Abstract
EGFR is a major anticancer drug target in human epithelial tumors. One effective class of agents is the tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib. These drugs induce dramatic responses in individuals with lung adenocarcinomas characterized by mutations in exons encoding the EGFR tyrosine kinase domain, but disease progression invariably occurs. A major reason for such acquired resistance is the outgrowth of tumor cells with additional TKI-resistant EGFR mutations. Here we used relevant transgenic mouse lung tumor models to evaluate strategies to overcome the most common EGFR TKI resistance mutation, T790M. We treated mice bearing tumors harboring EGFR mutations with a variety of anticancer agents, including a new irreversible EGFR TKI that is under development (BIBW-2992) and the EGFR-specific antibody cetuximab. Surprisingly, we found that only the combination of both agents together induced dramatic shrinkage of erlotinib-resistant tumors harboring the T790M mutation, because together they efficiently depleted both phosphorylated and total EGFR. We suggest that these studies have immediate therapeutic implications for lung cancer patients, as dual targeting with cetuximab and a second-generation EGFR TKI may be an effective strategy to overcome T790M-mediated drug resistance. Moreover, this approach could serve as an important model for targeting other receptor tyrosine kinases activated in human cancers.
Introduction
The EGFR is a membrane-bound receptor tyrosine kinase that belongs to a subfamily of 4 closely related receptors: HER1/EGFR/ERBB1, HER2/NEU/ERBB2, HER3/ERBB3, and HER4/ERBB4. Upon binding to extracellular ligands, the receptors undergo conformational changes that facilitate homo- or heterodimerization. Receptor dimerization leads to activation of downstream signaling pathways that regulate cell proliferation and survival (1).
Epithelial tumors often display aberrant expression of EGFR. Thus, a major focus of recent anticancer drug development has centered on agents that target the receptor (2). Current clinically available anti-EGFR therapies include antibodies that bind to the extracellular domain of the protein (e.g., cetuximab or panitumumab) or small-molecule tyrosine kinase inhibitors (TKIs; e.g., gefitinib or erlotinib) that selectively inhibit the kinase activity of the receptor. These agents have been FDA approved for use against colorectal, head and neck, and lung cancers. Notably, both antibodies and TKIs were originally developed to target wild-type EGFR.
In 2004, we and others reported that lung adenocarcinomas sensitive to gefitinib and erlotinib often harbor somatic mutations in exons encoding the tyrosine kinase domain of EGFR (3–5). Nearly 90% of these mutations occur as either multi-nucleotide in-frame deletions in exon 19 that eliminate 4 amino acids (LREA) or as single missense mutations that result in substitution of arginine for leucine at position 858 (L858R) (6). Both mutations lead to constitutive activation of the kinase. Expression of either mutant allele in mouse lung epithelia leads to the formation of lung tumors (7, 8). Mutant receptors also display increased affinity for drug and decreased affinity for ATP (9–11). The hypothesis that EGFR mutations are predictive of increased benefit from EGFR TKIs was recently validated in a phase III, randomized, open-label, first-line study of gefitinib versus chemotherapy (carboplatin/paclitaxel) in East Asian patients with advanced non–small cell lung cancer (NSCLC) (i.e., the IRESSA Pan Asia Study, also known as IPASS). Those with EGFR mutant tumors experienced longer progression-free survival (PFS) with gefitinib, and those without mutations had longer PFS with chemotherapy (EGFR mutation positive, hazard ratio (HR) 0.48 [95% CI, 0.36–0.64]; P < 0.0001 [favors gefitinib]; EGFR mutation negative, HR 2.85 [95% CI, 2.05–3.980]; P < 0.0001 [favors chemotherapy]) (12).
Unfortunately, after about 1 year on therapy, patients with drug-sensitive EGFR mutations whose tumors initially respond to gefitinib or erlotinib eventually develop acquired resistance (13, 14). In about half of the cases, tumors biopsied after disease progression contain a second-site mutation in the EGFR kinase domain (15–20). The most common (>90%) alteration involves a C→T change at nucleotide 2369 in exon 20, which results in substitution of methionine for threonine at position 790 (T790M). This substitution is analogous to the BCR-ABL T315I change found in patients with chronic myelogenous leukemias, who have developed acquired resistance to imatinib (Gleevec) (21). Based upon crystal structure analyses, the EGFRT790M substitution may impair binding of either gefitinib or erlotinib to the EGFR ATP-binding pocket (22). The change could also alter the relative affinity of ATP versus drug (23). An alternative mechanism of resistance — amplification of the gene encoding the MET tyrosine kinase — occurs in about 20% of patients with acquired resistance (24). MET amplification occurs independent of T790M status (25).
Like other drug-sensitizing EGFR mutations, the T790M change by itself has been shown to increase kinase activity and oncogenic potential when compared with wild-type protein (26). Induced expression of EGFRT790M in mouse lung epithelia leads to the formation of lung adenocarcinomas (27). Although somatic T790M mutations in patients who never received gefitinib or erlotinib are rarely detected by conventional mutational analyses (i.e., dideoxynucleotide sequencing), they can occasionally be found in tumors with primary drug resistance (28), and they exist at low frequency in untreated patients with metastatic disease (29). Certain cases of inherited susceptibility to lung cancer may also be associated with a germ line T790M mutation (30).
Preclinical studies have suggested that second-generation EGFR inhibitors may be able to overcome T790M-mediated resistance, at least in vitro (18, 31). Unlike gefitinib and erlotinib, which compete with ATP in a “reversible” manner, many of these new compounds form a covalent bond with EGFR and are thus considered “irreversible” inhibitors. Agents under evaluation include HKI-272 (32), BIBW-2992 (20, 33), and PF00299804 (34). However, no targeted agents have been clinically approved for use in patients with acquired resistance to current EGFR TKIs. The efficacy of anti-EGFR antibodies in EGFR mutant tumors also remains to be established.
