structural homology to Akt and it is activated downstream of PI3K in response to growth factors.39,40 Interestingly, Akt and SGK1 preferentially phosphorylate different combinations of sites within the FoxO factor.37 In FoxO3, for example, Akt and SGK1 both phosphorylate Thr-32, SGK1 preferentially phosphorylates Ser-315, whereas Akt phosphorylates Ser-253. These findings suggest that SGK1 and Akt coordinately modulate the function of FoxO factors, and these characteristics may enable FoxO factors to respond differently to a variety of stimuli.
Figure 1.
FoxO factors are regulated by the protein kinase Akt. Schematic representation of the generalized structure of FoxO forkhead factors and the conserved Akt-phosphorylation sites. Phosphorylation on these serine/threonine residues is considered to be inhibitory because it facilitates nuclear export and cytoplasmic retention/degradation of the FoxO factors. The forkhead/winged helix domain required for DNA binding and the transactivation domain necessary for interactions with other DNA-associated proteins are also illustrated.
After their phosphorylation by kinases such as Akt and SGK, nuclear FoxO factors are tagged for export to the cytoplasm. Several mechanisms have been proposed to mediate this regulatory step.41 Phosphorylation on a serine residue (ie, S256 in FoxO1) situated at the C-terminal boundary of the forkhead domain (Figure 1) occurs within the nuclear localization signal (NLS) which spans that area.42,43 It is possible that such a phosphorylation event could interfere with the ability of NLS to interact with an importin family member and thereby reduce the tendency of FoxO to re-enter the nucleus. Furthermore, members of the protein family 14-3-3 that bind to phospho-serine and phospho-threonine motifs have been also implicated in the nucleocytoplasmic shuttling of FoxO. After phosphorylation at a conserved motif at the N terminus of the forkhead domain (ie, T24 in FoxO1), 14-3-3 proteins present in the nucleus are recruited to bind the FoxO protein. The phosphorylated motif at the NLS is also targeted for 14-3-3 binding.42-45 It is suggested that the conformational changes induced by 14-3-3 binding exposes nuclear export signals (NES) present in the FoxO protein.44 Generally, NES can be recognized for binding by the chromosomal region maintenance protein 1 (Crm1/exportin). This protein in turn can interact with nuclear Ran-GTP which mediates active translocation of Crm1/FoxO complexes to the cytoplasm. Additionally, 14-3-3 proteins could also mediate FoxO nuclear exclusion via NLS masking and there is also evidence that 14-3-3 interferes with the DNA-binding activity of FoxO.46-48 Collectively, the complexity of the mechanisms described above highlights the significance of this posttranslational modification in the overall regulation of FoxO proteins in the cell.
Another important question in FoxO regulation is how these factors control the transcription of target genes. Early studies using comparative and PCR-assisted binding selection approaches identified the core consensus sequence TTGTTTAC as a binding site for FoxO.18,19 This sequence termed the Daf-16 family protein-binding element (DBE) is likely the optimal sequence that affords the strongest binding for all FoxO isoforms (FoxO1, FoxO3, FoxO4) and their C. elegans homolog Daf-16. This sequence occurs in the promoter regions of the human and worm genes encoding manganese superoxide dismutase (MnSOD),49 which has been identified as a downstream target of FoxO3.5 Recently, the crystal structure of the FoxO3 forkhead domain/DBE complex has been elucidated.50 Using a bioinformatics approach to search for genes containing DBE-like sequences, a number of genes from different species were identified as candidate FoxO targets.49 Moreover, sequences with similarity to the DBE are commonly found within insulin responsive elements.51 For example, the core insulin response sequence (IRS) is found in the promoters of glucose-6 phosphatase (G6Pase), phosphoenol- pyruvate carboxykinase (PEPCK) and the insulin-like growth factor binding protein-1.52,53 This IRS sequence [T(G/A)TTTTG] differs from the DBE in the last 2 bases and displays a weaker affinity for FoxO in in vitro DNA binding assays.19 This variability between optimal and suboptimal FoxO binding elements together with the tissue-specific expression pattern of the FoxO isoforms may contribute to a tightly controlled program of gene expression regulation. The list of candidate FoxO targets in the cardiovascular system is continuously expanding. A summary of confirmed FoxO target genes in vascular and cardiac cells can be found in the supplemental Table (available online at http://circres.ahajournals.org). Some of these genes are also discussed in other sections of the review.
On binding to target DNA sequences, FoxO factors can interact with other DNA/chromatin-associated proteins. The interaction between FoxO and the various cofactors is likely mediated by the transactivation domain (Figure 1). The LxxLL motif, found near the C-terminus of the transactivation domain, has been proposed to mediate the binding of FoxO with proteins from the family of nuclear receptors (NR).54 This is in agreement with studies demonstrating the functional interaction of FoxO with the estrogen receptor (ER), androgen receptor (AR), and retinoic acid receptor (RAR).54-56 Members from the NR family can bind directly to DNA and mediate either transcriptional activation or repression of their target genes, through modulating the chromatin structure around the promoter. Therefore, by facilitating the recruitment of NR family members to the promoters of target genes, FoxO can promote transcriptional activation or repression.
FoxO may also interact with other nuclear proteins to regulate transcription. For example, FoxO1 interacts with Smad transcriptional regulators to activate the transcription of p21cip.57 An interaction has been identified between FoxO and the transcriptional coactivator β-catenin in the context of oxidative stress in mammalian cells.58 FoxO1 has been shown to interact with the peroxisome proliferative activated receptor-γ coactivator 1 (PGC-1) to activate the expression of G6Pase and PEPCK in hepatocytes.10 Interestingly, FoxO1 has been also shown to functionally interact with the transcription factor CCAAT/enhancer binding protein β (C/EBPβ),59 a protein that can also be expressed in liver and is implicated in the transcriptional activation of PEPCK.60 Taken together, these findings document the complex interaction between FoxO and a variety of transcriptional regulators that allow the cell to control the expression of related sets of target genes in a coordinated manner.
FoxO transcriptional activity is also regulated by acetylation/deacetylation. Silent information regulator (Sir) 2 is a class III histone deacetylase (HDAC) that regulates longevity in lower organisms.61-63 Sirt1, a mammalian homolog of Sir2, has been shown to promote resistance to environmental stress and suppress apoptosis through a FoxO-dependent mechanism in mammalian cells.64,65 FoxO3 is acetylated on at least 5 lysine residues. Generally, Sirt1 deacetylase action promotes stress adaptation and cell cycle arrest (and longevity in lower organisms), whereas the acetylation of FoxO promotes its apoptotic action. Therefore, it appears that phosphorylation controls the nuclear translocation of FoxO, but whether FoxO functions to promote survival or apoptosis in the nucleus may depend on an interaction with Sirt1 and its status of lysine acetylation. Recently, it has been demonstrated that FoxO represent molecular targets of Sirt1 in endothelial cells (ECs).66 In this cellular context it was found that Sirt1 activity is associated with negative regulation of FoxO1, which is in contrast with other reports suggesting a positive effect of Sirt1-mediated deacetylation on FoxO transcriptional activity.65,67,68 In this study it is also proposed that HDAC, likely from group I/II, can be also involved in the deacetylation of FoxO to mediate transcriptional inactivation in ECs.66
FoxO in Cellular Life and Death Decisions
Numerous studies have examined the complex roles of the FoxO factors in cellular survival. Early studies showed that suppression of FoxO transcriptional activity by Akt-mediated phosphorylation led to enhanced cell survival.9 For example, activation of FoxO3 has been shown to induce apoptosis in neuronal cell lines and fibroblasts by upregulation of Fasligand expression and activation of the “extrinsic” death receptor pathway. Additionally, Dijkers et al have shown that the “intrinsic” mitochondrial pathway can promote apoptotic cell death in hematopoietic cells after activation of FoxO3 signaling.8 As discussed in greater detail below, FoxO action also promotes apoptosis in endothelial and smooth muscle cells through the combined actions of intrinsic and extrinsic pathways.69,70
In contrast, the Daf-16/Sir2 signaling axis in C. elegans has been shown to be important for cellular viability, adaptation to oxidative stress, and longevity.63,71 Similarly, studies in mammalian cells have shown that an important function of FoxO is the detoxification of ROS,5,6 a feature that is likely to contribute to its prosurvival and longevity-promoting actions. For example, a number of FoxO consensus binding sites occur in the catalase and MnSOD promoters, and both promoters are activated by FoxO in transient transfection assays in PC12 and 3T3-L6 cells, respectively.5,6 Brunet et al65 have reported that H2O2, but not UV irradiation or growth factor stimulation, promotes the deacetylation of FoxO, and this posttranslational modification promotes stress adaptation and cell cycle arrest versus apoptosis in mammalian cells. These data indicate that Sirt1 action changes FoxO-dependent responses from apoptosis-promoting to stress-resistance, thereby decreasing the stress-induced apoptosis that is associated with aging. Thus it appears that the ability of the FoxO factors to promote or inhibit apoptosis is highly dependent on the cellular environment. Under conditions of stimulation by mitogens, Akt signaling predominates and cells initiate a growth program. Under these conditions, the FoxO factors are inactivated and cellular survival is dependent on the PI3K/Akt signaling. On the other hand, FoxO signaling will predominate under conditions of quiescence, and the FoxO factors will activate pathways that ensure cell cycle arrest and long-term survival that is mediated by the ability of these transcription factors to activate ROS detoxification genes. Thus the opposing actions of Akt and the FoxO factors represent a checks and balances system that provides different mechanisms to ensure cell survival depending on whether the cell is actively growing in a nutrient rich environment (high levels of Akt signaling) or in a quiescent state where the ability to cope with oxidant stress over long periods is desirable (high levels of FoxO transcription).5 Given these considerations, it is reasonable to speculate that the apoptosis resulting from forced FoxO expression in many cellular contexts may be attributable to the generation of conflicting signals between Akt and FoxO regulatory programs.
