Abstract
Purpose
Lignans such as secoisolariciresinol diglucoside (SDG) in flaxseed, are metabolizes to bioactive mammalian lignans of END and ENL. Because mammalian lignans have chemical structural similarity to the natural estrogen, they are thought to behave like selective estrogen receptor modulators (SERM) and therefore have anticancer effect against hormone-related cancers. We isolated a series of lignan compounds, named as Vitexins, from the seed of Chinese herb Vitex Negundo.
Experimental Design
We purified several Vitexin lignan compounds. Cytotoxic and antitumor effects were analyzed in cancer cells and in tumor xenograft models. In vivo metabolism of Vitexins was determined in rat.
Results
Contrasts to the classical lignans, Vitexins were not metabolized to END and ENL. A mixture of Vitexins EVn-50 and purified Vitexin compound VB1 have cytotoxic effect on breast, prostate, and ovarian cancer cells and induces apoptosis with cleavage in PARP protein, up-regulation of Bax, and down-regulation of Bcl-2. This induction of apoptosis seems to be mediated by activation of caspases because inhibition of caspases activity significantly reduced induced apoptosis. We demonstrated a broad antitumor activity of EVn-50 on seven tumor xenograft models including breast, prostate, liver, and cervical cancers. Consistent with in vitro data, EVn-50 treatment induced apoptosis, down-regulated of Bcl-2, and up-regulated Bax in tumor xenografts.
Conclusion
Vitexin is a class of nature lignan compounds, whose action and anticancer effect is mediated by the mechanisms different from the classical lignans. Vitexin induced antitumor effect and cytotoxic activity is exerted through proapoptotic process, which is mediated by a decreased Bcl-2/Bax ratio and activation of caspases.
INTRODUCTION
Lignans are a group of complex polyphenolic antioxidants found in plants. Lignans such as secoisolariciresinol (SDG) found high in flaxseed, have antitumor effect. Clinical studies suggest that lignans are one of the most promising classes of dietary agents for testing in cancer prevention (1–3). In particular, lignan may prevent hormone-dependent diseases such as breast cancer and prostate cancer. Several lignans have been extensively investigated in pre-clinical tumor xenograft models, prospective and case-control epidemiological studies, and in some clinical trials (1, 2, 4–7). Many of the dietary plant lignans are converted by intestinal microbiota to mammalian metabolites of END and ENL (8–11). Mammalian lignans have been thought to be the major biologically active lignan, and suggested to be associated with low risk of cancer (4). Although there is no randomized clinical trial data exist indicating that lignan can reduce cancer growth, there are several biomarker-based neoadjuvant trials indicating that dietary intake of flaxseed lignan can inhibit tumor cell proliferation and induce apoptosis (2, 5). The ability to inhibit tumor growth kinetics in a neoadjuvant setting has previously been used as a measure of an agent's effectiveness in clinical circumstances (12–14). One neoadjuvant study, in a randomized double-blind placebo-controlled clinical trial, examined the effects of dietary flaxseed on tumor biological markers in postmenopausal patients with newly diagnosed breast cancer (5). In this study, patients were randomized to daily intake of either a 25 g flaxseed-containing muffin or a control (placebo) muffin. Dietary supplement of flaxseed lignans for 39 days resulted in a significant reduction of proliferation index (Ki67 labeling index) by 34% and increase in apoptotic index by 31%. Very interestingly, HER2 expression in breast cancer was significantly reduced by 71% in the lignan treated group.
Based on the current understanding on lignan metabolism, the primary benefit of plant lignan consumption is to increase blood levels of mammalian lignan END and ENL. The mechanism of anticancer action of mammalian lignans is not yet fully understood, but there is intriguing evidence for ENL as a modulator of steroid hormone metabolism and signaling. ENL functions via several mechanisms to reduce estrogen levels. First, ENL inhibits the aromatase enzyme (15–16). ENL and END inhibited the production of estrone and estradiol in MCF-7 breast cancer cells and reduced their proliferation (16). Second, ENL may also reduce estrogen levels by inhibiting its binding to its primary protein carrier sex hormone-binding globulin (17). This enables liver to more efficiently accelerate the natural metabolic clearance of estrogen. Third, among the three major estrogen hormones of estrone, estradiol, and estriol, estradiol (E2) is the most potent, and its levels are closely associated with high risk in breast cancer. ENL inhibits the enzyme 17-beta hydroxysteroid dehydrogenases, which are responsible for the conversion of estrone into estradiol (4). Other mechanisms relevant to breast cancer development are alteration of growth factor action (18–22), but there is little evidence that these effects occur in vivo in human subjects. END has been shown to inhibit angiogenesis in animal models of hormone-dependent cancers (23). Consistent with the hypothesis that mammalian lignans behave like SERMs and alternate estrogen metabolism and signaling, a recent large prospective study indicate that higher dietary lignan intakes is associated with a reduced risk of hormone-dependent breast cancer (7). In this study, women who had the highest dietary intakes of total plant lignans have a statistically significant lower risk of postmenopausal breast cancer compared with women who had the lowest intakes. However, these inverse associations are limited to ER- and PR-positive breast cancers, for which such inverse associations between breast cancer risk and the intakes of mammalian lignans are also observed.
We reported here an isolation of a unique class of lignan compounds named as Vitexins from the seed of Chinese herb Vitex Negundo. Contrast to the classical lignans, Vitexins did not go through metabolic activation to generate mammalian lignans. Although Vitex Negundo has been traditionally used as a folk medicine for cough, asthma, rheumatism, and arthritis and shown to have anti-inflammatory activities (24–26), we found that Vitexins activates caspases, induce apoptosis, and have broad anti-tumor activity in cancer xenograft models.
Materials and Methods
Extraction and isolation
Air-dried seeds of Vitex Negundo (10 kg) were extracted with 40% ethanol (2×10 L) and subjected to separation on polyamide chromatography column (30–60 mesh, 10×120 cm). After eluting with EtOH-H2O (0, 40%, 60% and 95%, 32 L each), the strongest cytotoxicity against MCF-7 was found to be present in the 40% ethanol. The 40% ethanol elution was partitioned between EtOAc and H2O. The EtOAc layer was concentrated to form a mixture of lignan extracts, EVn-50 (81 g). EVn-50 was first subjected to column chromatography over Sephadex LH-20 (5×70 cm) and eluted by increasing concentrations of MeOH (between 0 and 60%) in water to give 5 fractions. Fraction D was crystallized from MeOH to yield VB1.