To study further the biology of EGFR mutant lung tumors, our groups previously generated mouse tumor models that develop lung adenocarcinomas driven by EGFRL858R (8), EGFRT790M, or EGFRL858R+T790M (27). These models employ a tetracycline–inducible (tet-inducible) system, involving bitransgenic animals. One transgene carries a tet transactivator in lung epithelia (i.e., Clara cell secretory protein – reverse tetracycline transactivator [CCSP-rtTA], herein referred to as “C” mice). The 3 relevant strains are referred to as C/L858R, C/T790M, and C/L+T, respectively. As expected, tumors harboring EGFRL858R are sensitive to erlotinib, while tumors expressing EGFRT790M are resistant. Here, we used “clinical trials” in the animal models in conjunction with EGFR mutant cell lines, various anti-EGFR therapies, and multiple molecular biological techniques to identify a strategy to overcome T790M-mediated resistance. Surprisingly, we found that only dual targeting of EGFR with both an antibody (cetuximab) and a second-generation EGFR TKI (BIBW-2992) was effective at targeting T790M-driven tumors. These studies have immediate therapeutic implications for lung cancer patients. Moreover, these data provide new insights into the development of agents against EGFR that could serve as an important model for targeting other receptor tyrosine kinases activated in human cancers.
Results
Effect of BIBW-2992 in EGFR mutant mouse models of lung cancer.
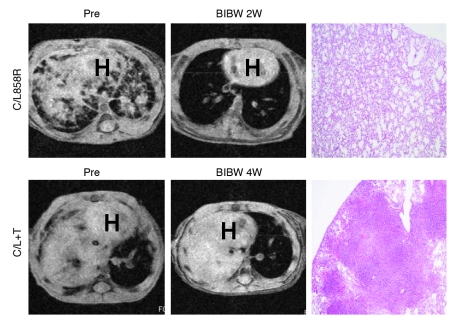
BIBW-2992 is one of several promising new irreversible EGFR inhibitors in clinical development. Enzymatic assays using recombinant human wild-type EGFR and HER2 indicate that the IC50 values are 0.5 and 14 nmol/l, respectively (20). The agent has been shown in patients to induce regressions of lung cancers with EGFR drug-sensitizing mutations (35) and has displayed modest activity against erlotinib-resistant EGFRT790M-harboring mouse lung tumor models (33). To confirm and extend reported results, we treated C/L858R, C/L+T and C/T790M animals with BIBW-2992. Mice were administered 25 mg/kg/d, the maximum tolerated dose (data not shown). Within days of treatment, 4 of 4 C/L858R mice displayed complete responses (CRs), as shown by a greater than 80% reduction in tumor volume on MRI (see Methods) after therapy (Figure 1 and Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI38746DS1). By contrast, 0 of 7 C/L+T animals displayed CRs to the same drug; 6 showed stable disease (SD) and 1 showed progressive disease (PD). Only 1 mouse could be treated for 4 weeks; this mouse showed PD. The 6 additional mice had to be sacrificed, because they showed modest signs of respiratory distress. Two C/T790M animals treated with BIBW-2992 also showed PD (Supplemental Figure 1 and Supplemental Table 2). Upon histological examination, all T790M mice that were treated with BIBW-2992 showed viable tumor (Figure 1 and Supplemental Tables 1 and 2).
Figure 1. BIBW-2992 induces radiographic CRs in lung tumor-bearing C/L858R but not C/L+T transgenic animals.
MRI images of lungs from tumor-bearing C/L858R and C/L+T mice pretreatment and after treatment with BIBW-2992 for 2 weeks (2W) and 4 weeks. Corresponding H&E-stained sections of lungs from treated mice (right panels) (original magnification, ×40). BIBW, BIBW-2992; H, heart.
Lung tumors from C/L+T mice express higher levels of the EGFR ligands, amphiregulin and epiregulin, compared with normal lung.
Since BIBW-2992 displayed limited activity against lung tumors in C/L+T and C/T790M animals, we sought to identify genes regulated by expression of mutant EGFRs whose products could potentially serve as additional targets for therapy. We performed mRNA expression profiling of lung tumors from C/L858R, C/T790M, and C/L+T mice and from corresponding normal lung tissue. Unsupervised clustering showed that tumors could readily be distinguished from normal lung (Figure 2A). A more detailed analysis of the mRNA profiles from these tumors and additional ones driven by other oncogenes will be presented elsewhere (K.A. Politi et al., unpublished observations).
Figure 2. EGFR mutant lung tumors display higher levels of the EGFR ligands, Areg and Ereg, compared with normal lungs.
(A) Unsupervised clustering of tumors from C/L858R, C/T790M, and C/L+T animals and normal lungs from control littermates fed a dox-containing diet form 2 separate groups. “Normal-1” lungs were derived from animals on a pure FVB background, and “normal-2” lungs were from animals on a mixed genetic background (see Methods for details). (B) RT-PCR for Ereg and Areg (and actin) was performed in the presence or absence of reverse transcriptase on mRNA extracted from 3 individual normal lungs and 3 separate macrodissected tumors from C/L+T, C/T790M, and C/L858R mice, respectively. h, human; m, mouse; NI, individual normal lung. (C) Levels of mouse epiregulin and amphiregulin were measured using ELISAs (see Methods) in lysates derived from 3 individual normal lungs and 3 separate macrodissected tumors from C/L+T, C/L858R, and C/T790M mice, respectively. Data represent mean ± SEM.
Relevant to this study, genes encoding the EGFR ligands, amphiregulin (Areg) and epiregulin (Ereg), were among those most highly expressed in tumors compared with normal lungs (Table 1 and Supplemental Table 3). Other genes did not appear to be obviously linked to EGFR signaling. In tumors from C/L+T mice, Ereg displayed approximately 91-fold higher levels of expression (false discovery rate [FDR] 5.61 × 10–9), while Areg levels were approximately 29-fold higher (FDR 1.33 × 10–7) (Table 1). These findings were grossly confirmed by RT-PCR (Figure 2B). Similar results were obtained when comparing tumors from C/L858R and C/T790M animals to normal lung (Table 1 and Figure 2B). ELISAs further confirmed that levels of mouse Ereg and Areg proteins were higher in tumors compared with normal tissue (Figure 2C). Thus, induction of mutant EGFRs in lung epithelia leads to increased EGFR ligand expression, irrespective of the specific EGFR genotype.
Table 1 .