FoxO Forkhead Factors in the Regulation of Endothelial Cell Biology
The role of FoxO factors in the developing vasculature has been highlighted by the observation that FoxO1-deficient mice die during embryogenesis and display malformations in major vessels of the embryo and yolk sac.25,26 In contrast, homozygous FoxO3 or FoxO4-null mice do not exhibit any overt embryonic abnormalities, survive until adulthood, and are largely similar to their wild type littermates.25,27 The abnormal development of the embryonic vasculature in FoxO1-deficient mice has partly been ascribed to an insufficiency of ECs to respond properly to VEGF.26 Furthermore, the transcription levels of connexins 37 and 40, which are necessary for the formation of gap junctions between endothelial cells,72 and the Eph receptor ligand ephrin-B2, which is implicated in vascular patterning and specification of arterial versus venous identities,73 were found to be reduced in FoxO1 deficient yolk sacs.26 Although these abnormalities could contribute to an impaired development of the vasculature, it is unknown whether they represent the primary cause of embryonic mortality.
The significance of FoxO factors in the postnatal vasculature has been further highlighted by the use of genetically engineered mice that allow the inducible ablation of FoxO genes.74 This system not only permits the study of homozygous null-FoxO1 mice that would otherwise die during embryogenesis, but also allows for different combinations of FoxO null alleles to be generated thereby revealing potential hierarchies and overlapping patterns of activity among these factors. Accordingly, generalized deletion of all 3 FoxO genes (FoxO1, FoxO3, and FoxO4) by the transient activation of Cre recombinase through the Mx1 promoter in 4- to 5-week-old mice results in the appearance of benign endothelial cell tumors termed hemangiomas. A less penetrant tumorigenic potential was also evident in mice rendered deficient in FoxO1, after Cre induction, with female mice developing uterine hemangiomas by the age of 60 weeks. Importantly, deficiency in either FoxO3 or FoxO4, or a compound FoxO3/FoxO4 gene deletion did not recapitulate the tumor prone phenotype observed in FoxO1-deficient mice, suggesting that in ECs, FoxO1 is the dominant factor required for the suppression of tumor formation. Downstream targets of FoxO in ECs identified in this study include the FGF-signaling antagonist Sprouty2, which is positively regulated by FoxO, and the homeobox-like transcription factor Pbx1, which is downregulated by the activity of FoxO.74 Collectively, these findings are consistent with the long purported role of FoxO factors in tumor suppression and they further illustrate the extensive functional redundancy of these factors. Moreover, they document the physiological significance of these factors in the maintenance of postnatal vasculature.
A number of studies have focused on FoxO factors in ECs as downstream targets of PI3K/Akt signaling (Figure 2). Several approaches have been used to activate Akt and modulate FoxO phosphorylation. In one study, vascular endothelial growth factor (VEGF) signaling was implicated in the regulation of FoxO transcriptional activity.75 Akt phosphorylation and activation was induced in cultured ECs treated with VEGF and this resulted in the phosphorylation, cytoplasmic sequestration, and transcriptional inactivation of FoxO factors. Moreover, VEGF stimulation induced the survival and proliferation of ECs whereas the adenoviral delivery of a FoxO3 isoform which is triply mutated at the conserved Akt phosphorylation sites (TM-FoxO3) abolished these effects. Concomitant treatment of cells with VEGF and transfection with TM-FoxO3 led to the identification of a number of VEGF-responsive genes in ECs that appear to be upregulated by the synergistic actions of both VEGF and FoxO activities.76 Examples of these genes include matrix metalloproteinase-10 (MMP-10), vascular endothelial cell adhesion molecule-1 (VCAM-1), endothelial specific molecule-1 (ESM-1), bone morphogenetic protein 2 (BMP-2), and CBP-interacting transactivator-2 (CITED-2). Additionally, the identification of putative FoxO responsive elements in the promoters of these genes suggests direct transcriptional regulation, although this merits further investigation. Another gene upregulated by VEGF and FoxO is MnSOD,75 a gene frequently shown to be upregulated by FoxO activity in many different cellular contexts and required for scavenging ROS. The fact that FoxO act in concert with VEGF to positively regulate the expression of target genes is paradoxical because of the documented ability of VEGF to induce the phosphorylation and nuclear exclusion of FoxO through activation of Akt signaling. Thus the regulation of genes by VEGF and FoxO is complex, and different degrees of phosphorylation or other posttranslational modifications may confer distinct effects. Furthermore, the interaction of FoxO with other transcription factors such as nuclear factor-κB (NF-κB) would likely contribute to this complexity.76
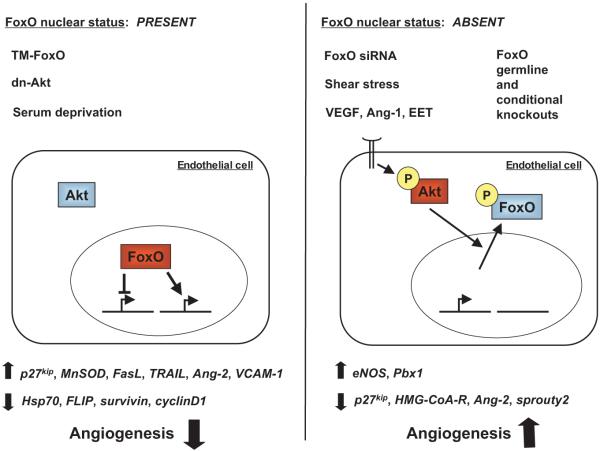
Figure 2.
The role of FoxO factors in endothelial cells. Left panel, FoxO nuclear localization can be conferred by incubating ECs in low serum media or by inhibiting PI3K/Akt signaling. Nuclear localization of FoxO has been frequently recapitulated in cultured ECs by means of ectopic expression of a mutant FoxO isoform mutated at the 3 conserved Akt sites (FoxO-TM). On nuclear localization, FoxO can activate or repress the transcription of several target genes as illustrated at the bottom. The overall effect of FoxO activation in ECs is a suppression of angiogenesis. Right panel, The classical route for FoxO nuclear exclusion involves shear stress or the stimulation of the PI3K/Akt pathway by different agonists. FoxO gene-targeting in mice has been also used to dissect their functions in vivo. FoxO inhibition is generally associated with angiogenesis. The generic term FoxO is used to describe properties identified in 1 or more of FoxO1, FoxO3, and FoxO4. See the text for details on isoform specific properties of the FoxO factors or the paradoxical synergism between FoxO and VEGF signaling. TM-FoxO indicates triple mutant FoxO; dn-Akt, dominant negative Akt; MnSOD, manganese superoxide dismutase; TRAIL, TNF-α related apoptosis-inducing ligand; Ang-2, angiopoietin-2; VCAM-1, vascular cell adhesion molecule-1; Hsp70, heat shock protein-70; FLIP, FLICE inhibitory protein; siRNA, small interfering RNA; VEGF, vascular endothelial growth factor; Ang-1, angiopoietin-1, EET, 11, 12-epoxyeicosatrienoic acid; eNOS, endothelial nitric oxide synthase; Pbx, pre-B cell acute leukemia homeobox gene; HMG-CoA-R, 3-hydroxyl 3-methylglutaryl-coenzyme A reductase.
In addition to VEGF, angiopoietin-1 (Ang-1) has also been reported to mediate inhibitory actions on FoxO in ECs.77 Ang-1 was shown to induce endothelial cell survival through the PI3K/Akt pathway,78 and unlike VEGF it does not induce vessel sprouting after EC proliferation but instead regulates vessel diameter.79 Ang-1 stimulation results in FoxO1 phosphorylation and inactivation, whereas transfection of primary ECs with constitutively-active FoxO1 (TM-FoxO1) promotes apoptosis.77 Microarray analysis of TM-FoxO1 transfected cells revealed regulation of several genes associated with either extracellular matrix remodeling (decorin, matrix metalloproteinase), angiogenesis (Flt-1, angiopoietin-2 [Ang-2]), apoptosis (TNFα-related apoptosis-inducing ligand [TRAIL], survivin) or cell cycle (cyclin D1).77 In this regard, it has been shown that Ang-2 secretion in ECs expressing TM-FoxO1 can activate the receptor Tie2.80 This, in turn, can stimulate PI3K/Akt signaling leading to phosphorylation and inhibition of FoxO. Accordingly, it is conceivable that under conditions of EC stress, excessive apoptosis conferred by the activation of FoxO1 can be bypassed by the initiation of a negative feedback loop involving autocrine stimulation of PI3K/Akt signaling by Ang-2.80
An important regulator of EC biology is fluid shear stress generated by blood flow, which leads to activation of Akt, eNOS, and AMP activated protein kinase (AMPK).81-83 Consistent with the regulation of FoxO factors by at least some of the above molecules, there is emerging evidence to suggest that FoxO factors may have functional implications in the response of ECs to shear forces.81,84 Under conditions of sustained fluid shear stress it has been shown that FoxO1 becomes rapidly phosphorylated and inactivated, likely involving an AMPK-dependent mechanism rather than Akt.81 Moreover, FoxO1 activity in this system has been associated with the expression of 3-hydroxyl 3-methylglutarylcoenzyme A reductase (HMG-CoA-R), which catalyzes the rate limiting step in the de novo biosynthesis of cholesterol. Indeed, knockdown of FoxO1 in endothelial cells results in reduced amounts of mRNA encoding HMG-CoA-R.81 The biological significance of HMG-CoA-R downregulation in the context of fluid shear stress remains elusive, although a proposed mechanism suggests that reduced enzymatic activity can result in attenuated isoprenylation of small guanosine triphosphatases (GTPase) such as Ras, which activate the MEK/ERK pathway. Nevertheless, inhibition of FoxO activity, mediated by AMPK can promote survival of ECs, based on the general proapoptotic potential of FoxO factors. FoxO1 downregulation in response to shear stress has been also documented independently, although in this case Akt is shown to mediate the phosphorylation/inactivation step.84 Here, FoxO1 inhibition was accompanied by a downregulation of p27kip, as well as Ang-2, which is consistent with previous studies demonstrating a dependence of Ang-2 expression on FoxO1 activity.77,80
In another study, 11, 12-epoxyeicosatrienoic acid (11, 12-EET), which has mitogenic effects on ECs,85 attenuated the expression of the cyclin dependent kinase (CDK) inhibitor p27kip.86 Further investigation revealed that PI3K/Akt signaling was activated in ECs on 11, 12-EET treatment, and this promoted the phosphorylation and transcriptional inactivation of FoxO1 and FoxO3.