EVn-50 and novel lignan compounds Vitexins
We isolated a mixture of lignan compounds from Vitex Negundo seed and named this lignan extract as EVn-50. We further purified a total of 15 lignan compounds, named as Vitexins, from the extract EVn-50 and identified two metabolites namely VB-M1 and VB-M2. Among these isolated Vitexins, there are 2 major lignans: VB1 (6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde); and VB2 (6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-5-methoxy-3,4-dihydro-2-naphthaldehyde), HPLC/mass spectrometry analyses showed that the extract EVn-50 contains mainly VB1 and VB2 and the total lignan compounds in the extract is approximately 70%. VB1 represents the most abundant lignan, accounting for 38% of total EVn-50; VB2 accounts for 17% of EVn-50 (Fig. 1A). In contrast to the classical lignans SDG or MAT (Fig. 1B&C), all these Vitexins isolated from EVn-50 have a different general structure and belong to the class of neolignan (Fig. 1D).
Fig. 1.
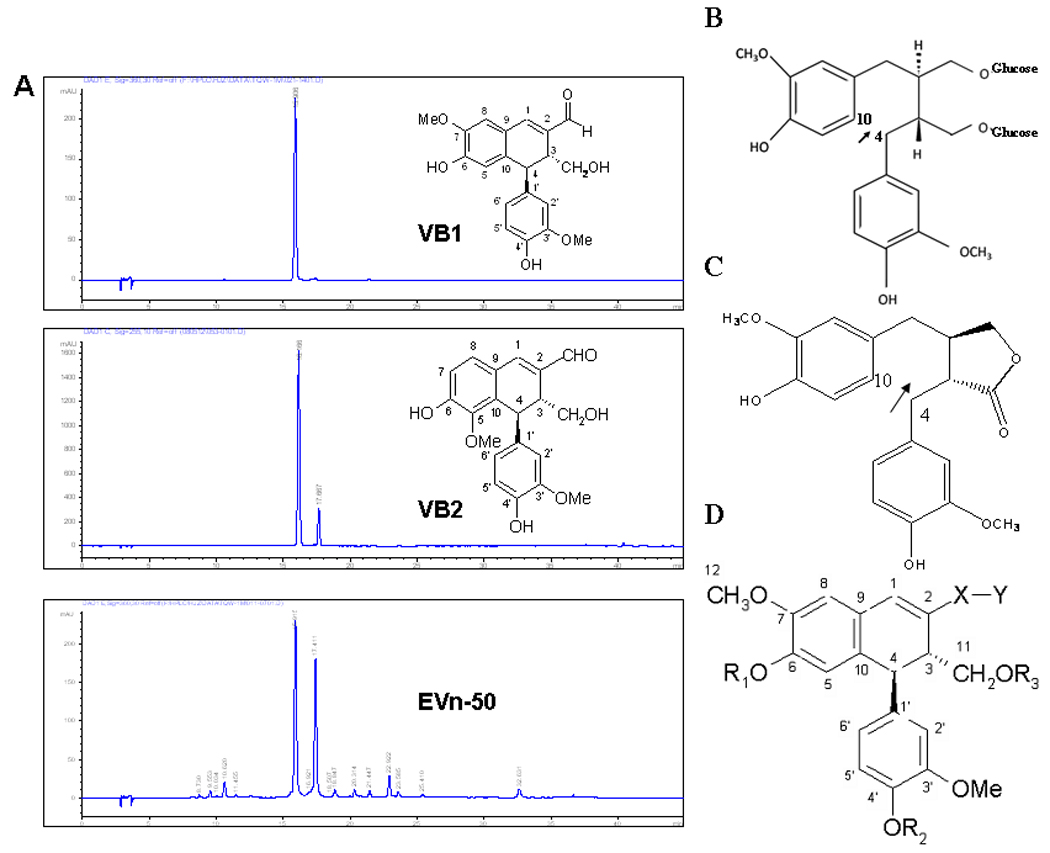
A. HPLC profiles of the Vitexin extract EVn-50 and the purified Vitexin lignan compounds. Inserts are chemical structures for purified Vitexin compound 1 (VB1) and Vitexin compound 2 (VB2). B. Lignan SDG from flaxseed. C. Lignan matairesionl (MAT). Both SDG and MAT have an open position 4 and 10 as arrows indicated. D. A general structure representing the isolated Vitexin lignan compounds from EVn-50.
Vitexin metabolism and identification of metabolites
Male Sprague-Dawley rats (n = 4) were given a single gavage of either EVn-50 (100 mg/kg) or SDG (20 mg/kg). All agents were dissolved in 5% Tween 80, 8% DMSO in PBS. Urine and feces within 0 to 48 h were collected through metabolic cages. Urine and fecal samples were extracted with methanol and filtered. The filtrate was concentrated under reduced pressure and subjected to macroporous resin AB-8 with H2O/ EtOH in gradient elution. The 10% EtOH and 20% EtOH elution were subjected to Sephadex LH-20 and HPLC on ODS column (C-18, 250×4.6 mm; Hypersil). Infrared spectra were determined on AVATAR 360 FT-IR spectrometer (Nicolet) in KBr pellets. UV spectra were measured on a Shimadzu UV-2450 spectrometer (Shimadzu). HRSI-MS was recorded with Finnigan LCQ-Advantage (Thermo, America). NMR spectra were measured on Varian-INOVA-400FT spectrometer (Varian, America) with tetramethylsilane as an internal standard.
Determination of apoptotic cells
Apoptotic cells were determined by propidium iodide stating and flow cytometry. Following treatments, cells were fixed and stained with 50 µg/ml propidium iodide for 20 min. The propidium iodide fluorescence was measured with flow cytometer. Quantification of apoptotic cells was determined by measurement of sub-G1 DNA content.
Cytotoxicity assay
Cells were cultured in 5% FCS and incubated with EVn-50 or purified Vitexin compounds for 72 h. To quantitate cytotoxic activities, metabolically active cells were determined by quantitative colorimetric method of MTT assay.
Tumor growth in athymic nude mice
A nude mouse tumorigenic assay was performed as we previously described (27–28). We used female athymic nude mice at the age of 6–7 weeks for MA782 murine breast cancer cells, MCF-7, T47D, MDA-MB-435s human breast cancer cell, and Hela human cervical cancer cells. For PC-3 prostate and HepG2 liver cancer cells, male mice at the same age were used. For hormone dependent T47D and MCF-7 tumor xenografts, 17β-estradiol pellets (0.72 mg/pellet, 60-day releasing, Innovative Research of America) were implanted subcutaneously one day before the injection of tumor cells. For all breast cancer xenografts, approximately 3 ×106 cells were injected. Each animal received two injections, one on each side, in the mammary fat pads between the first and second nipples. For other human tumor xenografts (PC-3, HepG2, Hela), approximately 2 × 106 cells were subcutaneously injected into the right and left flanks. When tumor xenografts were established, mice bearing tumors were randomly allocated to different treatment groups. Each group has 6 mice. Drug treatments were either given orally by gavage or administrated by i.p injection, 3 days/week. Tumor size was determined by three-dimensional measurements (mm) using a caliper.