Two of the most highly expressed genes in tumors versus normal lung tissue include Ereg and Areg
To determine whether EGFR mutant lung adenocarcinoma cells alone might be the source of EREG and AREG, we interrogated 2 other existing microarray datasets on relevant human cell lines: our own dataset comparing erlotinib-sensitive H3255 (EGFRL858R) cells after treatment with control (DMSO) or erlotinib (Y. Gong and W. Pao, unpublished observations) and a published dataset comparing erlotinib-resistant H1975 (EGFRL858R+T790M) cells after treatment with control or another irreversible EGFR inhibitor, CL-387-785 (36). After 12 hours of treatment with erlotinib, compared with control-treated cells, H3255 cells displayed approximately 7- and approximately 16-fold less AREG and EREG, respectively (data not shown), while after 24 hours, analogously treated H1975 cells displayed 4.85- and 4.71-fold less AREG and EREG, respectively. These data support the possibility that EGFR ligands are derived from tumor cells themselves and act in an autocrine manner. However, the results do not exclude the possibility that Ereg and Areg are also derived from surrounding cells and play a role in paracrine signaling. For example, EREG is highly expressed in peripheral blood leukocytes, which are often recruited to tumors as part of an inflammatory response (37).
Effect of cetuximab in EGFR mutant mouse models of lung cancer.
EGFR kinase domain mutants are constitutively activated but remain sensitive to further stimulation with EGFR ligands (3, 38). Increased expression of Areg and Ereg in the mouse lung tumors raised the possibility that these factors additionally contribute to EGFR mutant lung tumorigenesis. Cetuximab is a human-murine chimeric IgG1 monoclonal anti-EGFR antibody that has been shown to inhibit competitively the binding of EGF, TGF-α, and potentially other ligands to EGFR (39). To determine whether interfering with EGFR ligand binding might have a therapeutic effect in the EGFR mutant tumor models, we treated various lung tumor-bearing mice with the antibody. Cetuximab also induces downregulation and internalization of both wild-type (40) and mutant receptors (41).
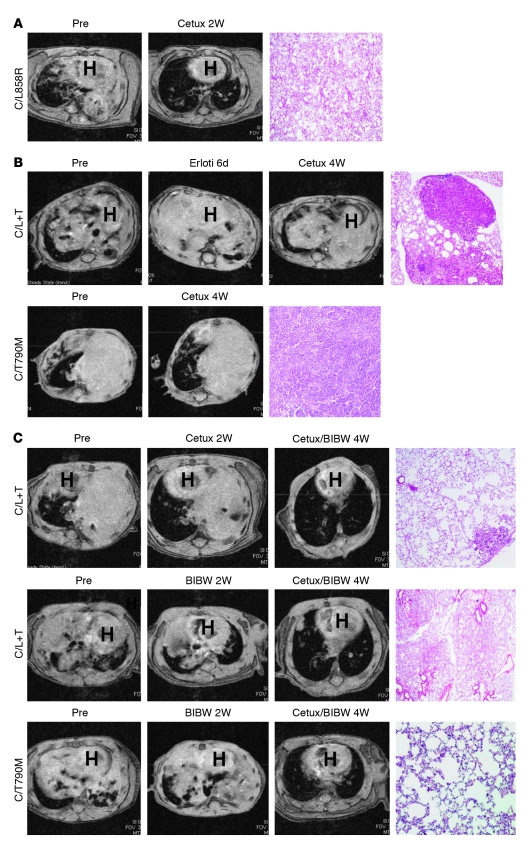
Tumors from 5 of 5 C/L858R mice completely responded to cetuximab within 2 weeks (1 mg i.p. every 3 days; Figure 3A, Figure 4, and Supplemental Table 4). Histologic analysis of lung tissue confirmed a lack of viable tumor in all 5 treated mice (Figure 3A and Supplemental Table 4). By contrast, among 7 C/L+T animals treated with the same regimen of cetuximab, no CRs were observed using MRI; 2 mice displayed partial responses (PRs) and 5 animals showed SD (Figure 3B, Figure 4, and Supplemental Table 4). Additionally, 2 C/T790M animals treated with the same regimen of cetuximab displayed SD (Figure 3B, Supplemental Figure 1, and Supplemental Table 2). Histological analysis of lung tissue from the 9 mice carrying the T790M mutation and treated with cetuximab showed viable tumor (Figure 3B and Supplemental Tables 2 and 4).
Figure 3. The combination of cetuximab and BIBW-2992 induces tumor regressions of mouse lung tumors driven by EGFRL858R+T790M.
(A) MRI images of lungs from a tumor-bearing C/L858R mouse pretreatment and after treatment with cetuximab for 2 weeks. H&E-stained section from treated C/L858R mouse (right panel) (original magnification, ×40). (B) MRI images of lungs from tumor-bearing C/L+T (top panels) and C/T790M (bottom panels) mice, pretreatment, after 6 days of erlotinib (erloti), and then after 4 weeks of cetuximab. H&E-stained section from treated C/L+T and C/T790M mice (right panels) (original magnification, ×40). (C) MRI images of lungs from tumor-bearing C/L+T and C/T790M mice, pretreatment, after treatment with either cetuximab for 2 weeks or BIBW-2992 for 2 weeks, and after treatment with cetuximab (cetux) and BIBW-2992 for 4 weeks. H&E-stained sections of lungs from mice treated with the drug combination (right panels) (original magnification, ×40). Representative images are shown from all studies.
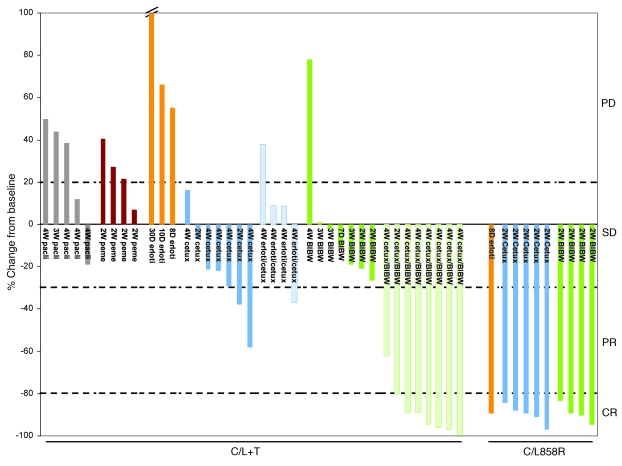
Figure 4. Change in radiographic tumor volume from baseline by treatment for individual lung tumor-bearing C/L858R and C/L+T animals.