Further analysis of the role of FoxO factors has revealed that different members of the subgroup have overlapping but not identical regulatory functions in ECs. For example, it has been reported that constitutively-active FoxO1 and FoxO3 but not FoxO4 reduce sprout formation and migration in vitro.87 Conversely, knockdown of endogenous FoxO1 and FoxO3 enhances the angiogenic activity of cultured ECs. Consistent with these observations, FoxO3-null mice exhibited enhanced neovascularization in ischemic hind limbs and matrigel implants.87 Analysis of downstream targets likely to be regulated by FoxO1 or FoxO3 demonstrated that these transcription factors were functionally redundant in ECs, although there are some gene-specific effects as well. For example, endothelial nitric oxide synthase (eNOS) expression was found to be negatively regulated by both FoxO1 and FoxO3, whereas Ang-2 was positively regulated by FoxO1 but not FoxO3. The negative effects of FoxO on eNOS are of particular interest with regard to the well documented significance of eNOS in vascular remodeling, vasodilation, and postnatal neovascularization. This study also demonstrated that both FoxO1 and FoxO3, but not FoxO4, can physically interact with a conserved forkhead responsive element in the promoter of the eNOS gene and inhibit the transcription of this gene.87
Additional studies in ECs demonstrated that FoxO factors regulate the extrinsic apoptotic pathway, mediated by the death-inducing signaling complex (DISC), or the intrinsic apoptotic pathway, mediated by the apoptosome.70,88 Caspase-8 (FLICE) performs the initiating phase of the extrinsic apoptotic cascade and its activity can be inhibited by FLICE inhibitory protein (FLIP), which in ECs has been demonstrated to be under the positive regulation of PI3K/Akt signaling.89 Conversely, transduction of ECs with adenovirus expressing TM-FoxO3 results in a reduction of FLIP mRNA and protein levels, and this is associated with increased caspase-8 activity and reduced EC viability.70 Furthermore, in a screen for downstream targets of PI3K/Akt signaling in ECs, heat shock protein (Hsp)-70 was found to be potently upregulated, an effect largely reversed by the ectopic expression of TM-FoxO3.88 Hsp70 downregulation as a result of TM-FoxO3 activity resulted in reduced EC viability. Hsp70 has been shown to inhibit Apaf-1 oligomerization and interfere with the recruitment of pro—caspase-9 to the apoptosome thereby blocking the intrinsic apoptotic pathway.90 Collectively, these studies indicate that FoxO3 can play a role in coordinating the processes that regulate EC apoptosis.
There is also evidence implicating FoxO factors in the inhibition of EC senescence mediated by the accumulation of ROS. In cultured primary ECs, it was shown that constitutively-active Akt signaling reduces cellular lifespan and is associated with premature growth arrest.91 Inhibition of FoxO3 by Akt was essential for growth arrest to occur. The growth arrest effect likely stems from the hyperactivation of the transcription factor p53 and subsequent accumulation of the cyclin-dependent kinase inhibitor p21cip. Although the connection between p53 and FoxO is not fully understood, this study suggests that activation of Akt results in the inhibition of FoxO3 and accumulation of ROS that eventually activate p53 and its downstream target p21cip.91
FoxO Forkhead Factors and Endothelial Progenitor Cells
A number of laboratories have reported that endothelial progenitor cells (EPCs) are present in the systemic circulation and home to sites of ischemic injury where they function to promote neovascularization.92,93 Circulating EPC levels are also an indicator of cardiovascular health.94,95 FoxO transcription factors have been detected in EPCs with the expression of FoxO4 being the most abundant.96,97 In contrast, FoxO1 and FoxO3 appear to be expressed predominantly in mature ECs.87 Treatment of EPCs with the HMG-CoA-R inhibitor atorvastatin promotes EPC survival under conditions of oxidative stress and induces the phosphorylation of FoxO4.97 Furthermore, FoxO4 phosphorylation is accompanied by reduced expression levels of Bcl-2-like protein Bim, a classical proapoptotic mediator shown to be regulated by FoxO factors.98,99 Therefore, it is proposed that the PI3K/Akt axis is functional in EPCs where it can repress the proapoptotic activity of FoxO under conditions of oxidative stress.97 However, in addition to regulating proapoptotic genes, FoxO factors have also been reported to reduce ROS through the induction of MnSOD and catalase in the mitochondria and peroxisomes respectively.5,6 In this regard, EPCs have been reported to express higher levels of MnSOD and catalase compared with mature ECs, and this was associated with increased survival on ROS challenge.92 These findings highlight the complexity of PI3K/Akt/FoxO signaling in EPCs. FoxO factors may either facilitate ROS detoxification and promote survival of EPCs, or they can induce apoptosis by upregulating proapoptotic genes. The direction of these effects is likely the result of the context-specific posttranslational signature of FoxO.
Pertinent to the findings suggesting that EPCs isolated from diabetic patients are largely dysfunctional,95,100 a recent study has established a link between glucotoxicity and FoxO activity in EPCs.96 In this study, it was shown that increased levels of glucose induce a distinct pattern of posttranslational modifications on FoxO1, characterized by reduced phosphorylation on Akt consensus sites, and enhanced acetylation on lysine residues. Under these conditions FoxO1 nuclear localization is accompanied by upregulation of the proapoptotic factors Bim and FasL. Furthermore, catalase expression is downregulated suggesting a switch in gene expression patterns from stress resistance to cell death. Indeed, EPCs treated with high levels of glucose show increased rates of apoptosis, and this effect was reversed by benfotiamine,96 a thiamine derivative shown to inhibit vascular damage under conditions of hyperglycemia.101 Therefore, it is suggested that aberrant activation of FoxO factors under conditions of glucose toxicity may be associated with the impaired ability of EPCs to differentiate and promote de novo tube formation.96 Taken together, studies where EPCs are challenged with high doses of H2O2 or glucose suggest that FoxO factors are intimately associated with induction of apoptosis, which can be at least in part inhibited by active PI3K/Akt signaling.
FoxO Forkhead Factors in Vascular Smooth Muscle Cells
Vascular smooth muscle cells (VSMCs) provide the vessel wall with resilience and contractility which are critical components in the regulation of vessel diameter and peripheral arterial pressure regulation. In addition, the ability of VSMCs to undergo proliferation and migration as a response to various physiological and pathological stimuli makes them potential targets for therapeutic intervention. Considering the roles of FoxO factors in the regulation of cell cycle, apoptosis, and cellular differentiation, it is not surprising that these factors have been shown to participate in different aspects of VSMC pathophysiology. Early studies examining apoptosis in VSMCs showed that the expression of FasL on the cell surface was an important determinant for cell death, under conditions of diminished PI3K/Akt signaling, and this could be modulated by the activity of FoxO factors.69 Indeed, transduction of VSMCs with dominant negative Akt, treatment with the PI3K-inhibitor wortmannin, or serum deprivation promoted expression of functional FasL on VSMC surface, and this was accompanied by enhanced cell death. Importantly, ectopic expression of constitutively-active FoxO3 in VSMC-induced FasL expression and promoted DNA fragmentation, and this effect was partially dependent on the activity of caspase-8. Although the details of FasL upregulation by FoxO in VSMCs were not fully explored in this study, it is possible that FoxO mediate FasL expression through direct interaction with the cognate promoter, as it has been reported in other experimental settings.9,102 Nevertheless, as it has been shown in the case of ECs,70,88 it seems likely that FoxO can mediate VSMC death by participating in both the extrinsic (death receptor mediated) or intrinsic (mitochondrial) apoptotic pathways.
The involvement of FoxO factors in regulating VSMC proliferation through modulation of cell cycle has also been documented. For example, cyclic strain has been shown to induce an integrin-dependent downregulation of p27kip, and this is associated with increased cell cycle entry.103 Under these conditions, p27kip downregulation requires the phosphorylation and activation of Akt, and this effect is reversed by transduction with TM-FoxO3 or TM-FoxO4. FoxO-mediated p27kip regulation in VSMCs has been also documented in other experimental models. Stimulation of coronary artery VSMCs with platelet-derived growth factor (PDGF), tumor necrosis factor-α (TNF-α), or insulin-like growth factor-1 results in the phosphorylation and nuclear exclusion of FoxO factors in VSMCs.104 Moreover, this study shows that transduction with TM-FoxO3 increases p27kip mRNA levels, suppresses proliferation induced by mitogens, and increases the number of apoptotic cells. Some of these effects appeared to be mediated by the upregulation of p27kip, as siRNA knockdown of the gene partly abrogated the anti-proliferative effects of active FoxO3.
Carotid artery balloon injury has been used to test the significance of FoxO on VSMC proliferation in vivo. Consistent with its antiproliferative effect in cultured cells, adenoviral delivery of TM-FoxO3 in arteries decreases the proliferation of VSMCs and reduces the intima/media ratio and these effects are accompanied by an increase in p27kip levels.104,105 Moreover, it was also shown that TM-FoxO3 delivered to the site of injury will increase the frequency of VSMC apoptosis,105 an effect that is consistent with earlier observations documenting the proapoptotic effects of active FoxO in cultured VSMCs.69 Collectively, findings from these studies point to FoxO factors in VSMCs as important regulators of neointimal hyperplasia after angioplasty.