Detection of tumor apoptotic cells by TUNEL assay
As previously described (28), tumor samples were harvested, fixed, and embedded in paraffin for immunohistochemical analysis. The two-step TdT in situ DAB apoptotic TUNEL assay (R&D System) was performed according to the manufacturers’ instructions. The apoptotic index was determined by the percentage of cells scored under a light microscope at 200-fold magnification. Three independent observers counted the positive cells and each observer randomly counted 3 fields. The numbers represent the average percentage of positive cells from 9 fields.
Statistical analysis
Results were reported as the mean ± SD for typical experiments done in three replicate samples and compared by the Student’s t test. Results were considered significantly different for p < 0.05. All experiments were done at least twice to ensure reproducibility of the results.
RESULTS
Different metabolism pathway
The biological efficacy of classical lignans is primarily mediated by their metabolized bioactive mammalian lignans END and ENL, which function like phytoestrogens. Since Vitexins have different general structure (Fig. 1) and belong to the new class of neolignan, we were interested in study whether these Vitexin lignans, like the classical plant lignans, are also metabolized to END and ENL. We fed rat with either SDG or lignan extract EVn-50 by gavage, collected urine and feces samples, and performed HPLC/Mass spectrometry analyses on the samples. As expected, two mammalian lignans END and ENL were found in the urine samples from SDG-treated rats (Fig. 2A). However, no END and ENL were found in the urine and feces samples from EVn50-treated rats; in contrast, among many metabolites, two major gluconate metabolites namely VB-Ml and VB-M2 were identified from EVn-50-treated rats (Fig. 2B). These data indicate that the new class of lignan compounds Vitexins undergoes a different metabolism and doesn’t generate mammalian lignans.
Fig. 2.
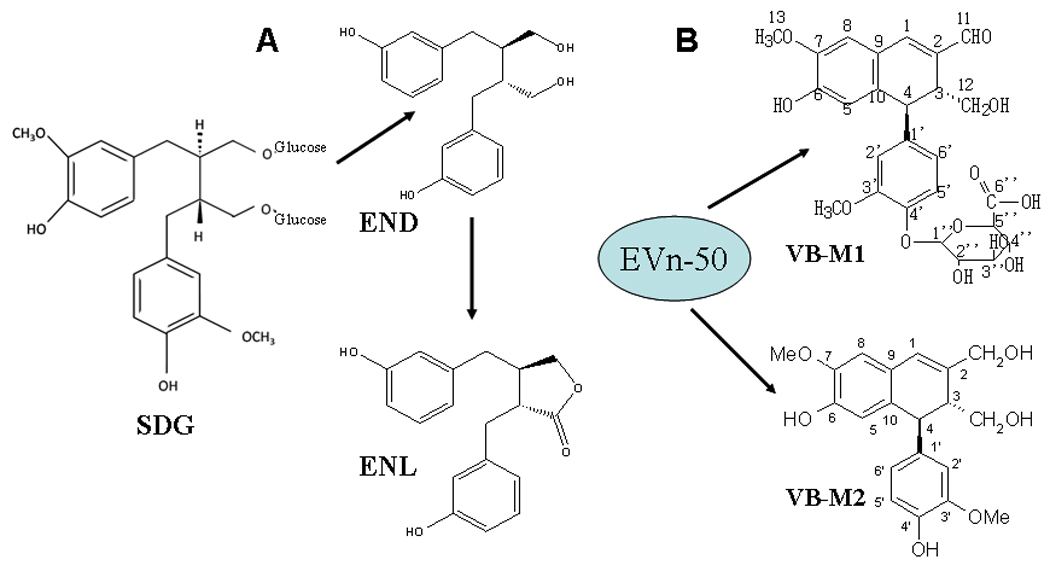
In vivo metabolism of Vitexins. Male Sprague-Dawley rats were given a single gavage of EVn-50 (100 mg/kg) or SDG (20 mg/kg) and the lignan metabolites were analyzed in urine and feces samples as described in Materials and Methods.
EVn-50 has cytotoxic effect and induces apoptosis
We first determined the cytotoxic effect of EVn-50 on a variety of different tumor cells. EVn-50 has cytotoxic effects on MCF-7, ZR-75-1, SK-BR-7, MDA-MB-231, and MDA-MB-435s breast cancer cells, PC-3 and LNCaP prostate cancer cells, and COC1 ovarian cancer cells (Table 1). COC1 is most sensitive cell line to EVn-50-mediated cytotoxicity; and SK-BR-7 is the least sensitive cell line. Since the total lignan compounds in the extract EVn-50 is 70%, it is important to exclude the possibility that the EVn-50-possessed cytotoxicity is not mediated by Vitexin lignans but via other unidentified components. To this end, purified Vitexin compounds have to be investigated. To start a biological study in individual purified Vitexin lignan compound, we picked up the Vitexin compound VB1, which is the most abundant Vitexin lignan and accounts for 38% of total EVn-50. We tested the cytotoxic effect of VB1 on MCF-7, ZR-75-1, MDA-MB-231, and COC1 cells. VB1 possessed strong cytotoxic effects with IC50 ranging from 0.39 to 3.2 µmol/L. These data suggest that Vitexin lignan per se are biologically active and that EVn-50-induced cytotoxicity may be mediated by Vitexin lignans, particularly VB1. Fig. 3A is representative cell morphology from VB1-treated MDA-MB-231 breast cancer cells. VB1 had a dose-dependent cytotoxic effect. At the dose range of 4–6 µg/ml, over 60% cells died. We also examined the effects of VB1 on induction of apoptosis. Treatment of MDA-MB-231 cells with VB1 induced a significant increase in apoptosis resulting in up to 43% of apoptotic cells (Fig. 3B).
Table 1.
Cytotoxicity of Vitexins. All cell lines, cultured with 10% FBS, were treated with EVn-50 (ranging from 0.05 to 20 µg/ml) or VB1 (50 nM to 30 µM) for 72 h. Cytotoxicy was measured by MTT assay. The IC50 values were determined by regression analysis. Data presented here are mean ± SEM of duplicate cultures from two separate experiments.
| Cell line | Cell type | Cytotoxicity IC50 | |
|---|---|---|---|
| EVn-50 (µg/ml) | VB-1 (µmol/L) | ||
| MCF-7 | Breast | 2.3 ± 0.19 | 3.2 ± 0.27 |
| ZR-75-1 | Breast | 0.95 ± 0.01 | 2.1 ± 0.15 |
| SK-BR-7 | Breast | 4.8 ± 0.24 | – |
| MDA-MB-435s | Breast | 2.1 ± 0.15 | – |
| MDA-MB-231 | Breast | 1.5 ± 0.11 | 1.8 ± 0.12 |
| LNCaP | Prostate | 1.3 ± 0.09 | – |
| PC-3 | Prostate | 0.8 ± 0.06 | – |
| COC1 | Ovarian | 0.25 ± .019 | 0.39 ± .023 |
Fig. 3.