Graphed is the percentage change in tumor volume, calculated for individual animals pretreatment and after treatment with paclitaxel (pacli), pemetrexed (peme), erlotinib, cetuximab, BIBW-2992, or combinations of erlotinib or BIBW-2992 with cetuximab. Cutoffs of 20% growth, 30% shrinkage, and 80% shrinkage (dotted lines) are shown to delineate PD, PR, and CR, respectively. Mice that displayed less than 20% growth and less than 30% shrinkage in tumor volume were considered to have SD (see Methods for details.) Statistical significance (calculated using Fisher’s exact test) of BIBW-2992/cetuximab–induced CRs in C/L+T animals versus paclitaxel (P = 0.0047), pemetrexed (P = 0.01), erlotinib (P = 0.02), cetuximab (P = 0.001), and cetuximab/erlotinib (P = 0.01).
Reasons for the discrepant responses observed with EGFRL858R- and EGFRL858R+T790M-driven lung tumors are currently unclear. We confirmed via immunoprecipitation of tumor lysates using cetuximab that the antibody is able to bind to both types of mutant receptors (Supplemental Figure 2). Further experiments to elucidate mechanistic differences are under investigation and outside the scope of this study (see Discussion).
Effect of combination treatment with BIBW-2992 and cetuximab in EGFR mutant models.
Previously, investigators have shown that AG1478, an experimental EGFR TKI, synergistically inhibits the growth of tumors overexpressing EGFR, when used in combination with the EGFR-specific mAb 806 (42). mAb 806, in preclinical development, binds only a transitional form of the receptor after it untethers but before forming the back-to-back, ligated, active oligomer. To determine whether analogous synergy could be achieved with BIBW-2992 and cetuximab, we treated tumor-bearing C/L+T and C/T790M animals with both drugs together for a maximum of 4 weeks. Eight of eight C/L+T animals displayed tumor shrinkage. Remarkably, 7 of these were CRs (Figure 3C, Figure 4, and Supplemental Table 1). Three of three C/T790M animals similarly showed CRs (Figure 3C, Supplemental Figure 1, and Supplemental Table 2). Histological analysis of lungs from animals displaying CRs after treatment showed either scant or no viable tumor cells (Figure 3C and Supplemental Tables 1 and 2). CRs were observed regardless of which drug was administered first as a single agent. By contrast, combinations of erlotinib plus cetuximab did not result in any CRs in C/L+T mice (Figure 4 and Supplemental Table 4). Such dramatic responses were not observed with any other attempted drug regimen, including with chemotherapy (pemetrexed or paclitaxel) (Figure 4 and Supplemental Table 5). Mice were not treated for longer periods of time or observed for tumor recurrence.
In vivo antitumor activity of BIBW-2992 with cetuximab against H1975 xenografts.
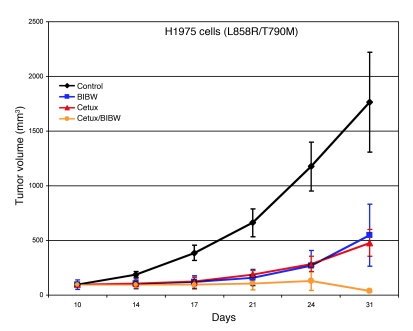
To assess the efficacy of the cetuximab/BIBW-2992 combination in a separate in vivo model, we treated mice bearing xenografts of H1975 cells. These lung adenocarcinoma cells harbor the EGFR L858R and T790M mutations in cis and are resistant to erlotinib in vitro (19). Consistent with results obtained from the mouse lung tumor model, the combination of cetuximab and BIBW-2992 was superior to either agent alone in 3 independent experiments (Figure 5).
Figure 5. The combination of cetuximab and BIBW-2992 induces regression of H1975 cell xenografts.
Athymic nude mice bearing established H1975 tumor cell xenografts were treated with placebo, cetuximab, BIBW-2992, or cetuximab with BIBW-2992 for 1 month. Five mice were treated in each group. Statistical analysis by a repeated measures ANOVA model is as follows: cetuximab versus control, P = 0.01; BIBW-2992 versus control, P = 0.09; BIBW-2992 plus cetuximab versus control, P = 0.006; BIBW-2992 versus cetuximab, P = 0.66; BIBW-2992 versus BIBW-2992 plus cetuximab, P = 0.13; cetuximab versus BIBW-2992 plus cetuximab, P = 0.02. Shown are representative results from 3 independent xenograft experiments. Data represent mean ± SEM.
Effect of the cetuximab/BIBW-2992 combination on EGFRL858R+T790M.
To assess how cetuximab and BIBW-2992 could overcome T790M-mediated resistance, we examined the status of EGFR in tumor lysates derived from C/L+T mice, briefly treated with either drug alone or the combination. Compared with placebo-treated samples, cetuximab-treated lungs displayed decreased levels of total EGFR, likely due to degradation induced by the antibody (Figure 6A). By contrast, BIBW-2992–treated lungs showed decreased levels of phospho-EGFR. When cetuximab and BIBW-2992 were used together, levels of both phospho-EGFR and total EGFR were markedly depleted (Figure 6A).
Figure 6. Effect of cetuximab plus BIBW-2992 on EGFRL858R+T790M.
(A) Tumor (T) lysates from C/L+T mice treated for 1 week with cetuximab (C), BIBW-2992 (B), or BIBW-2992 plus cetuximab (B+C) were probed by immunoblot analyses, using antibodies against the indicated phospho- (p-) or total (t) proteins and actin. Lysates from normal lung (N) were also examined. (B) Tumor lysates from H1975 xenograft models, treated as above for 1 week, were harvested for analogous immunoblot analyses. (C) NR6 mouse fibroblasts were stably transfected with cDNAs encoding EGFRL858R+T790M and then treated with cetuximab (20 μg/ml), BIBW-2992 (50 nmol), or the combination of drugs as indicated. Corresponding cell lysates were subjected to immunoblotting with the indicated antibodies.
To confirm and extend these data, we examined the effect of single and combination drug against H1975 xenografts. Again, compared with tumors treated with vehicle control or either drug alone, tumors treated with the combination of BIBW-2992 plus cetuximab displayed dramatic inhibition of both phospho-EGFR and total EGFR (Figure 6B).
Finally, we studied the effect of the drugs against mutant EGFR in vitro. NR6 mouse fibroblasts, an NIH 3T3 line devoid of EGFR (43), were stably transduced with retroviruses carrying EGFRL858R+T790M and treated with cetuximab, BIBW-2992, or both. NR6 transfectants treated with cetuximab showed degradation of EGFR, while cells treated with BIBW-2992 displayed lower levels of phosphorylated EGFR. The combination of cetuximab and BIBW-2992 enhanced depletion of both phosphorylated and total EGFR levels (Figure 6C). Collectively, these data suggest that CRs were induced by the double combination of anti-EGFR therapies in mice bearing EGFRT790M-driven tumors, because only the combination could achieve sufficient levels of EGFR inactivation.