Recent evidence also suggests that the upregulation of p27kip may not be the only mechanism by which FoxO factors inhibit the proliferation of VSMCs. Indeed, the cysteine-rich protein 61 (CYR61), a potent angiogenic factor rapidly secreted after angioplasty or angiotensin II stimulation, has been shown to be negatively regulated by FoxO in VSMCs.106 CYR61 is an extracellular matrix-associated protein that can interact with integrins to promote VSMC migration and adhesion, and it has been implicated in processes such as vascular development, postnatal neovascularization, atherosclerosis, and vascular restenosis.107-111 A functional association between FoxO and CYR61 was first noted after identification of a forkhead binding element in the promoter of CYR61 gene. Adenoviral delivery of constitutively-active FoxO3 was shown to suppress CYR61 expression, inhibit proliferation, and reduce cell viability.106 Overexpression of CYR61 partially reverses the antiproliferative but not the proapoptotic effect conferred by activated FoxO3. Furthermore, transduction with TM-FoxO3 interferes with the pathway that mediates activation of CYR61 by angiotensin II and suppresses the migration-stimulating effect of CYR61 in VSMCs in vitro. This study also showed that adenoviral delivery of constitutively-active FoxO3 in rat carotid arteries after balloon injury results in reduced neointimal hyperplasia, an effect that was paralleled by a reduction of endogenous CYR61 expression. Conversely, concomitant delivery of adenovirus expressing CYR61 reversed the intima-sparing effect of TM-FoxO3.106 Thus, this study illustrates another pathway through which FoxO factors can minimize the increase in cellularity in the vessel wall after acute injury.
Cyclins D1 and D2 are key regulators of cell proliferation that promote the transition from the G1 to the S phase of the cell cycle. Previous studies performed in primary embryonic fibroblasts and cancer cell lines showed that enforced FoxO4 expression resulted in the suppression of D-type cyclins, and this effect was associated with reduced CDK activity and subsequent cell cycle arrest independent of p27kip regulation.112 More recently, a correlation between FoxO activity and cyclin D expression was also reported in the regulation of VSMC proliferation.113 In this study, betacellulin (BTC), which acts as a ligand for the epidermal growth factor receptor, was shown to activate the PI3K/Akt signaling pathway leading to the phosphorylation of FoxO. BTC-stimulation also induced glycogen synthase kinase-3α/β (GSK-3α/β) phosphorylation and increased nuclear localization of β-catenin, which associates with T-cell factor (TCF) to upregulate downstream target genes such as Cyclin D. BTC-stimulated Akt signaling also promoted the phosphorylation of FoxO, and it was suggested that β-catenin nuclear localization in conjunction with FoxO phosphorylation is required for the upregulation of cyclin D1.113 Along these lines, there is emerging evidence from C. elegans to suggest that in conditions of oxidative stress β-catenin may interact directly with FoxO to activate its transcriptional activity.58 These findings support a model in which β-catenin can switch partners between FoxO and TCF to mediate cell cycle arrest or progression, respectively, depending on environmental conditions.
The well-documented association between FoxO and Mn-SOD expression has also been described in VSMCs.114 MnSOD protein levels and activity were shown to be reduced in VSMC upon aging. Further investigation demonstrated that FoxO activity is required for optimal MnSOD transcription and that reduced nuclear localization of FoxO occurs in VSMC derived from old animals. Moreover, the attenuated nuclear localization of FoxO factors could be ascribed to hyperactivation of the PI3K/Akt signaling. These data suggest that aged VSMCs may be in higher risk for oxidative damage because the pathway maintaining MnSOD expression has been impaired as a result of aberrant activation of Akt.
The transition of VSMCs from a contractile to a more proliferative phenotype has been recently shown to be modulated by FoxO4.115 FoxO4 localization in the nuclei of cultured aortic smooth muscle cells was associated with reduced expression of myogenic markers. Myogenic differentiation-specific genes likely to be repressed by nuclear FoxO4 include SMα-actin, SM-calponin, and SM-22α. These genes are under the positive regulation of myocardin,116 a transcription coactivator which is essential for the induction of differentiation in VSMCs.117,118 The physical interaction of FoxO4 with myocardin in cultured aortic smooth muscle cells was shown to repress the ability of myocardin to initiate the smooth muscle cell differentiation program.115 These findings suggest a model whereby the stimulation of VSMCs with growth factors, such as insulin-like growth factor-1, activates the PI3K/Akt pathway, which in turn inactivates FoxO4 and derepresses a myogenic program which is under the positive regulation of myocardin and its transcriptional partner serum response factor (SRF). Conversely, nuclear prevalence of FoxO4 is expected to suppress the differentiation program and promote a dedifferentiated and more proliferative phenotype. In agreement with this model, the nuclear translocation of FoxO4 was found to occur in proliferating VSMCs after in vivo vascular injury.115 These findings appear to be somewhat contradictory to the evidence described earlier, where constitutive nuclear localization of FoxO3 was shown to inhibit neointimal hyperplasia104,105 and that nuclear exclusion of FoxO3 is rapidly induced after carotid balloon injury.105 Nevertheless, it is possible that different FoxO factors may function in a distinct or even opposite manner in response to vascular injury, although no mechanistic evidence is currently available to support this notion. In this regard, it should be noted that FoxO4 was found to mediate its inhibitory effects on VSMC differentiation in a DNA-binding independent manner,115 suggesting that FoxO domains other than the forkhead domain may be responsible for this functional diversification.
In atherosclerotic lesions, TNF-α is released by macrophages and VSMCs and it enhances VSMC migration and promotes the expression of cell adhesion molecules and matrix metalloproteinases (MMP).119 Although the effects of TNF-α on vascular pathophysiology are likely to be pleiotropic, recent data suggest that these effects in VSMCs can be partially mediated by FoxO4 in a PI3K/Akt-independent manner.120 It has been demonstrated that FoxO4 genetic insufficiency (knockout or knockdown) in cultured aortic smooth muscle cells impairs their ability to migrate in response to TNF-α and this effect is partially reversed by the addition of MMP9 in the cultures. Consistent with a model whereby TNF-α activates MMP9 expression through FoxO4, depletion of FoxO4 in VSMCs blocks the induction of MMP9 after TNF-α stimulation. Moreover, FoxO4 ectopic expression in VSMCs was shown to activate MMP9 reporter constructs, and the DNA-binding activity of FoxO4 was dispensable for this effect.120 Further investigation demonstrated a specific interaction between FoxO4 and the transcription factor Sp1, suggesting that the formation of a ternary complex relying on the DNA-binding activity of Sp1 is necessary for the activation of MMP9 transcription. Finally, FoxO4-null mice subjected to carotid artery ligation exhibited reduced intimal formation compared with wild-type mice, and this effect was paralleled by a reduced expression of MMP9 in the knockout mice.120 This study implicates FoxO4 in the regulation of genes which are required for VSMC migration after inflammatory stimulation through its ability to supply transactivating domains, rather than directly engaging target DNA sites. This study also supports the notion that FoxO4 may have unique functions that are distinct from FoxO1 or FoxO3. Indeed, FoxO1 was not able to upregulate MMP9 in response to TNF-α, although it was shown to interact with Sp1. However, when the C-terminal region of FoxO4 was used to replace that of FoxO1, the resulting protein achieved comparable transactivation of MMP9. Thus, distinct functional domains between different FoxO factors may explain why in some studies FoxO3 activity is found to inhibit VSMC proliferation and neointimal hyperplasia, whereas in others FoxO4 activity promotes VSMC dedifferentiation and enhanced migration.