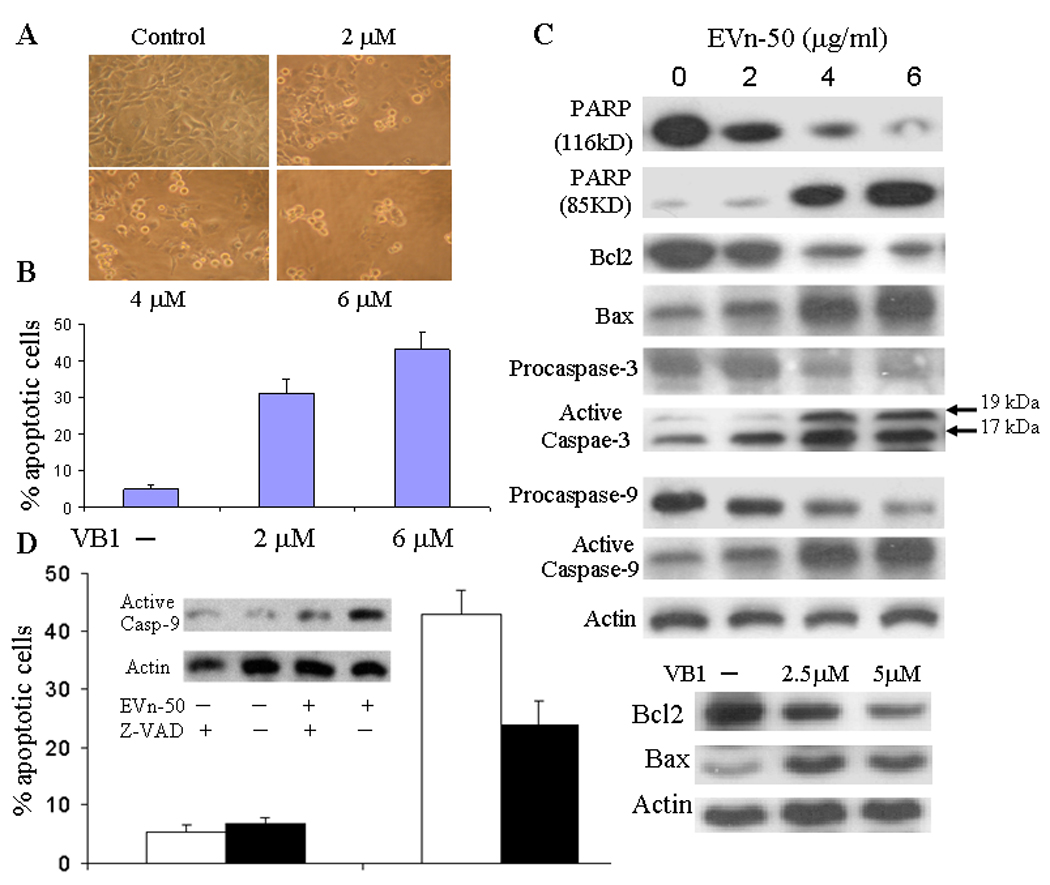
Induction of apoptosis and regulation of apoptotic biomarkers. A. Morphology of VB1 treated MDA-MB-231 cells. Cells were treated with VB1 at three doses as indicated for 48 hours. B. Analysis of apoptotic cells treated with or without VB1. MDA-MB-231 cells were treated VB1 for 48 hours and apoptotic cells were determined. The numbers represent means ± SD of three cultures. Statistical comparisons of treated vs. control indicate p < 0.001. C. Effects on apoptotic biomarkers. MDA-MB-231 cells were treated with EVn-50 or VB1 at different doses as indicated for 48 h. Equal amount of total cellular protein was subjected to Western analyses of PARP, cleaved PARP, Bcl-2, Bax, procaspase-3, active caspases-3, procaspase-9, and active caspases-9. D. Reversing EVn-50-indueced apoptosis by inhibiting caspases. MDA-MB-231 cells were pretreated with 12 µM of general caspases inhibitor Z-VAD-FMK for 2 h, followed by additional 48 h treatment with EVn-50 (5 µg/ml). Percentage of apoptotic cells were then determined. The numbers represent means ± SD of three cultures. Closed bars represent inhibitor treated cells and open bars represent non-treated cells. Inset is a Western blot for activated caspases-9.
During induction of apoptosis, caspase activates poly (ADP-ribose) polymerase (PARP) and leads to its cleavage into a C-terminal fragment, which is considered as an important biomarker of apoptosis. Western analysis showed a robotic dose-dependent increase in cleaved PARP in cells treated with EVn-50. As shown in Fig. 3C, we found that the full-size PARP protein (116 kDa) was cleaved to yield an 85 kDa fragment after treatment of cells with EVn-50 at the doses of 4 and 6 µg/ml for 48 h. Because Bax and Bcl-2 play crucial roles in apoptosis, we next studied the dose-dependent effects of EVn-50 on the protein levels of Bax and Bcl-2 in the treated cells. Treatment of the cells with EVn-50 induced a significant decrease in Bcl-2 expression with a concomitant increase in the protein level of Bax (Fig. 3C). This resulted in a substantial increase in Bax/Bcl-2 ratio, which favors apoptosis. Purified Vitexin VB1 had a similar effect on induction of Bax and reduction of Bcl-2 (Fig. 3C).
EVn-50 induces apoptosis via activation of caspases
To test whether caspases are involved in EVn50-induced apoptosis, we first evaluated the protein levels of caspase-3 and caspase-9 in EVn50-treated cells. In most cancer cells, caspases are present in the pro-forms and require site-specific cleavage of the protein to become active in the apoptotic process. As shown in Fig. 3C, EVn-50 treatment of MDA-MB-231 cells induced a significant and progressive increase in the levels of active caspase-3 and caspase-9 protein with a concomitant decrease in the pro-forms of caspase-3 and caspase-9. To study whether EVn-50-induced apoptosis is mediated by activation of caspases, we used a general caspases inhibitor Z-VAD-FMK and demonstrated that blocking caspases activity can partially inhibit EVn-50-induced apoptosis. As shown in Fig. 3D, while treatment of the cells with EVn-50 (5 µg/ml) resulted in a 43% apoptotic cells, blocking caspases activity significantly reduced EVn-50-mediated cytotoxic effect resulting in a 24% apoptotic cells. Western blot showed that EVn-50-induced activation of caspases-9 was significantly reduced after caspases inhibitor treatment (insert in Fig. 3D). These results suggest that activation of caspases may be one of the underlying mechanisms by which EVn-50 induces apoptosis.
