Abstract
Thymocytes lacking adenosine deaminase (ADA) activity, a purine metabolism enzyme, accumulate intracellular dATP and consequently undergo apoptosis during development. We have analyzed the effect of ADA enzyme inhibition in human thymocyte suspension cultures with regard to accumulation of intracellular dATP and induction of apoptosis. We demonstrate that while inhibition of deoxycytidine kinase will prevent the accumulation of dATP and induction of apoptosis to a large degree, inhibition of both deoxycytidine kinase and adenosine kinase completely abrogates the accumulation of dATP and significantly reduces the induction of apoptosis. Thus, both deoxynucleoside kinases are involved in this model of ADA deficiency.
Keywords: ADA deficiency, human thymocyte development, deoxynucleoside kinases, apoptosis
Introduction
Mutations disrupting the enzyme activity of adenosine deaminase (ADA), a purine metabolism enzyme that catalyzes the deamination of both adenosine (Ado) and deoxyadenosine (dAdo), cause severe combined immunodeficiency in humans. Patients lacking ADA have a profound T cell deficiency with varying degrees of B/NK lymphopenia, and die at an early age unless treated with enzyme replacement therapy, bone marrow transplantation, or gene therapy (reviewed in [1,2]).
Early studies of ADA deficiency addressed the biochemical mechanisms causing toxicity in cell culture models under conditions where ADA was inhibited, and an ADA substrate was supplied exogenously (either Ado or dAdo). In the absence of ADA enzyme activity, dAdo is phosphorylated by cellular deoxynucleoside kinases, leading to the formation of accumulated dATP. A number of studies concluded this was likely to be the most relevant biochemical process leading to toxicity in ADA-deficient developing lymphocytes [3,4]. However, some contention was held about the relative role of the two major deoxynucleoside kinases believed to be responsible for the initial phosphorylation of dAdo, deoxycytidine kinase (dCK) and adenosine kinase (AK). While dCK appears to be the major enzymatic activity to phosphorylate deoxynucleosides in cell extracts, AK also appeared to play a role, especially in whole cell studies [5].
A more recent approach has utilized the technique of murine fetal thymic organ culture (FTOC) to dissect the mechanism of inhibition of thymocyte development caused by ADA deficiency in a more physiological setting relevant to lymphocyte development. These studies showed that ADA deficiency induces an accumulation of intracellular dATP in developing murine thymocytes through the action of the AK, followed by the induction of mitochondrial-dependent apoptosis [6,7]. In murine FTOC, inhibition of AK enzyme activity alone was sufficient to ameliorate the toxicity associated with lack of ADA. Here we assess the pathways leading to dATP formation in developing human thymocytes and show that inhibition of multiple deoxynucleoside kinases during in vitro cultures of total and immature CD34+ thymocytes under ADA-deficient conditions is required for efficient prevention of both dATP accumulation and induction of apoptosis.
Methods
Human thymus tissue was obtained from infants undergoing corrective cardiac surgery (University of Oklahoma Children's Hospital; OKC, OK). Thymocyte suspensions were prepared by forcing the tissue through a 70μM mesh. Thymocytes were resuspended in Yssel's medium supplemented with 5% human serum and 7% FCS. CD34+ thymocytes were prepared using anti-CD34 beads (Dynal; CA). CD34+ thymocyte purity as assessed by staining with PE anti-CD34 (Caltag; CA) and flow cytometry on a FACSCalibur cytometer (BD Biosciences; CA) was ≥95%.
The specific ADA inhibitor, 2′-deoxycoformycin [8], dCF, was obtained from SuperGen (Dublin, CA). The adenosine kinase inhibitor, 5′-amino-5′-deoxyadenosine (5′A5′dAdo), and the nucleosides, 2′-deoxycytidine (dCyd) and dAdo were obtained from Sigma-Aldrich (St. Louis, MO).
Thymocyte suspensions at a density of 5×106 cells/ml were incubated overnight for approximately 24 h at 37°C, 5% CO2 with the indicated concentrations of each reagent, then harvested and processed for dATP quantitation and apoptosis analysis. Apoptosis was assessed by staining of cells with Annexin-V and propidium iodide followed by flow cytometry analysis on a FACSCalibur cytometer. Intracellular dATP was quantitated by extraction of cells with 60% methanol, followed by HPLC analysis using purified standards as calibration controls.