Non-FoxO Forkheads Implicated in Vascular Development and Homeostasis
Two closely related forkhead factors from the C subgroup, FoxC1 and FoxC2, have been reported to participate in the formation of the developing vasculature121 (Table). FoxC1 or FoxC2 null mutants die prenatally or perinatally because of multiple defects, including lesions in the cardiovascular system.122,123 The functions of the FoxC1 and FoxC2 genes during development appear largely redundant because elimination of both genes in compound homozygous animals results in more severe phenotype.124 Analysis of the developing vasculature in these embryos showed that FoxC1 and FoxC2 are required for the acquisition of arterial fate in endothelial cells.125 This effect is likely mediated by the direct transcriptional upregulation of Delta-like4 (Dll4), a ligand for Nothch1 signaling which functions downstream VEGF to determine arterial cell fate.126 Therefore, it is proposed that FoxC1 and FoxC2 operate downstream of VEGF, to activate Notch-1/Dll4 signaling necessary for the induction of arterial specification in endothelial cells.125
The role of FoxF1 in the lung vasculature has been highlighted by gene-targeting studies in mice. FoxF1 deficiency results in premature death before embryonic day 10 because of defects in extra-embryonic mesoderm (Table).127 In contrast, FoxF1 haploinsufficiency results in perinatal lethality attributable to lung malformations128 and hemorrhage,129 indicating that FoxF1 is essential for lung morphogenesis. Immunohistochemical studies showed that FoxF1 is expressed in alveolar endothelial cells positive for the platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), and this expression was shown to be partly dependent on the activity of the sonic hedgehog (Shh) signaling pathway.128 Further histological examination showed that the tight junctions formed between endothelial and epithelial cells were defective in FoxF1-deficient lungs, resulting in disruption of the alveolar capillaries.129 Additionally, lung microvasculature abnormalities associated to FoxF1 haploinsufficiency were paralleled by reduced levels of Notch-2 receptor, and it was shown that the FoxF1 can directly interact with putative binding sites situated in the promoter of the Notch-2 receptor gene.130 Collectively, these data suggest that FoxF1 expressed in lung endothelial cells can integrate multiple inputs from morphogens, such as Shh, BMP, and FGF, and mediate normal development of peripheral lung microvasculature through activation of Notch-2 receptor pathway. Of note, FoxF1 binding sites have been identified in the regulatory elements of genes that are critical for EC viability and maintenance of vascular homeostasis, such as the receptor tyrosine kinase Flk1, vascular endothelial cadherin, and PECAM-1.131
There is also evidence implicating FoxM1 transcription factor in lung vasculogenesis. Targeted deletion of FoxM1 gene in mice results in embryonic lethality attributable to multiple defects, including malformation of peripheral pulmonary capillaries and excessive hypertrophy of arteriolar smooth muscle cells.132 These defects were attributed to reduced proliferation rates of the lung mesenchyme and lower than normal expression levels of different endothelial specific molecules including Flt-1, PECAM-1, and FoxF1. Transgene-mediated overexpression of FoxM1 was associated with increased proliferation levels of pulmonary endothelial and smooth muscle cells and an improved response to lung repair after experimental injury.133 It is proposed that FoxM1 functions during lung development to enhance lung mesenchyme proliferation, including the vascular compartment, and its upregulation during adult life can be beneficial in injured lung, through enhanced proliferation of vasculature and other lung cells. These findings were further corroborated by studies using endothelial-restricted ablation of FoxM1.134 After LPS-induced microvascular injury in the lung, severe edema and increased mortality was observed in these mice, attributed to an inability to reestablish the endothelial barrier. Mechanistically, it is proposed that FoxM1 increases proliferative rates in endothelial cells by upregulating cyclin B1 and Cdc25C and by repressing p27Kip1 activities.134 Thus, in endothelial and vascular smooth muscle cells, FoxM1 may operate in an opposite manner from that of the FoxO proteins which must be inactivated to allow cell cycle progression.86,113 FoxM1 is also regulated at the level of cytoplasmic retention,135 a feature reminiscent to the regulation of FoxO proteins by Akt phosphorylation and their nuclear exclusion.9,34
Forkhead Factors and Lymphangiogenesis
Blood vessels and lymphatic vessels are functionally and structurally similar, and growth of both types of vessels is controlled by similar sets of signaling and transcriptional regulators.136 Along these lines, forkhead factors from the FoxC subgroup that are indispensable during blood vasculature development are also involved in the development and maintenance of the lymphatic vasculature (Table). FoxC2 is reported to be essential in the development of the lymphatic system. The analysis of families with the hereditary disorder called lymphedema-distichiasis syndrome revealed that mutations in the forkhead domain of FoxC2 are linked to the development of the disease.137,138 Mice with haploinsufficiency for FoxC2 (FoxC2+/-) display lymphatic vessel hyperplasia, lymphatic valve abnormalities, and other features reminiscent of the condition described in humans, suggesting that the FoxC2+/- mouse is a suitable model for the study of the disease.139 Further studies performed in FoxC2 null embryos (FoxC2-/-) revealed that lymphatic vessels display abnormal recruitment of pericytes and vascular smooth muscle cells. Furthermore, FoxC2 deficiency results in absence of lymphatic valves, suggesting that this factor is crucial for the development and maintenance of these valves.140 Mechanistically, it was found that FoxC2 activity in lymphatic endothelium depends on VEGFR-3 signaling, and this activity is responsible for the negative regulation of smooth muscle cell and pericyte recruitment.140 An increased recruitment of vascular smooth muscle cells and pericytes on the lymphatic capillaries may promote lymph backflow and defective drainage as observed in lymphedema patients. VEGFR-3 promotes lymphatic endothelial cell growth, migration, and survival by binding VEGF-C and VEGF-D.141 Additionally, platelet-derived growth factor-β (PDGF-β) is required for the recruitment of pericytes to the capillary wall.142 In FoxC2-/- mice, mRNA levels for PDGF-β were shown to be higher than normal.140 Therefore, it is suggested that VEGF signaling in lymphatic endothelial cells results in the activation of FoxC2 which in turn inhibits aberrant recruitment of mural cells, at least in part by repressing PDGF signaling. Intriguingly, the function of FoxC2 was also shown to be required for the development and maintenance of venous valves,143 suggesting that extended parallels exist between lymphatic and venous valve morphogenesis.
FoxO Factors in Cardiac Hypertrophy
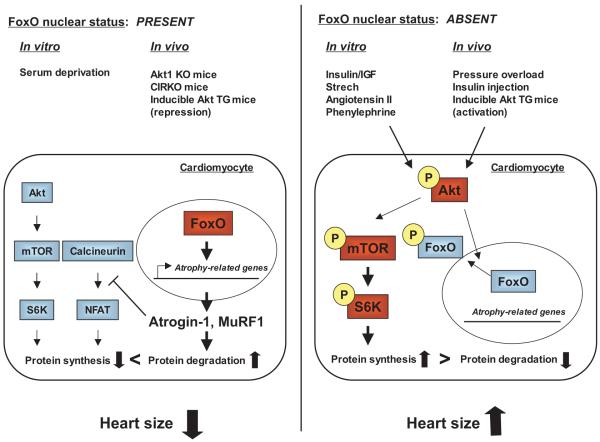
FoxO subfamily members are present in cardiac myocytes and are regulated by phosphorylation in response to multiple hypertrophic stimuli.17a In cultured cardiac myocytes, the FoxO factors are phosphorylated in response to both physiological (insulin like growth factor-1 and insulin) and pathological (angiotensin II) hypertrophic growth stimuli, and their subcellular localization is regulated by Akt-mediated phosphorylation (Figure 3). In vivo, phosphorylation of FoxO is increased in the heart in response to pressure overload, insulin injection, or Akt overexpression in cardiac myocytes. Conversely, FoxO phosphorylation is decreased in cardiac insulin receptor knockout (CIRKO) mice and Akt1 KO mice. Adenovirus-mediated overexpression of wild-type or constitutively-active FoxO3 inhibits growth factor or mechanical stretch-induced hypertrophy in vitro.17a Adenovirus-mediated overexpression of FoxO3 also reduces myocyte size in vivo. These findings indicate that FoxO factors function downstream of Akt signaling to negatively regulate heart size. Similarly, FoxO transcription factors are likely to be important determinants of skeletal muscle hypertrophy.15,16
Figure 3.
The role of Akt-FoxO pathway in regulation of heart size. Left, FoxO factor phosphorylation is decreased under conditions of Akt inactivation. Under these conditions, FoxO factors remain in the nucleus and activate atrophy-related genes, including the ubiquitin ligases atrogin-1 and MuRF-1, which promote protein degradation. Atrogin-1 also interacts with and represses calcineurin by targeting calcineurin for ubquintin-mediated proteolysis, leading to inhibition of cardiac growth. Right, Various physiological or pathological hypertrophic growth stimuli result in the activation of Akt through phosphorylation. Akt activiation leads to inactivation of FoxO factors through phosphorylation and nuclear exclusion, thereby limiting the expression of atrophy-related genes. Akt activation also increases protein synthesis through the mammalian target of rapamycin (mTOR)/S6 linase (S6K)-dependent signaling pathways.
Target genes thought to mediate the antihypertrophic actions of FoxO factors include atrogin-1/MAFbx and MuRF1 E3 ubiquitin ligases, which promote proteasome-mediated protein degradation.16 In the heart, atrogin-1/MAFbx mRNA is downregulated by activation of an Akt1 transgene in myocytes and upregulated after Akt1 transgene repression.17a,144 These data suggest that Akt signaling simultaneously activates protein synthesis and inhibits protein degradation in the heart. This notion is consistent with the observation that termination of Akt transgene expression after 2 weeks of induction results in an extremely rapid decrease in heart size in an inducible Akt1 transgenic mouse model.145 This rapid decrease in heart size is presumably attributable to combinatorial effects of decreased protein synthesis and increased protein degradation.
In the heart, atrogin-1 is reported to interact with and repress calcineurin by targeting calcineurin for ubiquitin-mediated proteolysis, leading to inhibition of cardiac hypertrophy in response to pathologic stimuli.146 Ni et al reported that overexpression of either wild-type and constitutively-active forms of FoxO1 or FoxO3 reduces calcineurin phosphatase activity and suppresses the expression of the modulatory calcineurin interacting protein exon 4 isoform MCIP1.4, a direct target of the calcineurin/NFAT cascade.147 They also documented that FoxO3-deficient mice showed ≈10% increase in heart weight compared with wild-type mice at baseline, but both strains displayed similar degrees of cardiac hypertrophy when subjected to pressure overload. Interestingly, it was recently reported that FoxO1 and FoxO3 can be direct targets of atrogin-1 ubiquitination in the heart.148 The ubiquitination does not target FoxO factors for proteasomal degradation but enhances their transcriptional activity. These findings suggest a feed-forward mechanism involving FoxO and atrogin-1 and provide additional insights about the complex regulation of FoxO factors during postnatal heart growth.
Alcendor et al have shown that moderate levels of Sirt1 overexpression protect the heart from oxidative stress and apoptosis through a FoxO1-dependent mechanism.149 They also showed that overexpression of either Sirt1 or constitutively-active FoxO1 stimulated expression of catalase, whereas transduction of dominant negative FoxO1 in cultured cardiac myocytes resulted in reduced catalase expression. On the other hand, it was also reported that overexpression of FoxO1 activated transcription of TRAIL in cultured cardiac myocytes, and this response was enhanced by phenylephrine and inhibited by epidermal growth factor.150
Non-FoxO Forkheads Involved in Cardiac Development
Other subgroups of forkhead transcription factors have been implicated in cardiac development.121,151-153 Von Both et al152 showed that FoxH1 is required for formation of the secondary, or anterior, heart-forming field. Targeted disruption of FoxH1 gene in mouse embryos leads to severe defects in outflow tract and right ventricle formation that resemble the defects seen in Mef2c-deficient heart.154 They also showed that Mef2c expression in the anterior heart-forming field is FoxH1-dependent. Likewise, it is reported that FoxC1 and FoxC2 are required for the proper development of the outflow tract and the right ventricle.121 Inactivation of FoxP1 in embryos results in multiple severe defects in heart morphogenesis, including cushion defects, thin ventricular myocardial compact zone, defects in valvular formation, and lack of proper ventricular septation, which lead to embryonic death at E14.5.151 Finally, FoxM1 is essential for the proliferation of cardiomyocytes during heart development (see Table).153 Disruption of FoxM1 results in ventricular hypoplasia, diminished DNA replication, and mitosis in cardiomyocytes during development.
