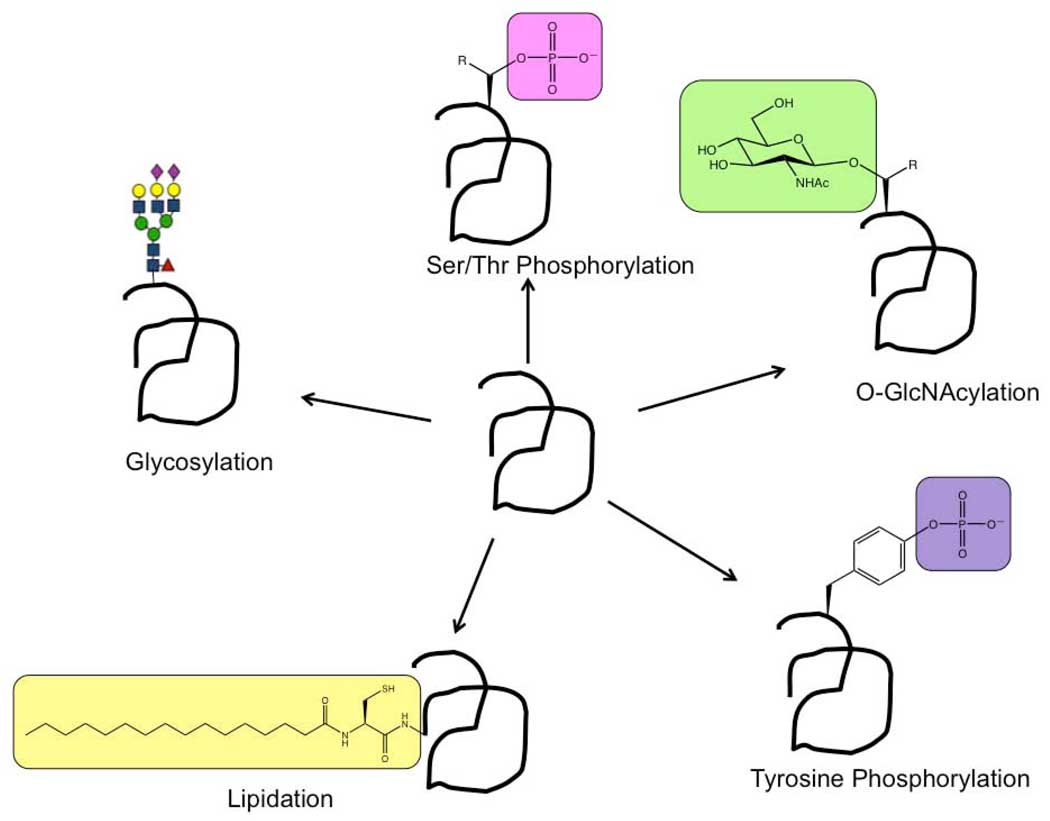
The development of proteomics has been one of the great achievements in analytical chemical biology in the past ten years. The determination of the total protein constituents in biological samples has allowed new and unprecedented insights into cellular processes and diseases. To make proteomics routine and ubiquitous, significant improvements in mass spectrometry, bioinformatics, and separation science were realized through unprecedented international collaborative and competitive efforts. The next stage of this evolution is even more difficult as we face the issue of posttranslational modifications (PTMs). Proteomics approaches have often neglected PTMs not purposely but out of necessity. PTMs introduce a whole new level of analytical challenge due to the complexity presented. For example, a protein that is glycosylated at three sites with ten different glycans at each sight can result in 1000 different glycoforms of that protein. Glycosylation is only one form among of scores of different types of PTMs (Figure 1). In this issue we focus on emerging methods for the studies and analyses of specific PTMs ranging from glycosylation to lipidation. The analytical challenge of PTMs has inspired a wide variety of approaches to their study; These include incorporating new chemistry, such as in the mechanistic probes presented by Dr. Amy Barrios for profiling tyrosine phosphatases and the metabolic labels discussed by Dr. Howard Hang for protein lipidation. Creating new methods for in vivo tracking of PTMs, such as the fluorescent biosensors presented by Dr. Jin Zhang. The utilization of new methodologies for understanding the functions and signaling properties of PTMs and their binding proteins, such as the protein microarrays discussed by Dr. Gavin MacBeath and the glycan microarrays presented by Dr. Jeff Gildersleeve. and The development of new methods for comprehensive analysis, presented in reviews on phosphorylation by Dr. Albert Hect, and glycosylation analysis at both the site-specific and global levels (reviews by Dr. Carlito Lebrilla on mass spectrometry techniques and Dr. Lara Mahal on lectin microarray techniques, respectively). Clearly, there will be no single method for the analysis of all PTMs, but several methods each specific for the type of PTM. The reviews presented here illustrate not only the challenges of PTM analysis but also the significant progress in these areas.
Figure 1.
Posttranslational modifications for which analytical techniques are highlighted within this issue.
Biographies
Lara K. Mahal is an Associate Professor of Chemistry at New York University. She is currently a Sloan Foundation Fellow and the recipient of both an NIH Director’s New Innovator Award and an NSF CAREER Award. Professor Mahal obtained her B. A. from the University of California at Santa Cruz, her Ph.D. at the University of California at Berkeley and was a Jane Coffins Child Postdoctoral Fellow at Sloan Kettering Research Center. She has recently moved from her previous academic position at the University of Texas at Austin to New York City.
Carlito B. Lebrilla is a professor at University of California, Davis in the Department of Chemistry and Biochemistry and Molecular Medicine at the School of Medicine. He is currently the Chair of the Chemistry Department. He received his BS degree from the University of California, Irvine and Ph.D. from the University of California, Berkeley. He was an Alexander von Humboldt Fellow and a NSF-NATO Fellow at the Technical University in Berlin. He returned to the UC Irvine as a President’s Fellow and has been at UC Davis since 1989. His research is in Analytical Chemistry, primarily mass spectrometry with applications to clinical glycomics and bioanalytical chemistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.