Results and Discussion
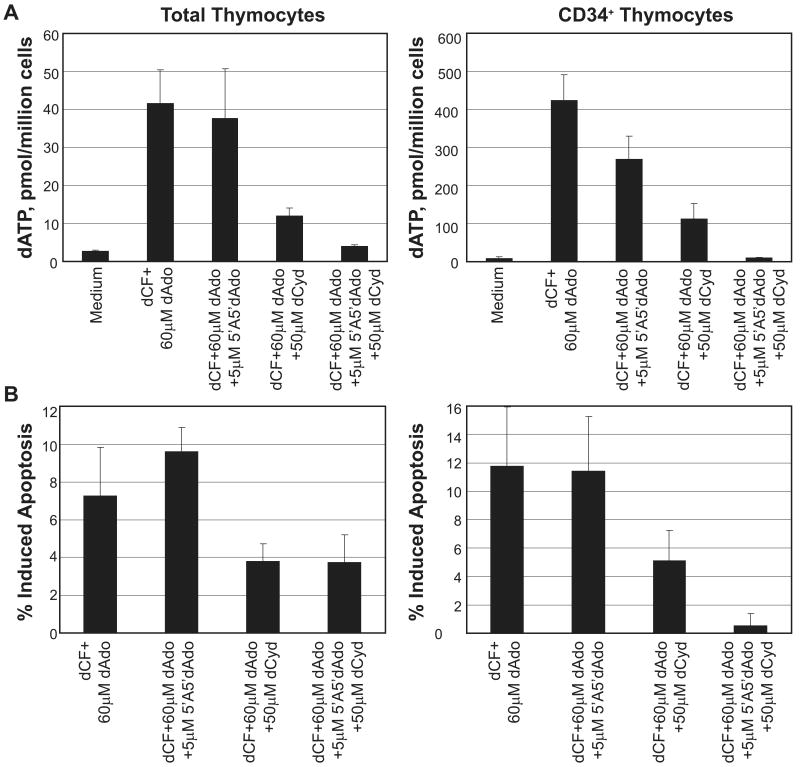
Human thymocyte suspensions were incubated overnight in the presence of dCF to inhibit ADA enzyme activity, and dAdo to mimic the accumulation of substrate under conditions where dAdo is not metabolized by ADA. The AK inhibitor, 5′A5′dAdo, was used to inhibit AK activity [9], whereas 50μM dCyd was used to inhibit the activity of dCK [10]. The graphs in Figure 1A show the accumulation of intracellular dATP under ADA-inhibited conditions in both total thymocytes (comprised mainly of double positive thymocytes expressing surface CD4 and CD8; left), and immature CD34+ thymocytes (right). dATP levels were elevated in total thymocytes under ADA-deficient conditions on average at least 20-fold relative to the medium control. The addition of an AK inhibitor reduced the level of accumulated dATP only slightly in total thymocyte suspensions. However, the addition of dCyd substantially reduced the accumulated dATP level in these cells (by approximately 75%). CD34+ thymocytes showed a somewhat unique profile of dATP accumulation under ADA-inhibited conditions. First, they accumulated on average approximately 10-fold higher levels of dATP on a per cell basis than total thymocytes. The reasons for the exaggerated dATP accumulation in immature thymocytes relative to total thymocytes are currently unknown, although it is not due to a higher dAdo kinase activity, at least as measured in cell extracts (data not shown). Second, they displayed a more effective response to the inhibition of AK alone than did total thymocytes, with a 25-30% reduction in dATP. Finally, although the response to dCK inhibition was similar to that in total thymocytes, the additive effect of both inhibitors appeared to be more complete in CD34+ thymocytes than in total thymocytes, normalizing the dATP to the level of the medium control (compared to a remaining nearly 2-fold elevation in total thymocyte suspensions).
Figure 1.
Assessment of dATP accumulation and induction of apoptosis in total and CD34+ human thymocytes. A. Total or CD34+ thymocyte suspensions were incubated as described in Methods with the indicated reagents and harvested for dATP analysis by HPLC. Results are expressed as pmol dATP/million cells (mean ± SE; n=4); note the difference in scale between total and CD34+ thymocytes. B. Thymocytes (total or CD34+) as in part A were analyzed for induction of apoptosis by Annexin-V/PI staining as described in Methods. Results are expressed as % Induced apoptosis, indicating the percentage of cells that were Annexin-V+ above the media control (mean ± SE; n=4).