Summary and Future Directions
Numerous studies in many experimental organisms have shown that the forkhead transcription factors are involved in development, immune regulation, tumorigenesis, metabolism, and longevity. In the cardiovascular system, most attention has focused on the FoxO subgroup of factors that control diverse biological functions including detoxification of ROS,5,6 cell cycle progression,7 apoptosis,8,9 DNA repair,7 and metabolism.10-12 These factors are under the regulatory control of the serine/threonine protein kinase Akt which phosphorylates and inactivates FoxO. This mode of regulation is essential for the maintenance of cellular homeostasis, a notion supported by the finding that this pathway is evolutionarily conserved in species ranging from C. elegans up to humans. Further posttranslational regulation of FoxO is conferred by acetylation and deacetylation. In this regard, removal of acetyl residues by the deacetylase Sirt1 enables FoxO to protect the cell from oxidative insults. In vascular cells, FoxO factors are generally involved in the suppression of cellular proliferation, quiescence, and stress endurance. However, in more extreme conditions they launch an apoptotic program. In the heart, FoxO factors have been shown to function as negative regulators of cardiac myocyte growth through their ability to activate the expression of atrophy-related genes.
Gene targeting in mice has greatly contributed to our understanding of the function of FoxO in the vasculature. Mice simultaneously deficient for FoxO1, FoxO3, and FoxO4 display a hyperproliferative phenotype in ECs that ultimately leads to formation of hemangiomas. In addition, gene targeting has shown that members of forkhead subgroups other than FoxO participate in the regulation of the developing heart and vasculature. Factors from the subgroup FoxC are involved in arterial specification during development, lymphatic vessel morphogenesis, and in the patterning of the outflow tract and right ventricle in the developing heart. Moreover, factors from the subgroup FoxM are involved in the regulation of cell proliferation of lung peripheral microvasculature while they also participate in the coordinated proliferation and growth of cardiac and vascular myocytes.
Although great progress has been made in our understanding of forkhead factors, many questions remain to be answered. The study of forkheads in vivo has been greatly hampered by the fact that some homozygous knockout mice display embryonic lethality attributable to severe developmental defects in the heart and vasculature. Thus, greater use of both spatial and temporal conditional knockouts will permit a better dissection of the in vivo functions of these factors which so heavily impact the cardiovascular system.
With regard to the established roles of FoxO, it is apparent that the regulation conferred by these factors is very complex, and they can activate opposing cell fates (eg, apoptosis and stress-resistance) depending on their degree of posttranslational modification and cellular context. The regulation by Akt or Sirt1 may be the most pronounced examples of a complex network of FoxO protein modifications that regulate their function. Additional complexity may derive from the ability of these factors to either cooperate or antagonize the activity of other transcription factors or DNA-associated proteins. Additionally, complexity exists within the FoxO group itself. In vivo and in vitro studies have shown that despite a general functional redundancy, FoxO isoforms may also exhibit individual functions that are conferred by protein regions outside of the forkhead domain.
With regard to EPCs, future studies should test the hypothesis that FoxO transcription factors control the function of these cells by regulating their resistance to oxidative stress. Presumably, FoxO transcription factors activate a genetic program that confers quiescence and resistance to stress when EPCs are present in their bone marrow niche. However, under conditions of EPC mobilization a new program that confers proliferation and differentiation is activated through an increase in Akt signaling and an inhibition of the FoxO transcriptional program.
With regard to the heart, it will be interesting to test whether FoxO factors regulate autophagy, an intracellular degradation process for protein and organelles. Importantly, autophagy is increased by stresses in the heart, and this process appears to be dysregulated in various cardiac pathologies. Finally, myocardial substrate metabolism plays a key role in the maintenance of contractile function particularly under ischemic conditions. Because FoxO factors regulate metabolism in various tissues, the analysis of FoxO in acute or chronic myocardial ischemia could provide interesting insights.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH grants HL86785, HL77774, and HL81587.
Footnotes
Disclosures
None.
References
- 1.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 2.Lehmann OJ, Sowden JC, Carlsson P, Jordan T, Bhattacharya SS. Fox’s in development and disease. Trends Genet. 2003;19:339–344. doi: 10.1016/S0168-9525(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 4.Delated in proof.
- 5.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 6.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 7.Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ, Jr, DiStefano PS, Chiang LW, Greenberg ME. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 8.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 9.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 10.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 11.Nakae J, Biggs WH, 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 12.Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imae M, Fu Z, Yoshida A, Noguchi T, Kato H. Nutritional and hormonal factors control the gene expression of FoxOs, the mammalian homologues of DAF-16. J Mol Endocrinol. 2003;30:253–262. doi: 10.1677/jme.0.0300253. [DOI] [PubMed] [Google Scholar]
- 14.Furuyama T, Kitayama K, Yamashita H, Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem J. 2003;375:365–371. doi: 10.1042/BJ20030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 16.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, Lecker S, Taegtmeyer H, Goldberg AL, Walsh K. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 2005;280:20814–20823. doi: 10.1074/jbc.M500528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17b.Dejana E, Taddei A, Randi AM. Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim Biophy Acta. 2007;1775:298–312. doi: 10.1016/j.bbcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Biggs WH, 3rd, Cavenee WK, Arden KC. Identification and characterization of members of the FKHR (FOX O) subclass of winged-helix transcription factors in the mouse. Mamm Genome. 2001;12:416–425. doi: 10.1007/s003350020002. [DOI] [PubMed] [Google Scholar]
- 19.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoekman MF, Jacobs FM, Smidt MP, Burbach JP. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns. 2006;6:134–140. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ, 3rd, Emanuel BS, Rovera G, Barr FG. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5:230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 22.Corral J, Forster A, Thompson S, Lampert F, Kaneko Y, Slater R, Kroes WG, van der Schoot CE, Ludwig WD, Karpas A, et al. Acute leukemias of different lineages have similar MLL gene fusions encoding related chimeric proteins resulting from chromosomal translocation. Proc Natl Acad Sci U S A. 1993;90:8538–8542. doi: 10.1073/pnas.90.18.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics. 1998;47:187–199. doi: 10.1006/geno.1997.5122. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem. 2003;278:35959–35967. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- 25.Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Ikeda K, Motoyama N, Mori N. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 27.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 28.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 29.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 30.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 31.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 32.Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- 33.Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 34.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 36.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angio-genesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 37.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J. 1999;339(Pt 2):319–28. [PMC free article] [PubMed] [Google Scholar]
- 40.Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. Embo J. 1999;18:3024–3033. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Gan L, Pan H, Guo S, He X, Olson ST, Mesecar A, Adam S, Unterman TG. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem. 2002;277:45276–45284. doi: 10.1074/jbc.M208063200. [DOI] [PubMed] [Google Scholar]
- 43.Brownawell AM, Kops GJ, Macara IG, Burgering BM. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol Cell Biol. 2001;21:3534–3546. doi: 10.1128/MCB.21.10.3534-3546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunet A, Kanai F, Stehn J, Xu J, Sarbassova D, Frangioni JV, Dalal SN, DeCaprio JA, Greenberg ME, Yaffe MB. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J Cell Biol. 2002;156:817–828. doi: 10.1083/jcb.200112059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obsilova V, Vecer J, Herman P, Pabianova A, Sulc M, Teisinger J, Boura E, Obsil T. 14-3-3 Protein interacts with nuclear localization sequence of forkhead transcription factor FoxO4. Biochemistry. 2005;44:11608–11617. doi: 10.1021/bi050618r. [DOI] [PubMed] [Google Scholar]
- 46.Rena G, Prescott AR, Guo S, Cohen P, Unterman TG. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem J. 2001;354:605–612. doi: 10.1042/0264-6021:3540605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cahill CM, Tzivion G, Nasrin N, Ogg S, Dore J, Ruvkun G, Alexander-Bridges M. Phosphatidylinositol 3-kinase signaling inhibits DAF-16 DNA binding and function via 14-3-3-dependent and 14-3-3-independent pathways. J Biol Chem. 2001;276:13402–13410. doi: 10.1074/jbc.M010042200. [DOI] [PubMed] [Google Scholar]
