Abstract
Secondary hyperparathyroidism often occurs in chronic kidney disease (CKD) and vitamin D deficiency, resulting in increased fractures and mortality. Understanding factors that stimulate parathyroid hormone (PTH) synthesis is important for devising methods to treat this condition. Previous work has demonstrated that murine Pth mRNA levels are regulated by proteins that bind AU-rich elements (AREs) within the 3′ UTR region of Pth mRNA and influence Pth mRNA stability. In this issue of the JCI, Nechama et al. demonstrate that in murine secondary hyperparathyroidism associated with CKD or Ca deficiency, the activity of Pin1, a peptidyl-prolyl isomerase, is reduced (see the related article beginning on page 3102). Reduced Pin1 activity resulted in the phosphorylation and degradation of an ARE-binding protein, K-homology splicing regulator protein (KSRP), which normally enhances the degradation of Pth mRNA. The activity of other ARE-binding proteins, such as AU-rich binding factor 1 (AUF1), that increase Pth mRNA stability, was increased, thereby increasing PTH synthesis. This work suggests new ways by which to regulate PTH synthesis in secondary hyperparathyroidism.
RNA processing in the cytoplasm regulates RNA concentrations
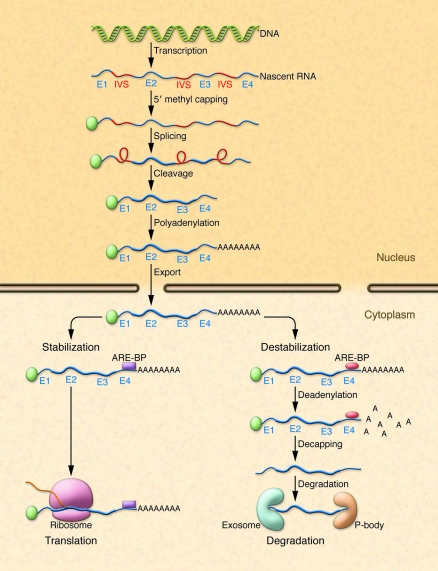
Following transcription, nascent RNA is processed by 5′ methyl capping, splicing, cleavage, and polyadenylation in the nucleus (1–4) (Figure 1). RNA is exported from the nucleus and associates with various cellular structures prior to association with the ribosome. In the cytoplasm, RNA transcripts interact with RNA-binding proteins that influence RNA half-life and stability within the cell (5–8) (Figure 1). RNA-binding proteins (Table 1) associate with sequence-specific elements (adenine- and uridine-rich elements [AREs]) either within the coding or, more usually, within the 3′ UTRs of RNA. AREs regulate the rate at which mRNAs are degraded in cells and were first described as important elements involved in the regulation of the stability and half-life of protooncogene and cytokine mRNAs (1, 9–12). AREs often contain overlapping adenine- and uridine-containing AUUUA pentamers that are found in U-rich regions within the 3′ UTRs of various genes (13). Three classes of AREs have been described: class I AREs contain several copies of the AUUUA motif dispersed within U-rich regions; class II AREs possess at least two overlapping UUAUUUA(U/A) nonamers; and class III AREs are less well defined and generally do not contain an AUUUA sequence (1, 10, 13). Whether an mRNA species containing an ARE bound to ARE-binding proteins (ARE-BPs) is degraded or stabilized is partly dependent upon the cellular milieu, physiological circumstances, and the relative amounts of different bound stabilizing or destabilizing ARE-BPs. Following binding of ARE-BPs to an ARE, RNAs are targeted for translation or degradation. RNAs targeted for degradation undergo deadenylation, decapping, and degradation in a large multiprotein complex, the exosome, or in cytoplasmic compartments known as GW bodies or processing bodies (P-bodies) (14–16).
Figure 1. Cellular processing of RNA.
Following transcription, nascent RNA comprised of exons (E1–E4) and intervening sequences (IVS) is processed in the nucleus by 5′ methyl capping, splicing, cleavage, and polyadenylation. Processed RNA is exported from the nucleus and binds various structural elements and binding proteins. ARE-BPs (purple box and red oval) bind to AREs within the 3′ region of RNA and stabilize or destabilize mRNA. Stabilized RNA undergoes translation in ribosomes, whereas destabilized RNA undergoes deadenylation, decapping, and degradation in exosomes or P-bodies.
Table 1 .
Effect of ARE-BPs on mRNA stability
Parathyroid hormone serum concentrations are dependent on parathyroid hormone secretion and synthesis
The parathyroid (PT) glands play a central role in Ca homeostasis by regulating bone resorption and formation, the synthesis of 1α, 25-dihydroxyvitamin D in the renal proximal tubule, and the reabsorption of Ca2+ in the distal nephron of the kidney (17–21). Changes in serum Ca2+ concentrations are sensed by PT chief cells via a cell-surface, G protein–coupled receptor, the Ca2+-sensing receptor, and result in rapid (within minutes) alterations in parathyroid hormone (PTH) secretion (22, 23). More long-term changes in serum Ca2+ concentrations (over several hours) result in increases or decreases in PTH synthesis and PTH mRNA concentrations in the PT gland (24, 25).