EVn-50 suppresses tumor growth of mammary xenografts
To investigate whether EVn-50 inhibits tumor growth, we first studied the tumor suppressing effect of EVn-50 on MA782 murine breast cancer cells using an orthotopic nude mouse model. Twelve days after implantation of MA782 cells, mice bearing MA782 tumor xenografts were treated with different doses of EVn-50 (day 0). For positive controls, we also treated the mice with either Adriamycin (ADM) or Tamoxifen (Tam). As illustrated in Fig. 4A, treatment of mice with ADM and Tam inhibited tumor growth by 95% and 91%, respectively. EVn-50 had a dose-dependent tumor growth inhibition with the maximum tumor inhibition reached to 92%, which is compatible to the levels of growth inhibition achieved by ADM. Oral administration of mice with EVn-50 caused 56%, 86%, and 92% tumor inhibition at 20, 40, and 80 mg/kg, respectively. Similar tumor suppressions were also observed when the inhibitions were calculated based on the tumor weights. Fig. 4B shows the real tumor size when mice were sacrificed at day 20 following the treatment.
Fig. 4.
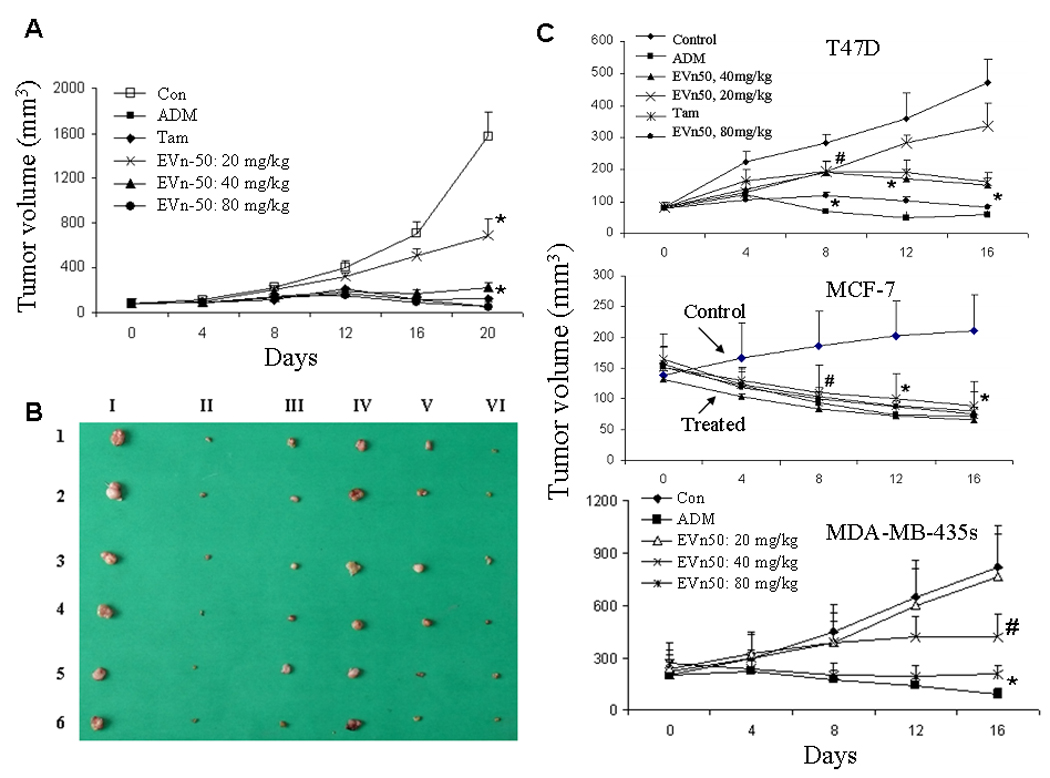
Suppression of growth of mammary tumor xenografts by EVn-50. A. Inhibition of growth of MA782 marine mammary tumor. After 12 days of cell injection, when tumors reached to ≥ 75 mm3, mice bearing similar size tumors were divided into 6 groups. Each group has 6 mice. Tam (20 mg/kg) and Evn-50 were administrated by gavage and ADM (1 mg/kg) was administrated by i.p. injection. All treatment groups received drugs every other day until the termination of the experiment. All mice were sacrificed at day 20 after the first treatment. Each point represents the mean of tumors ± SE. Statistical comparisons for tumor size in treated mice relative to control mice indicate: * p < 0.001. B. MA782 tumor xenograft sizes. I. Control. II. ADM. III. Tam. IV. EVn-50 low dose (20 mg/kg). V. EVn-50, medium dose (40 mg/kg). VI. EVn-50 high dose (80 mg/kg). C. Antitumor effect of EVn-50 on human breast cancer xenografts. Mice bearing established T47D, MCF-7, or MDA-MB-435s tumor xenografts were treated EVn-50 (20, 40, and 80 mg/kg). For positive controls, all three tumor xenografts were treated with chemotherapy drug ADM (1 mg/kg). For hormone-dependent T47D and MCF-7 xenografts, we also used tamoxifen (Tam, 20 mg/kg). The treatments were same as described for MA782 tumor xenograft. All mice were sacrificed at day 16 after the first treatment. Each point represents the mean of tumors ± SE. Statistical comparisons for tumor size in treated mice relative to control mice indicate: # P <0.05, *P <0.01.
We next extend the study on EVn-50-mediated antitumor effect to three human breast cancer xenografts: T47D, MCF-7, and MBA-MB-435s (Fig. 4C). Mice bearing established tumors were treated with ADM as a positive control and with different doses of EVn-50. For hormone-dependent MCF-7 and T47D xenografts, mice were also treated with Tam. As expected, treatment of mice with ADM inhibited tumor growth by 85%, 73%, and 87% in T47D, MCF-7, and MDA-MB-435s xenografts, respectively. Tam treatment also significantly suppressed T47D and MCF-7 tumor growth resulting in a 61%–68% growth inhibition. EVn-50 had a dose-dependent tumor growth inhibition with the maximum tumor inhibition reached to 75% to 82% (by volume) at the end of dosing period of 80 mg/kg. Similar tumor suppressions were also observed when the inhibitions were calculated based on the tumor weights. Oral administration of EVn-50 was well-tolerated in mice with no overt clinical sign of toxicity.