Discussion
The second-site EGFRT790M mutation is found in about half of patients whose EGFR mutant tumors develop acquired resistance to gefitinib or erlotinib. Many second-generation irreversible EGFR inhibitors, such as HKI-272 (32), BIBW-2992 (33), and PF00299804 (34), are being developed to overcome T790M-mediated resistance. However, by modeling acquired resistance in vitro, others have shown that HKI-272 can overcome T790M-mediated resistance only at suprapharmacologic concentrations (44), and we have obtained analogous results with BIBW-2992 (J. Chmielecki and W. Pao, unpublished observations). Consistent with these findings, in mice bearing tumors with EGFRL858R+T790M that were treated with BIBW-2992, we and others (33) have not observed any CRs. Moreover, in a clinical trial of BIBW-2992 in patients with acquired resistance to an EGFR TKI, only modest activity has been observed (35). Thus, use of these agents alone may not be as effective as originally anticipated.
To find additional targets for therapy, we compared the mRNA expression profiles of lung tumors from EGFRL858R+T790M mutant transgenic animals versus normal lung. We found that the EGFR ligands, Areg and Ereg, were more highly expressed in tumors from C/L+T mice as well as from C/T790M and C/L858R animals, at both the mRNA and protein levels, when compared with expression of control animals. Notably, our animal models were generated with transgenes encoding human EGFRs, and at least murine Ereg has been shown to bind readily to human EGFR (45).
To determine whether interfering with the binding of such ligands might have an antitumor effect, we treated tumor-bearing mice with cetuximab. This anti-EGFR antibody has been approved by the FDA for use in treating colorectal and head and neck cancers, respectively (46, 47), and addition of the antibody to systemic chemotherapy conferred a modest but superior survival over chemotherapy alone in the treatment of chemo-naive patients with unselected non–small cell lung cancer (48). The effect of cetuximab against EGFR mutant tumors is less clear. In preclinical models, although most EGFR mutant lung cancer cell lines are sensitive to treatment with EGFR TKIs, only 1 line (with an exon 19 deletion) thus far has been shown to be sensitive to treatment with the antibody (49, 50). In mouse models of lung cancer driven by EGFRL858R, others have found, similar to data presented here, that cetuximab can induce dramatic tumor regressions (7). Data derived from patients is still relatively scant. In a report of 4 patients with tumors bearing EGFR exon 19 deletions, cetuximab induced only SD (50). After developing disease progression, all patients had PRs on treatment with gefitinib. To our knowledge, no response data to cetuximab have yet been reported for patients whose tumors harbor EGFRL858R.
In the animals treated with cetuximab in this study, we did observe some antitumor activity. In a total of 9 mice bearing T790M mutant-positive tumors (2 C/T790M and 7 C/L+T), 7 displayed SD and 2 showed PRs. However, we did not observe any CRs. This result suggests that ligands contribute to EGFR mutant lung tumorigenesis but that antibody treatment alone is insufficient to induce substantial tumor shrinkage in the majority of cases. Consistent with this finding, some of us have found that the presence of EGFR ligands can raise the threshold for drug sensitivity of EGFR mutant lung cancer cells (I. Vivanco et al., unpublished observations).
Because dual targeted therapy against EGFR has been shown to be synergistic, at least with preclinical compounds (i.e., AG1478 with mAb806 [ref. 42]), we next tested the combination of BIBW-2992 with cetuximab in the mouse models. Remarkably, the administration of both drugs together induced CRs in 7 of 8 C/L+T animals and 3 of 3 C/T790M animals. This difference versus the effect of cetuximab (0 of 9) or BIBW-2992 (0 of 8) alone was statistically significant (P = 0.003 and P = 0.0007, respectively; Fisher’s exact test). No other treatments tested were effective at overcoming T790M-mediated resistance, including the combination of erlotinib with cetuximab. BIBW-2992 plus cetuximab was similarly synergistic in a separate relevant xenograft model.
Analysis of 3 separate biological systems (primary mouse lung tumors, human xenografts, and NR6 transfectants) revealed that the drug combination overcomes T790M-mediated resistance by targeting the mutant receptor more effectively than either agent alone. While the antibody (cetuximab) induces receptor degradation, it is insufficient to inhibit the ligand-independent activity of the mutant receptors. The kinase inhibitor (BIBW-2992) inhibits phospho-EGFR activity but only incompletely at the doses administered. Only the combination of both agents together induced depletion of both phosphorylated and total EGFR, resulting in the induction of CRs. Multiple mechanisms could explain this observation. One possibility is that BIBW-2992 increases binding of cetuximab to the cell surface. Consistent with this, AG1478 increases binding of mAb 806 to the cell surface through 2 distinct mechanisms: an immediate effect on the conformation of EGFR and a longer-term increase in cell surface underglycosylated EGFR, an event known to increase mAb 806 reactivity (51). As a consequence of increased binding, EGFR could be degraded more efficiently. A second possibility is that cetuximab and BIBW-2992 target different receptor pools. Consistent with this, cetuximab alone induces degradation of total EGFR without significantly affecting levels of phospho-EGFR, while BIBW-2992 dephosphorylates EGFR without inducing degradation of the receptor. The combination allows BIBW-2992 to inhibit more efficiently any residual kinase activity. A third possibility, in vivo at least, is that cetuximab binding leads to enhanced antibody-dependent cellular cytotoxicity (52).