- 48.Obsil T, Ghirlando R, Anderson DE, Hickman AB, Dyda F. Two 14-3-3 binding motifs are required for stable association of Forkhead transcription factor FOXO4 with 14-3-3 proteins and inhibition of DNA binding. Biochemistry. 2003;42:15264–15272. doi: 10.1021/bi0352724. [DOI] [PubMed] [Google Scholar]
- 49.Xuan Z, Zhang MQ. From worm to human: bioinformatics approaches to identify FOXO target genes. Mech Ageing Dev. 2005;126:209–215. doi: 10.1016/j.mad.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 50.Tsai KL, Sun YJ, Huang CY, Yang JY, Hung MC, Hsiao CD. Crystal structure of the human FOXO3a-DBD/DNA complex suggests the effects of post-translational modification. Nucleic Acids Res. 2007 doi: 10.1093/nar/gkm703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Brien RM, Granner DK. Regulation of gene expression by insulin. Physiol Rev. 1996;76:1109–1161. doi: 10.1152/physrev.1996.76.4.1109. [DOI] [PubMed] [Google Scholar]
- 52.Streeper RS, Svitek CA, Chapman S, Greenbaum LE, Taub R, O’Brien RM. A multicomponent insulin response sequence mediates a strong repression of mouse glucose-6-phosphatase gene transcription by insulin. J Biol Chem. 1997;272:11698–11701. doi: 10.1074/jbc.272.18.11698. [DOI] [PubMed] [Google Scholar]
- 53.O’Brien RM, Noisin EL, Suwanichkul A, Yamasaki T, Lucas PC, Wang JC, Powell DR, Granner DK. Hepatic nuclear factor 3- and hormone-regulated expression of the phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein 1 genes. Mol Cell Biol. 1995;15:1747–1758. doi: 10.1128/mcb.15.3.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao HH, Herrera RE, Coronado-Heinsohn E, Yang MC, Ludes-Meyers JH, Seybold-Tilson KJ, Nawaz Z, Yee D, Barr FG, Diab SG, Brown PH, Fuqua SA, Osborne CK. Forkhead homologue in rhabdomyosarcoma functions as a bifunctional nuclear receptor-interacting protein with both coactivator and corepressor functions. J Biol Chem. 2001;276:27907–27912. doi: 10.1074/jbc.M104278200. [DOI] [PubMed] [Google Scholar]
- 55.Schuur ER, Loktev AV, Sharma M, Sun Z, Roth RA, Weigel RJ. Ligand-dependent interaction of estrogen receptor-alpha with members of the forkhead transcription factor family. J Biol Chem. 2001;276:33554–33560. doi: 10.1074/jbc.M105555200. [DOI] [PubMed] [Google Scholar]
- 56.Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol Cell Biol. 2003;23:104–118. doi: 10.1128/MCB.23.1.104-118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 58.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 59.Christian M, Zhang X, Schneider-Merck T, Unterman TG, Gellersen B, White JO, Brosens JJ. Cyclic AMP-induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein beta in differentiating human endometrial stromal cells. J Biol Chem. 2002;277:20825–20832. doi: 10.1074/jbc.M201018200. [DOI] [PubMed] [Google Scholar]
- 60.Arizmendi C, Liu S, Croniger C, Poli V, Friedman JE. The transcription factor CCAAT/enhancer-binding protein beta regulates gluconeogenesis and phosphoenolpyruvate carboxykinase (GTP) gene transcription during diabetes. J Biol Chem. 1999;274:13033–13040. doi: 10.1074/jbc.274.19.13033. [DOI] [PubMed] [Google Scholar]
- 61.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 62.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 63.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 64.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 65.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 66.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004;101:10042–10047. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1) J Biol Chem. 2004;279:28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 69.Suhara T, Kim H-S, Kirshenbaum LA, Walsh K. Suppression of Akt signaling induces Fas ligand expression: involvement of caspase and Jun kinase activation in Akt-mediated Fas ligand regulation. Mol Cell Biol. 2002;22:680–691. doi: 10.1128/MCB.22.2.680-691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skurk C, Maatz H, Kim HS, Yang J, Abid MR, Aird WC, Walsh K. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem. 2004;279:1513–1525. doi: 10.1074/jbc.M304736200. [DOI] [PubMed] [Google Scholar]
- 71.Berdichevsky A, Viswanathan M, Horvitz HR, Guarente L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell. 2006;125:1165–1177. doi: 10.1016/j.cell.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 72.Simon AM, McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol. 2002;251:206–220. doi: 10.1006/dbio.2002.0826. [DOI] [PubMed] [Google Scholar]
- 73.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 74.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abid MR, Guo S, Minami T, Spokes KC, Ueki K, Skurk C, Walsh K, Aird WC. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:294–300. doi: 10.1161/01.ATV.0000110502.10593.06. [DOI] [PubMed] [Google Scholar]
- 76.Abid MR, Shih SC, Otu HH, Spokes KC, Okada Y, Curiel DT, Minami T, Aird WC. A novel class of vascular endothelial growth factor-responsive genes that require forkhead activity for expression. J Biol Chem. 2006;281:35544–35553. doi: 10.1074/jbc.M608620200. [DOI] [PubMed] [Google Scholar]
- 77.Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai KM, Lin HC, Ioffe E, Yancopoulos GD, Rudge JS. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18:1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 79.Thurston G, Wang Q, Baffert F, Rudge J, Papadopoulos N, Jean-Guillaume D, Wiegand S, Yancopoulos GD, McDonald DM. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period. Development. 2005;132:3317–3326. doi: 10.1242/dev.01888. [DOI] [PubMed] [Google Scholar]
- 80.Daly C, Pasnikowski E, Burova E, Wong V, Aldrich TH, Griffiths J, Ioffe E, Daly TJ, Fandl JP, Papadopoulos N, McDonald DM, Thurston G, Yancopoulos GD, Rudge JS. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15491–15496. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fisslthaler B, Fleming I, Keseru B, Walsh K, Busse R. Fluid shear stress and NO decrease the activity of the hydroxy-methylglutaryl coenzyme A reductase in endothelial cells via the AMP-activated protein kinase and FoxO1. Circ Res. 2007;100:e12–21. doi: 10.1161/01.RES.0000257747.74358.1c. [DOI] [PubMed] [Google Scholar]
- 82.Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol Scand. 2000;168:81–88. doi: 10.1046/j.1365-201x.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- 83.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 84.Chlench S, Mecha Disassa N, Hohberg M, Hoffmann C, Pohlkamp T, Beyer G, Bongrazio M, Da Silva-Azevedo L, Baum O, Pries AR, Zakrzewicz A. Regulation of Foxo-1 and the angiopoietin-2/Tie2 system by shear stress. FEBS Lett. 2007;581:673–680. doi: 10.1016/j.febslet.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 85.Fleming I, Fisslthaler B, Michaelis UR, Kiss L, Popp R, Busse R. The coronary endothelium-derived hyperpolarizing factor (EDHF) stimulates multiple signalling pathways and proliferation in vascular cells. Pflugers Arch. 2001;442:511–518. doi: 10.1007/s004240100565. [DOI] [PubMed] [Google Scholar]
- 86.Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278:29619–29625. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- 87.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim HS, Skurk C, Maatz H, Shiojima I, Ivashchenko Y, Yoon SW, Park YB, Walsh K. Akt/FOXO3a signaling modulates the endothelial stress response through regulation of heat shock protein 70 expression. Faseb J. 2005;19:1042–1044. doi: 10.1096/fj.04-2841fje. [DOI] [PubMed] [Google Scholar]
- 89.Suhara T, Mano T, Oliveira BE, Walsh K. Phosphatidylinositol 3-kinase/Akt signaling controls endothelial cell sensitivity to Fas-mediated apoptosis via regulation of FLICE-inhibitory protein (FLIP) Circ Res. 2001;89:13–19. doi: 10.1161/hh1301.092506. [DOI] [PubMed] [Google Scholar]
- 90.Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 91.Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. Embo J. 2004;23:212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 93.Rumpold H, Wolf D, Koeck R, Gunsilius E. Endothelial progenitor cells: a source for therapeutic vasculogenesis? J Cell Mol Med. 2004;8:509–518. doi: 10.1111/j.1582-4934.2004.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-ki-nase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 96.Marchetti V, Menghini R, Rizza S, Vivanti A, Feccia T, Lauro D, Fukamizu A, Lauro R, Federici M. Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes. 2006;55:2231–2237. doi: 10.2337/db06-0369. [DOI] [PubMed] [Google Scholar]
- 97.Urbich C, Knau A, Fichtlscherer S, Walter DH, Bruhl T, Potente M, Hofmann WK, de Vos S, Zeiher AM, Dimmeler S. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. Faseb J. 2005;19:974–976. doi: 10.1096/fj.04-2727fje. [DOI] [PubMed] [Google Scholar]
- 98.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 99.Dijkers PF, Birkenkamp KU, Lam EW, Thomas NS, Lammers JW, Koenderman L, Coffer PJ. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J Cell Biol. 2002;156:531–542. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 101.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 102.Ciechomska I, Pyrzynska B, Kazmierczak P, Kaminska B. Inhibition of Akt kinase signalling and activation of Forkhead are indispensable for upregulation of FasL expression in apoptosis of glioma cells. Oncogene. 2003;22:7617–7627. doi: 10.1038/sj.onc.1207137. [DOI] [PubMed] [Google Scholar]
- 103.Sedding DG, Seay U, Fink L, Heil M, Kummer W, Tillmanns H, Braun-Dullaeus RC. Mechanosensitive p27Kip1 regulation and cell cycle entry in vascular smooth muscle cells. Circulation. 2003;108:616–622. doi: 10.1161/01.CIR.0000079102.08464.E2. [DOI] [PubMed] [Google Scholar]
- 104.Abid MR, Yano K, Guo S, Patel VI, Shrikhande G, Spokes KC, Ferran C, Aird WC. Forkhead transcription factors inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia. J Biol Chem. 2005;280:29864–29873. doi: 10.1074/jbc.M502149200. [DOI] [PubMed] [Google Scholar]
- 105.Park KW, Kim DH, You HJ, Sir JJ, Jeon SI, Youn SW, Yang HM, Skurk C, Park YB, Walsh K, Kim HS. Activated forkhead transcription factor inhibits neointimal hyperplasia after angioplasty through induction of p27. Arterioscler Thromb Vasc Biol. 2005;25:742–747. doi: 10.1161/01.ATV.0000156288.70849.26. [DOI] [PubMed] [Google Scholar]