EVn-50 suppresses growth of liver, prostate, and cervical tumor xenografts
Since the biological efficacy of classical flaxseed lignan SDG is primarily mediated by its metabolized mammalian metabolites END and ENL, which occurs at the gut and converted by colon microflora (4), the antitumor effect of SDG is usually observed when it is administrated orally. The antitumor effect of EVn-50 on breast cancer xenografts was demonstrated when the drug administrated orally. We were interested in studying whether we can achieve a similar antitumor effect when EVn-50 is administrated non-orally. Here we demonstrated an antitumor effect EVn-50 on PC-3 prostate, HepG2 liver, and Hela cervical tumor xenograft models when administrated non-orally but by i.p injection (Fig. 5). For positive controls, we used chemotherapy drugs ADM, 5-fluorouracil (5-FU), and Cisplatin (DDP) for PC-3, HepG2, and Hela xenografts, respectively. As expected, all three chemotherapy drugs induced significant tumor suppression. Repeated i.p. administration of EVn-50 (once every other day for 16 days) also produced significant antitumor activity in mice bearing established PC-3, HepG2, and Hela xenografts. Maximal inhibition (by volume) of 62% and 71% was observed at the end of highest dosing period of 20 mg/kg for Hela and HepG2 (Fig. 5D) xenografts, respectively. For PC-3 prostate tumor xenograft, a maximal tumor growth inhibition of 65% was observed at the end of highest dosing period of 30 mg/kg (Fig, 5A). Similar tumor suppressions were also observed when the inhibitions were calculated based on the tumor weights: 67% for Hela, 56% for PC-3, and 69% for HepG2.
Fig. 5.
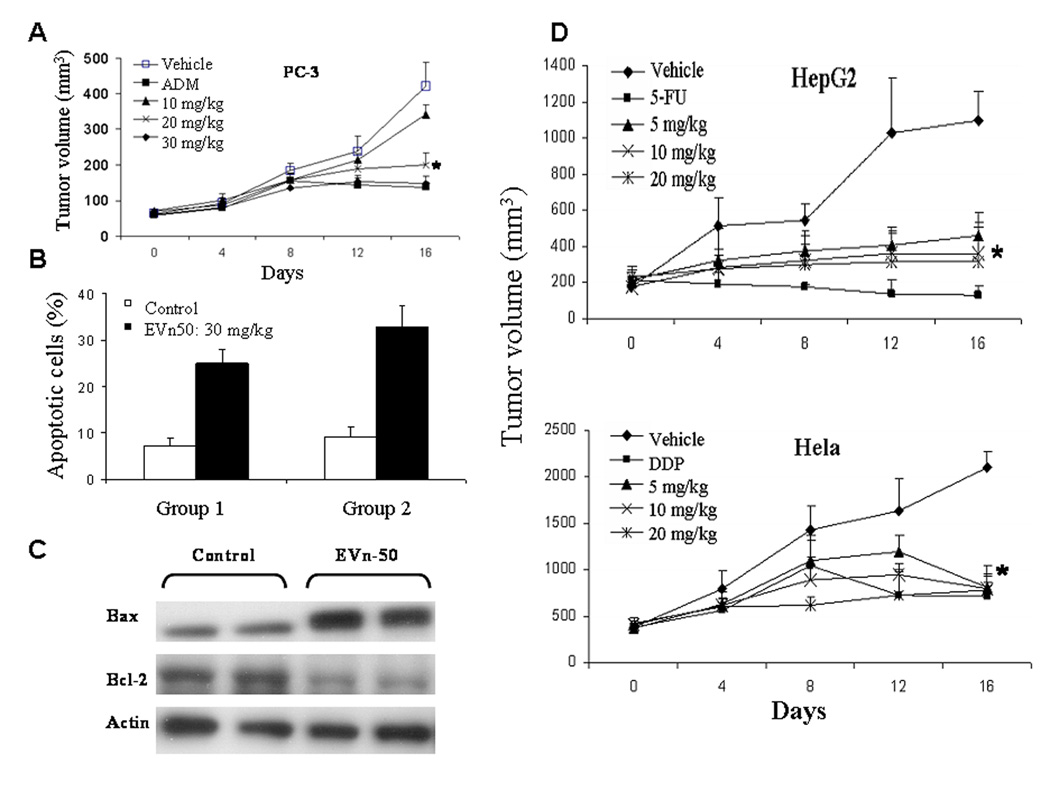
Antitumor effect of EVn-50 on human prostate, cervical, and liver cancer xenografts. Mice bearing established PC-3 (A), HepG2 (D), or Hela (D) human tumor xenografts were treated with vehicle or EVn-50 (i.p. injection). For positive controls, we used chemotherapy drugs ADM (1 mg/kg) for PC-3, Cisplatin (DDP, 4 mg/kg) for Hela, and 5-fluorouracil (5-FU, 20 mg/kg) for HepG2 cells. Treatment groups received drugs every other day until the termination of the experiment. All mice were sacrificed at day 16 after the first treatment. Each point represents the mean of tumors ± SE. Statistical comparisons for tumor size in treated mice relative to control mice indicate: *P <0.01. B. Histological sections of PC-3 tumors from two control and two treated mice were analyzed for apoptosis (TUNEL assay). The apoptotic index of the tumor cells increased 3.3–3.6 fold (p<0.005) in the EVn-50 treated mice. C. Regulation of Bax and Bcl-2 protein expression in PC-3 tumor xenografts. Tumors from two control and two treated mice were harvested at day 16, 12 hours following the last EVn-50 treatment. Total protein was subjected to Western analyses of Bax and Bcl-2 and normalized with actin.
We also analyzed apoptotic index in EVn-50-treated PC-3 tumor xenografts. Immunohistochemical analysis of apoptotic cells using TUNEL reaction showed a significant increase in tumor cell apoptosis in EVn-5-treated PC-3 xenograft, resulting in an average of 3.6-fold increase in apoptotic cells over control xenografts (Fig. 5B). Because EVn-50 treatment was observed to modulate the expression levels of Bax and Bcl-2 in vitro, we determined the effect of EVn-50 on the expression levels of Bax and Bcl-2 in tumors from control and treated xenografts. Consistent with the in vitro data on decreased Bcl-2/Bax ratio and in vivo data on increased apoptosis in tumor xenograft, EVn-50 treatment also significantly increased Bax protein expression and decreased Bcl-2 expression in PC-3 tumor xenograft (Fig. 5C).
Discussion
Studies suggest that lignans may be helpful for cancer prevention and treatment, particularly on the malignancy of breast (1, 7). Vitex Negundo is a native to China and its seed is used as anti-asthmatics and a cough remedy. Moreover, Vitex Negundo seed is also used, in some regions of China, for the treatment of gynecological cancers. However, few antineoplastic constituents from Vitex Negundo were ever reported up to now. In the present study, we have isolated a series of novel lignan compounds Vitexins from the seed of Vitex Negundo. We showed that a mixture of Vitexin lignans EVn-50 and the purified Vitexin compound VB1 possessed a broad cytotoxic effect against many different cancer cells. Our data demonstrated that Vitexin treatment of breast cancer cells resulted in 1) cytotoxic effect; 2) apoptosis in a dose-dependent fashion with cleavage in PARP protein, up-regulation of Bax, and down-regulation of Bcl-2; and 3) induction of apoptosis by activation of caspases. These are important observations because regulations of Bax/Bcl-2 and caspases activity become appreciated targets for cancer intervention. Our data suggest that Vitexins-induced cytotoxic effect is through the induction of apoptosis, which is mediated by activation of caspases.