At this juncture, we cannot explain why tumors in C/L858R animals respond to single-agent cetuximab, while tumors in C/L+T mice remain mostly stable. One explanation is that cetuximab-induced receptor downregulation is different for EGFRL858R versus EGFRL858R+T790M. Others have demonstrated that cetuximab in vitro degrades mutant EGFRs to a greater degree in lung tumor cells harboring drug-sensitive mutations than in cells harboring the double mutation (53). Interestingly, mice bearing tumors driven by EGFRT790M alone also did not radiographically respond to single-agent cetuximab (n = 2) but did display CRs after treatment with BIBW-2992/cetuximab (n = 3). This result suggests that the difference in responses may be in part due to the T790M change itself and may not be a property of the double-mutant EGFR. Perhaps the T790M change induces conformational changes within the receptor that lead to differential partnering of mutant receptor, either with itself (homodimers) or with other EGFR-related family members (heterodimers). Another possibility is that although both mutant receptors are constitutively active, the L858R single mutant is less active but more responsive to ligand activation, while the double mutant is more active but less responsive to ligands. In this context, cetuximab is able to lower the threshold for EGFR activation in the single but not the double mutant. Finally, it is possible that the T790M change alters the amount of mutant receptor that reaches the cell surface compared with that with the L858R mutant alone. Although our immunoprecipitation studies with cetuximab showed that the antibody bound to either mutant receptor (Supplemental Figure 2), the studies were qualitative, not quantitative. We plan in future studies to address this issue in more detail.
Based upon the preclinical data shown here, we believe a trial is warranted of BIBW-2992 in combination with cetuximab for patients with EGFR mutant tumors and acquired resistance to gefitinib or erlotinib. The animals treated with both drugs appeared to tolerate the regimen without difficulty (data not shown). However, in humans we acknowledge that such treatment could lead to excess skin toxicity. Dual targeting of EGFR will also likely enrich for EGFR-independent mechanisms of acquired resistance, such as MET amplification (24, 25). Thus, future studies will need to address how to overcome resistance that develops due to both T790M and MET amplification together.
Finally, mutant receptor tyrosine kinases have served as tractable substrates for targeted cancer therapy. The dual targeting approach presented here, with both a TKI and an antibody, could serve as an important model for targeting other receptor tyrosine kinases activated in various human cancers.
Methods
Animals.
The generation of dox-inducible EGFRL858R, EGFRT790M, and EGFRL858R+T790M mice has been previously described (8, 27). All animals were housed in specific pathogen-free housing, with abundant food and water, and treated with various drugs under guidelines approved by the MSKCC IACUC and Research Animal Resource Center. CCSP-rtTA mice were previously described (54). Dox was administered by feeding mice with drug-impregnated food pellets (625 ppm; Harlan-Teklad).
Drug trials in transgenic animals.
BIBW-2992 was synthesized by the Organic Synthesis Core Facility (MSKCC), using a modification of the published procedure (55). The drug was suspended in 0.5% (w/v) methylcellulose and administered i.p., 25 mg/kg/d. The stock solution was reconstituted every week and stored at 4°C. Cetuximab (1 mg every 3 days; Erbitux; Bristol-Myers Squibb and Eli Lilly Pharmaceuticals), pemetrexed (100 mg/kg twice per week; Alimta; Eli Lilly Pharmaceuticals), and paclitaxel (30 mg/kg/d once per week; Taxol; Mayne Pharma) were provided by the MSKCC Pharmacy and injected i.p. Erlotinib (50 mg/kg/d; synthesized by the Organic Synthesis Core Facility) was suspended in 0.5% (w/v) methylcellulose and injected i.p.
For xenograft studies, 8-week-old nu/nu athymic male mice (Taconic) were injected subcutaneously with 10 million H1975 cells together with Matrigel (BD Biosciences). Once tumor volumes reached 100 mm3, mice were randomized to receive either vehicle control, BIBW-2992 alone (25 mg/kg/d, 5 days per week, by oral gavage), cetuximab alone (1 mg/mouse every 3 days i.p.), or BIBW-2992 and cetuximab together. Tumor size was measured twice weekly using calipers. The average tumor volume in each group was expressed in cubic millimeters and calculated using the formula π / 6 × (large diameter) × (small diameter)2. At the end of the study, mice were euthanized by CO2 asphyxiation. Experiments were performed 3 independent times and were carried out under an IACUC-approved protocol, and institutional guidelines for the proper and humane use of animals were followed.
Gene expression profiling.
mRNA was extracted from pulverized lung samples using TRIzol (Invitrogen) and then hybridized to MOE 430 2.0 chips (Affymetrix) using standard hybridization techniques. The following samples were analyzed: macrodissected visible tumor nodules from bitransgenic animals (C/L858R, n = 4; C/L+T, n = 5; and C/T790M, n = 5) fed a dox-containing diet for 3 to 6 months and normal lung tissue (n = 10) derived from transgene-negative or mono-transgenic littermates on dox. Samples were submitted to the MSKCC Genomics Core Lab at 5 different points in time. To minimize batch effects, very strict standard operating procedures were followed for the RNA extraction, labeling, and array hybridization/washing/scanning. The same technician handled the entire project. We analyzed normal lung tissue derived from littermates from 2 sets of mice (n = 5, each), because C/L+T and C/T790M mice were derived on a pure FVB background, while C/L858R mice were derived on a mixed genetic background. Although these normal sets formed 2 separate groups by unsupervised clustering, they were still clearly distinct from clusters of tumor tissue.
We used the Robust Multichip Average method for data preprocessing, and the empirical Bayes method for differential expression analysis of the results. P values were adjusted for multiple comparisons using the Benjamini and Hochberg method of controlling FDR; P values of less than 0.05 were considered significant. The cutoff criteria to select a subset of genes of interest were as follows: FDR of less than or equal to 1% and absolute fold change of more than or equal to 2. Hierarchical clustering was performed to identify natural groupings of the samples using the average linkage method. The distance metric used was one minus correlation. Notably, the 2 sets of normal tissue sets clustered together, despite being processed in different batches. Conversely, tumors from C/L+T mice and the second normal set clustered in separate groups, despite being processed in the same batch (data not shown). Thus, the clusters separated primarily due to tissue differences rather than batch effects. All datasets are available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE17373.
Histology.
Animals were sacrificed as per institutional guidelines. After excision of an individual animal, both lungs were inflated with PBS. The left lung was flash-frozen in liquid nitrogen. The right lung was placed in 4% paraformaldehyde in PBS, fixed overnight at room temperature, placed in 70% ethanol, and sent for paraffin embedding and sectioning (Histoserv). In some animals, gross tumor nodules were macrodissected and flash-frozen, and the remaining lung tissues were processed for histological analysis. All lungs were sectioned in the same manner: 5 steps were taken, 100 microns apart. All steps were evaluated to determine whether tumors were present. Slides were reviewed by a board-certified pathologist with expertise in lung cancer (M.F. Zakowski).
RT-PCR analysis.