The amount of induced apoptosis in thymocyte suspensions treated with dCF and dAdo was analyzed by assessment of Annexin-V+ cells compared to controls. The graphs in Figure 1B show the percentage of total thymocytes (left) and CD34+ thymocytes (right) induced to undergo apoptosis under ADA-deficient conditions in the presence and absence of deoxynucleoside kinase inhibitors. In general, in vitro ADA-deficient conditions induced less apoptosis in total thymocyte suspensions than in CD34+ thymocytes, perhaps due to the fact that total thymocytes already have high levels of spontaneous apoptosis. The addition of the AK inhibitor gave no correction to induced apoptosis in either thymocyte preparation, but the addition of the dCK inhibitor reduced the induction of apoptosis substantially in both types of thymocytes (by about 50% in both cases). Interestingly, the addition of both inhibitors had a much more substantial effect on reduction of apoptosis in CD34+ thymocytes, than in total thymocytes. The presence of both inhibitors in immature thymocytes essentially completely abolished the induction of apoptosis under in vitro ADA-deficient conditions, compared with no effect over dCyd alone in total thymocytes. In summary, multiple enzymatic activities appear to be involved in the accumulation of dATP and induction of apoptosis in human thymocytes in vitro. Chimeric human/mouse FTOC studies should help reveal the impact of ADA deficiency on developing human thymocytes, and the contribution of each of the kinase activities assessed here to the toxicity induced by lack of ADA activity.
Acknowledgments
This work was supported by the NIH grant HD36044 to L.F.T. L.F.T. holds the Putnam City Schools Distinguished Chair in Cancer Research.
References
- 1.Hershfield MS, Mitchell BS. Immunodeficiency Diseases Caused by Adenosine Deaminase Deficiency and Purine Nucleoside Phosphorylase Deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. Vol. 2. McGraw-Hill, Inc.; New York: 1995. Chapter 52. [Google Scholar]
- 2.Blackburn MR, Kellems RE. Adenosine Deaminase Deficiency: Metabolic Basis of Immune Deficiency and Pulmonary Inflammation. Adv Immunol. 2005;86:1–41. doi: 10.1016/S0065-2776(04)86001-2. [DOI] [PubMed] [Google Scholar]
- 3.Carson DA, Wasson DB, Lakow E, Kamatani N. Possible Metabolic Basis for the Different Immunodeficient States Associated With Genetic Deficiencies of Adenosine Deaminase and Purine Nucleoside Phosphorylase. Proc Natl Acad Sci USA. 1982;79:3848–3852. doi: 10.1073/pnas.79.12.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell BS, Mejias E, Daddona PE, Kelley WN. Purinogenic Immunodeficiency Diseases: Selective Toxicity of Deoxyribonucleosides for T Cells. Proc Natl Acad Sci USA. 1978;75:5011–5014. doi: 10.1073/pnas.75.10.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ullman B, Levinson BB, Hershfield MS, Martin DW., Jr A Biochemical Genetic Study of the Role of Specific Nucleoside Kinases in Deoxyadenosine Phosphorylation by Cultured Human Cells. J Biol Chem. 1981;256:848–852. [PubMed] [Google Scholar]
- 6.Thompson LF, Van De Wiele CJ, Laurent AB, Hooker SW, Vaughn JG, Jiang H, Khare K, Kellems RE, Blackburn MR, Hershfield MS, Resta R. Metabolites From Apoptotic Thymocytes Inhibit Thymopoiesis in Adenosine Deaminase-Deficient Fetal Thymic Organ Cultures. J Clin Invest. 2000;106:1149–1157. doi: 10.1172/JCI9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van De Wiele CJ, Vaughn JG, Blackburn MR, Ledent C, Jacobson M, Jiang H, Thompson LF. Adenosine Kinase Inhibition Promotes Survival of Fetal Adenosine Deaminase-Deficient Thymocytes by Blocking DATP Accumulation. J Clin Invest. 2002;110:395–402. doi: 10.1172/JCI15683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal RP, Spector T, Parks RE., Jr Tight-Binding Inhibitors-IV. Inhibition of Adenosine Deaminases by Various Inhibitors. Biochem Pharmacol. 1977;26:359–367. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- 9.Ugarkar BG, DaRe JM, Kopcho JJ, Browne CE, III, Schanzer JM, Wiesner JB, Erion MD. Adenosine Kinase Inhibitors. 1. Synthesis, Enzyme Inhibition, and Antiseizure Activity of 5-Iodotubercidin Analogues. J Med Chem. 2000;43:2883–2893. doi: 10.1021/jm000024g. [DOI] [PubMed] [Google Scholar]
- 10.Arner ES, Eriksson S. Mammalian Deoxyribonucleoside Kinases. Pharmacol Ther. 1995;67:155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]