To establish the relevance of these in vitro findings to in vivo situation, we studied the antitumor effect of EVn-50 on 8 tumor xenograft models, which include 4 breast cancer (MA782, MCF-7, T47D, MDA-MB-435s) xenografts, PC-3 prostate, HepG2 liver, and Hela cervical tumor xenografts. Notably, we observed a significant and dose-dependent broad antitumor effect on all studied tumor xenograft models. For many of the studied tumor xenografts, the maximum tumor inhibition reached to 62–92%, which is compatible to the levels of growth inhibition achieved by chemotherapy drugs such as adriamycin, 5-fluorouracil, and Cisplatin. We provided direct experimental evidence that EVn-50 has potent and broad antitumor efficacy in preclinical models of ectopic growth of breast, prostate, liver, and cervical cancer cells in nude mice (Fig 4–Fig 5). Using PC3 tumor xenograft, we demonstrated that in vivo tumor-suppressing effect of EVn-50 could be correlated well with the induction of apoptosis. A significant increase in apoptotic cells and an increase in Bax protein expression with a concomitant decrease in Bcl-2 protein expression in EVn-50-treated PC-3 tumor xenograft suggest the involvement of similar molecular events as those observed in the in vitro studies.
High dietary intakes of plant lignans and high exposure to mammalian lignans are associated with reduced risks of ER- and PR-positive breast cancer, suggesting an antitumor effect of lignans on hormone-dependent cancers (7). Although acting as phytoestrogens, mammalian lignans alternates steroid metabolism and signaling, the exact mechanisms for antitumor effect are poorly understood. The antitumor effect of flaxseed lignan often dependents on the actual amount of END and ENL, which can be affected by many factors such as the activity of intestinal bacteria and the use of antibiotics. Although Vitexins are structurally quite like classical lignan, e.g., SDG (Fig. 1), the mechanism of action of Vitexin is different from classical lignans. Very importantly, compared with classical plant lignans, e.g., SDG and MAT, these new lignan Vitexin compounds have several unique characteristics. First, as illustrated in Fig. 1, the new lignan compounds Vitexins have a unique structure, in which the position 4 and 10 are connected. Lignans belong to this general structure (Fig. 1D) are classified as neolignans. In contrast, the classical lignans have an open position at 4 and 10 (Fig. 1B&C). Second, while classical lignans are precursor of active mammalian lignans, which are converted by the colonic bacteria to the two mammalian lignans of END and ENL, isolated Vitexins are converted to different metabolites but not END and ENL. Furthermore, we demonstrated that EVn-50-mediated antitumor effects on tumor xenografts can be achieved both by oral gavage and by i.p. injection, suggesting that, in contrast to classical lignans, the efficacy of Vitexins doesn’t require in vivo metabolic activation by colonic bacteria. Third, although classical lignans act as phytoestrogens, the exact mechanisms are poorly understood. The new lignan compounds Vitexins induce apoptosis by activation of caspases-3 and -9, up-regulation of Bax, and down-regulation of Bcl-2.
Members of the Bcl-2 family proteins are critical regulators of the apoptotic pathway (29). Bcl-2 is an upstream effector molecule in the apoptotic pathway and has been identified as a potent suppressor of apoptosis (30). Bcl-2 is found at inappropriately high levels in more than half of all human tumors (29) thereby rendering tumors escaping apoptosis and undermining therapy. Bcl-2 forms a heterodimer with the apoptotic protein Bax and thereby neutralizes its apoptotic effects. Alternation of Bax/Bcl-2 ratio in favor Bax is a decisive factor to determine whether cells will undergo apoptosis (31). In our study, EVn-50 treatment of MDA-MB-231 cells induced a decrease in Bcl-2 protein expression with an increase in Bax protein expression. Such Bax/Bcl-2 ratio change in favor for apoptosis was also observed EVn-50-treated tumor xenografts (Fig. 5C). Therefore, Vitexins-possessed broad antitumor effect and cytotoxicity might be mediated by alternation of Bax/Bcl-2 ratio in favor Bax and by activation of caspases.
We are well aware that EVn-50 contains 70% of Vitexin lignan compounds, and thus may be multifunctional and affect multiple signaling pathways relevant for cancer growth and progression. Nevertheless, we demonstrated that purified Vitexin compound VB1, the most abundant Vitexin compound in EVn-50, has potent cytotoxic effect, regulates Bax/Bcl-2, and induces apoptosis. In general, an extract from a plant contains multiple known and unknown ingredients and it is not uncommon that the plant extract may present same or even better therapeutic efficacy and often less toxic effect than pure compound isolated from the extract. We have demonstrated that EVn-50 has broad anti-tumor activity on breast and many other different tumor xenograft models. Developments of EVn-50 or individual Vitexin compound for potential cancer intervention agents warrant further investigation.
ACKNOWLEDGMENTS
This study was supported in part by grants W81XWH-04-1-0569 and W81XWH-07-1-0375 from the United States Army Medical Research and Development Command, and a grant 2008SK2005 from the Key Projects in 2008 Science & Technology Pillar Program of Hunan Province.
Abbreviations
- END
enterodiol
- ENL
enterolactone
- EVn-50
a proprietary lignan extract from the seed of Chinese herb Vitex Negundo
- MAT
matairesionl
- SERM
selective estrogen receptor modulator
- SDG
secoisolariciresinol
- Tam
tamoxifen
- Vitexin
lignan compound isolated from Vitex Negundo
- VB1
purified Vitexin compound 1
Footnotes
Translational Relevance
The biological efficacy of classical lignans, e.g., SDG in flaxseed, is primarily mediated by their metabolized bioactive mammalian lignans END and ENL. The antitumor effect of flaxseed lignan often dependents on the actual amount of END and ENL, which can be affected by the activities of intestinal bacteria and the use of antibiotics.
We isolated a series of new lignan compounds Vitexins, which belong to a new class of neolignan. In contrast to classical lignans, Vitexins are not metabolized to END and ENL, and thus, the antitumor efficacy may not require intestinal activation process. Although acting as phytoestrogens, classical lignans alternates steroid biosynthesis and metabolism, the exact mechanisms are poorly understood. Vitexins induce apoptosis by activation of caspases-3 and -9, up-regulation of Bax, and down-regulation of Bcl-2, and have a potent and broad antitumor effect. Vitexins may prove to be a better lignan compounds for cancer intervention.