RNA was extracted from pulverized tissue samples using TRIzol. RNA was treated with DNase I (Sigma-Aldrich) to eliminate contaminating DNA. cDNA was synthesized using oligo-dT primers (Invitrogen) and the SuperScript III First-Strand cDNA Synthesis Kit (Invitrogen). RT-PCR reactions were performed using the HotstarTaq Master Mix Kit (Qiagen) as per manufacturer’s instructions and the following primers for mouse epiregulin, forward, 5′-TGGCTCAAGTGCAGATTACA-3′, and reverse, 5′-AATGAGAATCACTGTCAACG-3′, and mouse amphiregulin, forward, 5′-GAAAAAGAATCCATGCACTG-3′, and reverse, 5′-GGCAGAGACAAAGATAGTGA-3′. Control reactions were performed in the absence of reverse transcriptase.
ELISAs.
Mouse epiregulin and amphiregulin ELISA Kits (R&D Systems) were performed as per manufacturer’s instructions.
MRI.
Mice were imaged in a Bruker 4.7T Biospec scanner (Bruker Biospin Inc.) as previously published (27). Tumor volume (cm3) per animal was quantified by calculating the area of visible lung opacities present in each axial image sequence (usually 20–22 per mouse), using ParaVision 3.0.2 imaging software, and then multiplying the total sum of the areas by 0.09 cm (the distance between each MRI sequence). Prior to treatment, mice were always scanned at least twice, 1 week apart, to confirm the presence of growing lung nodules and to avoid treating false-positive animals.
The following criteria (slightly modified from ref. 27) were used to classify tumor responses to treatment: (a) for CR, at least an 80% decrease in the volume of target lesions, taking as reference the baseline tumor volume; (b) for PR, at least a 30% decrease in the volume of target lesions, taking as reference the baseline tumor volume; (c) for PD, at least a 20% increase in the volume of target lesions, taking as reference the baseline tumor volume, and (d) for SD, neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the baseline tumor volume. The 80% decrease in tumor volume was chosen as the cutoff for CRs based upon multiple experiments, showing that virtually no viable tumors remained in mice displaying this amount of reduction in tumor volume.
Immunoblotting and immunoprecipitation assays.
Pulverized tissue was lysed with NP-40 lysis buffer supplemented with 40 mM NaF, 100 μM Na3VO4, and Complete Protease Inhibitor (Roche). Tissue lysates containing 0.5 mg protein were preincubated for 1 hour with 2 μg cetuximab, followed by overnight incubation with 30 μl protein A/G agarose beads (Santa Cruz Biotechnology Inc.). Solutions were pelleted and washed 3 times with PBS. The captured immunocomplexes were boiled in 2x SDS sample buffer for 5 minutes, and proteins were resolved by SDS-PAGE gel electrophoresis on 4%–20% gradient gels. Subsequent immunoblots were probed with an antibody against total EGFR (Santa Cruz Biotechnology Inc.).
For all other immunoblotting experiments, established protocols were performed (5), using antibodies that recognize phospho-EGFR Y1092 (Biosource; Invitrogen), total EGFR (catalog no. 1005; Santa Cruz Biotechnology Inc.), and actin (Sigma-Aldrich). Note that 2 numbering systems exist for EGFR. The first denotes the initiating methionine in the signal sequence as amino acid –24. The second, used here, denotes the methionine as amino acid +1.
Derivation of NR6 transfectants.
NR6 cell lines were maintained at 37°C in a humidified incubator with 5% CO2 in DMEM (MSKCC Media Core Facility), supplemented with 5% FBS and 1% Penicillin:Streptomycin Solution (both from Gemini Bio-Products). To derive stably transduced NR6 cells, 293T cells were cotransfected, using the calcium phosphate method, with 15 μg of an amphotropic packaging plasmid, with 15 μg of pLNCX-EGFRL858R+T790M. Viral particles were collected 36 and 60 hours after transfection and used to sequentially infect NR6 cells. Seventy-two hours after the first round of infection, cells were selected with 1 mg/ml G418. For immunoblotting studies, NR6 cells were serum starved for 12 hours prior to treatment with cetuximab and/or BIBW-2992.
Statistics.
Changes in radiographic tumor volume from baseline by treatment for individual lung tumor-bearing C/L858R and C/L+T animals were compared using Fisher’s exact test. P values were calculated using 2-tailed Student’s t test. P values of less than 0.05 were considered statistically significant. Microarray data were analyzed as described above (see Methods).
Supplementary Material
Acknowledgments
We thank Mihaela Lupu and Dov Winkelman for assistance with animal MRI, Dennis Grossano for providing chemotherapeutic agents, and Gregory Riely for critical reading of the manuscript. This work was supported by the American Cancer Society (to K.A. Politi); the Labrecque Foundation (to K.A. Politi); NIH National Cancer Institute (NCI) grants K08-CA097980 (to W. Pao), R01-CA121210 (to W. Pao), K99-CA131488 (to K.A. Politi), and R01-CA120247 (to H. Varmus for K.A. Politi); Joan’s Legacy: The Joan Scarangello Foundation to Conquer Lung Cancer (to Y. Gong and W. Pao); the MSKCC Experimental Therapeutics Core (to W. Pao); the MSKCC Translational and Integrative Medicine Research Fund (to W. Pao); the Elaine Terner Cooper Fellowship (to W. Pao for L. Regales); the Doris Duke Foundation (to I.K. Mellinghoff); the Sontag Foundation (to I.K. Mellinghoff); the Sidney Kimmel Foundation (to I.K. Mellinghoff); and the Golfers against Cancer Foundation (I.K. Mellinghoff). Services provided by the Genomics Core Facility were partially supported by an NCI CCSG award to MSKCC (P30-CA008748). W. Pao received additional support from Vanderbilt’s Specialized Program of Research Excellence in Lung Cancer grant (CA90949) and the VICC Cancer Center Core grant (P30-CA68485).
Footnotes
Conflict of interest: The rights to a patent application on the testing of EGFR T790M mutations have been licensed on behalf of K.A. Politi, W. Pao, and others by MSKCC to MolecularMD.
Citation for this article: J. Clin. Invest. 119:3000–3010 (2009). doi:10.1172/JCI38746.
William Pao’s present address is: Vanderbilt-Ingram Cancer Center, Nashville, Tennessee, USA.