- 106.Lee HY, Chung JW, Youn SW, Kim JY, Park KW, Koo BK, Oh BH, Park YB, Chaqour B, Walsh K, Kim HS. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2007;100:372–380. doi: 10.1161/01.RES.0000257945.97958.77. [DOI] [PubMed] [Google Scholar]
- 107.Grzeszkiewicz TM, Lindner V, Chen N, Lam SC, Lau LF. The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin alpha(6)beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology. 2002;143:1441–1450. doi: 10.1210/endo.143.4.8731. [DOI] [PubMed] [Google Scholar]
- 108.Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hilfiker A, Hilfiker-Kleiner D, Fuchs M, Kaminski K, Lichtenberg A, Rothkotter HJ, Schieffer B, Drexler H. Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II. Circulation. 2002;106:254–260. doi: 10.1161/01.cir.0000021426.87274.62. [DOI] [PubMed] [Google Scholar]
- 110.Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu KJ, Yee A, Zhu NL, Gordon EM, Hall FL. Characterization of differential gene expression in monkey arterial neointima following balloon catheter injury. Int J Mol Med. 2000;6:433–440. [PubMed] [Google Scholar]
- 112.Schmidt M, Fernan dez deMattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, Medema RH. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shin HS, Lee HJ, Nishida M, Lee MS, Tamura R, Yamashita S, Matsuzawa Y, Lee IK, Koh GY. Betacellulin and amphiregulin induce upregulation of cyclin D1 and DNA synthesis activity through differential signaling pathways in vascular smooth muscle cells. Circ Res. 2003;93:302–310. doi: 10.1161/01.RES.0000086803.64109.9E. [DOI] [PubMed] [Google Scholar]
- 114.Li M, Chiu JF, Mossman BT, Fukagawa NK. Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J Biol Chem. 2006;281:40429–40439. doi: 10.1074/jbc.M606596200. [DOI] [PubMed] [Google Scholar]
- 115.Liu ZP, Wang Z, Yanagisawa H, Olson EN. Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin. Dev Cell. 2005;9:261–270. doi: 10.1016/j.devcel.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 116.Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol. 2003;23:2425–2437. doi: 10.1128/MCB.23.7.2425-2437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34:1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 118.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2003;100:9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li H, Liang J, Castrillon DH, DePinho RA, Olson EN, Liu ZP. FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol Cell Biol. 2007;27:2676–2686. doi: 10.1128/MCB.01748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seo S, Kume T. Forkhead transcription factors, Foxc1 and Foxc2, are required for the morphogenesis of the cardiac outflow tract. Dev Biol. 2006;296:421–436. doi: 10.1016/j.ydbio.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 122.Iida K, Koseki H, Kakinuma H, Kato N, Mizutani-Koseki Y, Ohuchi H, Yoshioka H, Noji S, Kawamura K, Kataoka Y, Ueno F, Taniguchi M, Yoshida N, Sugiyama T, Miura N. Essential roles of the winged helix transcription factor MFH-1 in aortic arch patterning and skeletogenesis. Development. 1997;124:4627–4638. doi: 10.1242/dev.124.22.4627. [DOI] [PubMed] [Google Scholar]
- 123.Kume T, Deng KY, Winfrey V, Gould DB, Walter MA, Hogan BL. The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell. 1998;93:985–996. doi: 10.1016/s0092-8674(00)81204-0. [DOI] [PubMed] [Google Scholar]
- 124.Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001;15:2470–2482. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294:458–470. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 126.Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 127.Mahlapuu M, Ormestad M, Enerback S, Carlsson P. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development. 2001;128:155–166. doi: 10.1242/dev.128.2.155. [DOI] [PubMed] [Google Scholar]
- 128.Mahlapuu M, Enerback S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. doi: 10.1242/dev.128.12.2397. [DOI] [PubMed] [Google Scholar]
- 129.Kalinichenko VV, Lim L, Stolz DB, Shin B, Rausa FM, Clark J, Whitsett JA, Watkins SC, Costa RH. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev Biol. 2001;235:489–506. doi: 10.1006/dbio.2001.0322. [DOI] [PubMed] [Google Scholar]
- 130.Kalinichenko VV, Gusarova GA, Kim IM, Shin B, Yoder HM, Clark J, Sapozhnikov AM, Whitsett JA, Costa RH. Foxf1 haploinsufficiency reduces Notch-2 signaling during mouse lung development. Am J Physiol Lung Cell Mol Physiol. 2004;286:L521–L530. doi: 10.1152/ajplung.00212.2003. [DOI] [PubMed] [Google Scholar]
- 131.Kalinichenko VV, Zhou Y, Shin B, Stolz DB, Watkins SC, Whitsett JA, Costa RH. Wild-type levels of the mouse Forkhead Box f1 gene are essential for lung repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1253–L1265. doi: 10.1152/ajplung.00463.2001. [DOI] [PubMed] [Google Scholar]
- 132.Kim IM, Ramakrishna S, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV. The forkhead box m1 transcription factor is essential for embryonic development of pulmonary vasculature. J Biol Chem. 2005;280:22278–22286. doi: 10.1074/jbc.M500936200. [DOI] [PubMed] [Google Scholar]
- 133.Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, Yoder HM, Costa RH. Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J Biol Chem. 2003;278:37888–37894. doi: 10.1074/jbc.M305555200. [DOI] [PubMed] [Google Scholar]
- 134.Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, Malik AB. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006;116:2333–2343. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ye H, Holterman AX, Yoo KW, Franks RR, Costa RH. Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Mol Cell Biol. 1999;19:8570–8580. doi: 10.1128/mcb.19.12.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 137.Ferrell RE. Research perspectives in inherited lymphatic disease. Ann N Y Acad Sci. 2002;979:39–51. doi: 10.1111/j.1749-6632.2002.tb04866.x. discussion 76-9. [DOI] [PubMed] [Google Scholar]
- 138.Finegold DN, Kimak MA, Lawrence EC, Levinson KL, Cherniske EM, Pober BR, Dunlap JW, Ferrell RE. Truncating mutations in FOXC2 cause multiple lymphedema syndromes. Hum Mol Genet. 2001;10:1185–1189. doi: 10.1093/hmg/10.11.1185. [DOI] [PubMed] [Google Scholar]
- 139.Kriederman BM, Myloyde TL, Witte MH, Dagenais SL, Witte CL, Rennels M, Bernas MJ, Lynch MT, Erickson RP, Caulder MS, Miura N, Jackson D, Brooks BP, Glover TW. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Hum Mol Genet. 2003;12:1179–1185. doi: 10.1093/hmg/ddg123. [DOI] [PubMed] [Google Scholar]
- 140.Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 141.Karkkainen MJ, Alitalo K. Lymphatic endothelial regulation, lymphoedema, and lymph node metastasis. Semin Cell Dev Biol. 2002;13:9–18. doi: 10.1006/scdb.2001.0286. [DOI] [PubMed] [Google Scholar]
- 142.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 143.Mellor RH, Brice G, Stanton AW, French J, Smith A, Jeffery S, Levick JR, Burnand KG, Mortimer PS. Mutations in FOXC2 are strongly associated with primary valve failure in veins of the lower limb. Circulation. 2007;115:1912–1920. doi: 10.1161/CIRCULATIONAHA.106.675348. [DOI] [PubMed] [Google Scholar]
- 144.Schiekofer S, Shiojima I, Sato K, Galasso G, Oshima Y, Walsh K. Microarray analysis of Akt1 activation in transgenic mouse hearts reveals transcript expression profiles associated with compensatory hypertrophy and failure. Physiol Genomics. 2006;27:156–170. doi: 10.1152/physiolgenomics.00234.2005. [DOI] [PubMed] [Google Scholar]
- 145.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest. 2004;114:1058–1071. doi: 10.1172/JCI22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, Gerard RD, Rothermel BA, Hill JA. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114:1159–1168. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li HH, Willis MS, Lockyer P, Miller N, McDonough H, Glass DJ, Patterson C. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest. 2007;117:3211–3223. doi: 10.1172/JCI31757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 150.Morris JB, Kenney B, Huynh H, Woodcock EA. Regulation of the proapoptotic factor FOXO1 (FKHR) in cardiomyocytes by growth factors and alpha1-adrenergic agonists. Endocrinology. 2005;146:4370–4376. doi: 10.1210/en.2005-0162. [DOI] [PubMed] [Google Scholar]
- 151.Wang B, Weidenfeld J, Lu MM, Maika S, Kuziel WA, Morrisey EE, Tucker PW. Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477–4487. doi: 10.1242/dev.01287. [DOI] [PubMed] [Google Scholar]
- 152.von Both I, Silvestri C, Erdemir T, Lickert H, Walls JR, Henkelman RM, Rossant J, Harvey RP, Attisano L, Wrana JL. Foxh1 is essential for development of the anterior heart field. Dev Cell. 2004;7:331–345. doi: 10.1016/j.devcel.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 153.Ramakrishna S, Kim IM, Petrovic V, Malin D, Wang IC, Kalin TV, Meliton L, Zhao YY, Ackerson T, Qin Y, Malik AB, Costa RH, Kalinichenko VV. Myocardium defects and ventricular hypoplasia in mice homozygous null for the Forkhead Box M1 transcription factor. Dev Dyn. 2007;236:1000–1013. doi: 10.1002/dvdy.21113. [DOI] [PubMed] [Google Scholar]
- 154.Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131:3931–3942. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- 155.Winnier GE, Kume T, Deng K, Rogers R, Bundy J, Raines C, Walter MA, Hogan BL, Conway SJ. Roles for the winged helix transcription factors MF1 and MFH1 in cardiovascular development revealed by nonallelic noncomplementation of null alleles. Dev Biol. 1999;213:418–431. doi: 10.1006/dbio.1999.9382. [DOI] [PubMed] [Google Scholar]
- 156.Hoodless PA, Pye M, Chazaud C, Labbe E, Attisano L, Rossant J, Wrana JL. FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev. 2001;15:1257–1271. doi: 10.1101/gad.881501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Korver W, Schilham MW, Moerer P, van den Hoff MJ, Dam K, Lamers WH, Medema RH, Clevers H. Uncoupling of S phase and mitosis in cardiomyocytes and hepatocytes lacking the winged-helix transcription factor Trident. Curr Biol. 1998;8:1327–1330. doi: 10.1016/s0960-9822(07)00563-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.