References
- 1.Thompson LU, Chen JM, Li T, Strasser-Weippl K, Goss PE. Dietary flaxseed alters tumor biological markers in postmenopausal breast cancer. Clin Cancer Res. 2005;11:3828–3835. doi: 10.1158/1078-0432.CCR-04-2326. [DOI] [PubMed] [Google Scholar]
- 2.Demark-Wahnefried W, Price DT, Polascik TJ, et al. Pilot study of dietary fat restriction and flaxseed supplementation in men with prostate cancer before surgery: exploring the effects on hormonal levels, prostate-specific antigen, and histopathologic features. Urology. 2001;58:47–52. doi: 10.1016/s0090-4295(01)01014-7. [DOI] [PubMed] [Google Scholar]
- 3.Kuijsten A, Arts ICW, Hollman PCH, Veer P, Kampman E. Plasma enterolignans are associated with lower colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2006;15:1132–1136. doi: 10.1158/1055-9965.EPI-05-0991. [DOI] [PubMed] [Google Scholar]
- 4.Adlercreutz H. Lignans and human health. Crit Rev Clin Lab Sci. 2007;44:483–525. doi: 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- 5.Bergman Jungeström M, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13:1061–1067. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 6.Chen LH, Fang J, Li H, Demark-Wahnefried W, Lin X. Enterolactone induces apoptosis in human prostate carcinoma LNCaP cells via a mitochondrial-mediated, caspase-dependent pathway. Mol Cancer Ther. 2007;6:2581–2590. doi: 10.1158/1535-7163.MCT-07-0220. [DOI] [PubMed] [Google Scholar]
- 7.Touillaud MS, Thiébaut AC, Fournier A, Niravong M, Boutron-Ruault MC, Clavel-Chapelon F. Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J Natl Cancer Inst. 2007;99:475–486. doi: 10.1093/jnci/djk096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setchell KDR, Lawson AM, Borriello SP, et al. Lignan formation in man-microbial involvement and possible roles in relation to cancer. Lancet. 1981;2:4–7. doi: 10.1016/s0140-6736(81)90250-6. [DOI] [PubMed] [Google Scholar]
- 9.Borriello SP, Setchell KDR, Axelson M, Lawson AM. Production and metabolism of lignans by the human faecal flora. J Appl Bacteriol. 1985;58:37–43. doi: 10.1111/j.1365-2672.1985.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 10.Axelson M, Setchell KD. The excretion of lignans in rats - evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett. 1981;123:337–342. doi: 10.1016/0014-5793(81)80322-5. [DOI] [PubMed] [Google Scholar]
- 11.Heinonen S, Nurmi T, Liukkonen K, et al. In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem. 2001;49:3178–3186. doi: 10.1021/jf010038a. [DOI] [PubMed] [Google Scholar]
- 12.Miller WR, Dixon JM, Macfarlane L, Cameron D, Anderson TJ. Pathological features of breast cancer response following neoadjuvant treatment with either letrozole or tamoxifen. Eur J Cancer. 2003;39:462–468. doi: 10.1016/s0959-8049(02)00600-7. [DOI] [PubMed] [Google Scholar]
- 13.Miller WR, Dixon JM, Cameron DA, Anderson TJ. Biological and clinical effects of aromatase inhibitors in neoadjuvant therapy. J Steroid Biochem Mol Biol. 2001;79:103–107. doi: 10.1016/s0960-0760(01)00149-2. [DOI] [PubMed] [Google Scholar]
- 14.Ellis MJ, Coop A, Singh B, et al. Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res. 2003;53:6523–6531. [PubMed] [Google Scholar]
- 15.Adlercreutz H, Bannwart C, Wähälä K, et al. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol. 1993;44:147–153. doi: 10.1016/0960-0760(93)90022-o. [DOI] [PubMed] [Google Scholar]
- 16.Brooks JD, Thompson LU. Mammalian lignans and genistein decrease the activities of aromatase and 17 beta-hydroxy steroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol. 2005;94:461–467. doi: 10.1016/j.jsbmb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Hutchins AM, Martini MC, Olson BA, Thomas W, Slavin JL. Flaxseed consumption influences endogenous hormone concentrations in postmenopausal women. Nutr Cancer. 2001;39:58–65. doi: 10.1207/S15327914nc391_8. [DOI] [PubMed] [Google Scholar]
- 18.Adlercreutz H. Phyto-oestrogens and cancer. Lancet Oncol. 2002;3:364–373. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- 19.Boccardo F, Lunardi GL, Petti AR, Rubagotti A. Enterolactone in breast cyst fluid: correlation with EGF and breast cancer risk. Breast Cancer Res Treat. 2003;79:17–23. doi: 10.1023/a:1023356209478. [DOI] [PubMed] [Google Scholar]
- 20.Rickard SE, Yuan YV, Thompson LU. Plasma insulin-like growth factor I levels in rats are reduced by dietary supplementation of flaxseed or its lignan secoisolariciresinol diglycoside. Cancer Lett. 2000;161:47–55. doi: 10.1016/s0304-3835(00)00592-9. [DOI] [PubMed] [Google Scholar]
- 21.Vrieling A, Voskuil DW, de Mesquita HB, et al. Dietary determinants of circulating insulin-like growth factor (IGF)-I and IGF binding proteins 1, -2 and -3 in women in the Netherlands. Cancer Causes Control. 2004;15:787–796. doi: 10.1023/B:CACO.0000043429.51915.c6. [DOI] [PubMed] [Google Scholar]
- 22.Prasad K. Antioxidant activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int J Angiol. 2000;9:220–225. doi: 10.1007/BF01623898. [DOI] [PubMed] [Google Scholar]
- 23.Bergman JM, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13:1061–1067. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 24.Dharmasiri MG, Jayakody JR, Galhena G, Liyanage SS, Ratnasooriya WD. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethnopharmacol. 2003;87:199–206. doi: 10.1016/s0378-8741(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 25.Alam MI, Gomes A. Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J Ethnopharmacol. 2003;86:75–80. doi: 10.1016/s0378-8741(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 26.Tandon VR, Gupta RK. Vitex negundo Linn (VN) leaf extract as an adjuvant therapy to standard anti-inflammatory drugs. Indian J Med Res. 2006;124:447–450. [PubMed] [Google Scholar]
- 27.Jiang Y, Liu YE, Goldberg ID, Shi YE. γ Synuclein, a novel heat shock protein-associated chaperone, stimulates ligand-dependent estrogen receptor α signaling and mammary tumorigenesis. Cancer Res. 2004;64:4539–4546. doi: 10.1158/0008-5472.CAN-03-3650. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, Wang M, Celiker MY, et al. Stimulation of mammary tumorigenesis by systemic tissue inhibitor of matrix metalloproteinase 4 gene delivery. Cancer Res. 2001;61:2365–2370. [PubMed] [Google Scholar]
- 29.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 30.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 31.Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]