References
- 1.Yarden Y., Sliwkowski M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn J., Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 3.Lynch T.J., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Paez J.G., et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. . Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Pao W., et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pao W., Miller V.A. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J. Clin. Oncol. 2005;23:2556–2568. doi: 10.1200/JCO.2005.07.799. [DOI] [PubMed] [Google Scholar]
- 7.Ji H., et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Politi K., et al. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey K.D., et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66:8163–8171. doi: 10.1158/0008-5472.CAN-06-0453. [DOI] [PubMed] [Google Scholar]
- 10.Yun C.H., et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Gureasko J., Shen K., Cole P.A., Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Mok T.S., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009 doi: 10.1056/NEJMoa0810699. Online publication ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Jackman D.M., et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin. Cancer Res. 2006;12:3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 14.Riely G.J., et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin. Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 15.Balak M.N., et al. Novel D761Y and common secondary T790M mutations in EGFR-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. . Clin. Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S., et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. . N. Engl. J. Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 17.Kosaka T., et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin. Cancer Res. 2006;12:5764–5769. doi: 10.1158/1078-0432.CCR-06-0714. [DOI] [PubMed] [Google Scholar]
- 18.Kwak E.L., et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pao W., et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bean J., et al. Acquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinoma. Clin. Cancer Res. 2008;14:7519–7525. doi: 10.1158/1078-0432.CCR-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorre M.E., et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 22.Clark J., Cools J., Gilliland D.G. EGFR inhibition in non-small cell lung cancer: resistance, once again, rears its ugly head. PLoS Med. 2005;2:e75. doi: 10.1371/journal.pmed.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yun C.H., et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelman J.A., et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 25.Bean J., et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl. Acad. Sci. U. S. A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godin-Heymann N., et al. Oncogenic activity of epidermal growth factor receptor kinase mutant alleles is enhanced by the T790M drug resistance mutation. Cancer Res. 2007;67:7319–7326. doi: 10.1158/0008-5472.CAN-06-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regales L., et al. Development of new mouse lung tumor models expressing EGFR T790M mutants associated with clinical resistance to kinase inhibitors. PLoS ONE. 2007;2:e810. doi: 10.1371/journal.pone.0000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inukai M., et al. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res. 2006;66:7854–7858. doi: 10.1158/0008-5472.CAN-06-1951. [DOI] [PubMed] [Google Scholar]
- 29.Maheswaran S., et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell D.W., et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat. Genet. 2005;37:1315–1316. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 31.Carter T.A., et al. Inhibition of drug-resistant mutants of ABL, KIT and EGF receptor kinases. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11011–11016. doi: 10.1073/pnas.0504952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D., et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Li D., et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engelman J.A., et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–11932. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 35.Yang, C., et al. 2008. Use of BIBW 2992, a novel irreversible EGFR/HER2 TKI, to induce regression in patients with adenocarcinoma of the lung and activating EGFR mutations: Preliminary results of a single–arm phase II clinical trial [abstract 8026]. Presented at the 2008 American Society of Clinical Oncology Annual Meeting. May 30–June 3. Chicago, Illinois, USA. http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=31779.
- 36.Kobayashi S., et al. Transcriptional profiling identifies cyclin D1 as a critical downstream effector of mutant epidermal growth factor receptor signaling. Cancer Res. 2006;66:11389–11398. doi: 10.1158/0008-5472.CAN-06-2318. [DOI] [PubMed] [Google Scholar]
- 37.Toyoda H., Komurasaki T., Uchida D., Morimoto S. Distribution of mRNA for human epiregulin, a differentially expressed member of the epidermal growth factor family. Biochem. J. 1997;326:69–75. doi: 10.1042/bj3260069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greulich H., et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2005;2:e313. doi: 10.1371/journal.pmed.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham J., Muhsin M., Kirkpatrick P. Cetuximab. Nat. Rev. Drug Discov. 2004;3:549–550. doi: 10.1038/nrd1445. [DOI] [PubMed] [Google Scholar]
- 40.Sunada H., Magun B.E., Mendelsohn J., MacLeod C.L. Monoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 1986;83:3825–3829. doi: 10.1073/pnas.83.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doody J.F., et al. Inhibitory activity of cetuximab on epidermal growth factor receptor mutations in non small cell lung cancers. Mol. Cancer Ther. 2007;6:2642–2651. doi: 10.1158/1535-7163.MCT-06-0506. [DOI] [PubMed] [Google Scholar]
- 42.Johns T.G., et al. Antitumor efficacy of cytotoxic drugs and the monoclonal antibody 806 is enhanced by the EGF receptor inhibitor AG1478. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15871–15876. doi: 10.1073/pnas.2036503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Fiore P.P., et al. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987;51:1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- 44.Godin-Heymann N., et al. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol. Cancer Ther. 2008;7:874–879. doi: 10.1158/1535-7163.MCT-07-2387. [DOI] [PubMed] [Google Scholar]
- 45.Toyoda H., et al. Epiregulin. A novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J. Biol. Chem. 1995;270:7495–7500. doi: 10.1074/jbc.270.13.7495. [DOI] [PubMed] [Google Scholar]
- 46.Bonner J.A., et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 47.Jonker D.J., et al. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 48.Pirker R., et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 49.Amann J., et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226–235. [PubMed] [Google Scholar]
- 50.Mukohara T., et al. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J. Natl. Cancer Inst. 2005;97:1185–1194. doi: 10.1093/jnci/dji238. [DOI] [PubMed] [Google Scholar]
- 51.Gan H.K., et al. The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor AG1478 increases the formation of inactive untethered EGFR dimers. Implications for combination therapy with monoclonal antibody 806. J. Biol. Chem. 2007;282:2840–2850. doi: 10.1074/jbc.M605136200. [DOI] [PubMed] [Google Scholar]
- 52.Kurai J., et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin. Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 53.Perez-Torres M., Guix M., Gonzalez A., Arteaga C.L. Epidermal growth factor receptor (EGFR) antibody down-regulates mutant receptors and inhibits tumors expressing EGFR mutations. . J. Biol. Chem. 2006;281:40183–40192. doi: 10.1074/jbc.M607958200. [DOI] [PubMed] [Google Scholar]
- 54.Tichelaar J., Lu W., Whitsett J. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 55.Solca, F. 2008. Method for treating cancer harboring EGFR mutations. World International Property Organization patent WO/2008/034776, filed September 14, 2007, and issued March 27, 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.