Pathogenesis of secondary hyperparathyroidism
Secondary hyperparathyroidism occurs in the clinical context of vitamin D deficiency, Ca deficiency, and chronic kidney disease (CKD) (26, 27). The pathogenesis of secondary hyperparathyroidism in CKD is multifactorial and includes phosphate retention and hyperphosphatemia, hypocalcemia, 1α, 25-dihydroxyvitamin D deficiency, intestinal Ca malabsorption, the reduction in vitamin D receptor concentrations within the PT gland, and reduced Ca2+-sensing receptor amounts in PT tissue (28–30). Not only is PTH synthesis increased with concomitant increases in serum PTH concentrations, but PT hyperplasia often occurs as well (29, 30). In CKD and dialysis patients, uncontrolled secondary hyperparathyroidism is associated with an increased incidence of fractures and increased mortality (31–34). Numerous methods, including the control of serum phosphate concentrations, the administration of vitamin D analogs, Ca supplementation, and the administration of calcimimetics, have been developed to control PTH levels in CKD and dialysis patients (35–38), but secondary hyperparathyroidism in CKD remains a significant problem. Additional methods for the treatment of this condition would therefore be of value.
Pth mRNA amounts are regulated by Ca2+ and Pi by posttranscriptional mechanisms
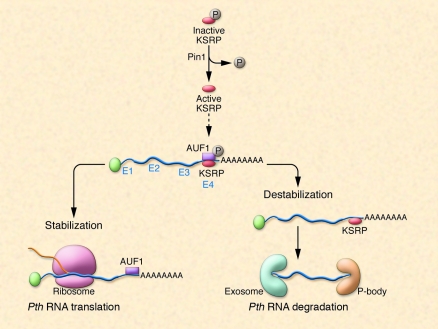
In murine PT glands, changes in Pth mRNA concentrations following alterations in serum Ca2+ (or inorganic phosphate, Pi) concentrations are due to alterations in Pth mRNA stability rather than changes in Pth mRNA transcription (39, 40). Such changes are brought about by the binding of proteins to the terminal portion of the 3′ UTR of Pth mRNA (Figure 2). A 63-nt ARE in the 3′ UTR regulates Pth mRNA stability in response to changes in Ca2+ and Pi concentrations (39, 41, 42). The 63-nt element consists of a core 26-nt minimal binding sequence and adjacent flanking regions. The Pth RNA ARE does not contain AUUUA sequences and falls into the class III category of AREs (42, 43). Two proteins, AU-rich binding factor 1 (AUF1) and K-homology splicing regulator protein (KSRP), bind the ARE in the 3′ UTR of Pth mRNA (43, 44). AUF1 increases Pth mRNA half-life, whereas KSRP has the opposite effect (43, 44). Both proteins are regulated by changes in serum Ca2+ and Pi concentrations and are altered by CKD (43, 45).
Figure 2. Processing of Pth mRNA.
Murine Pth mRNA is bound by ARE-BPs, which either stabilize or destabilize Pth mRNA, thereby altering Pth mRNA half-life. The ratio of activities of stabilizing/destabilizing ARE-BPs bound to Pth mRNA determines the half-life of a given Pth mRNA molecule. KSRP is a Pth mRNA–destabilizing ARE-BP that is active in its dephosphorylated state. In their new study in this issue of the JCI, Nechama et al. (46) report that the peptidyl-prolyl isomerase Pin1 is responsible for the dephosphorylation of KSRP. In CKD, Pin1 activity is reduced, and as a result, less dephosphorylated (active) KSRP is available. As a consequence, a stabilizing ARE-BP, AUF1, is active and Pth mRNA is degraded to a lesser extent, resulting in higher intracellular Pth mRNA levels, more PTH synthesis, and secondary hyperparathyroidism. P, phosphate.
Pin1, a peptidyl-prolyl isomerase, alters KSRP phosphorylation and binding of KSRP to the Pth ARE
In the accompanying article, Nechama et al. have elegantly dissected the manner in which Pth mRNA is degraded via AREs and cognate ARE-BPs in secondary hyperparathyroidism in rodents and have shown a role for the enzyme peptidyl-prolyl cis/trans isomerase NIMA-interacting 1 (Pin1) in this process (46) (Figure 2). The authors demonstrate that the secondary hyperparathyroidism associated with CKD or Ca deficiency is due in part to reduced Pin1 activity in the PT glands. The reduction in Pin1 activity reduced the ratio of the ARE-BPs, KSRP, and AUF1, which normally exert opposite effects on Pth mRNA stability. As a result, Pth mRNA half-life and stability were increased due to unopposed AUF1 activity. The data suggest that it is possible to modulate Pth mRNA half-life and stability by altering the activity of Pin1 and by changing KSRP concentrations within the PT cell.
Pin1 is a peptidyl-prolyl isomerase that specifically binds serine/threonine-protein motifs and catalyzes the cis-trans isomerization of peptide bonds, thereby changing the activity of proteins (47, 48). Previous work from other laboratories has shown that Pin1 interacts with AUF1 and stabilizes GMCSF and TGFB mRNAs (49, 50). Nechama et al. (46) hypothesized that Pin1 might also alter Pth mRNA stability and play a role in the pathogenesis of secondary hyperparathyroidism seen in CKD. They showed the presence of Pin1 epitopes and Pin1 enzymatic activity in PT glands and PT extracts. Induction of secondary hyperparathyroidism by feeding rats a diet low in Ca or by inducing CKD with adenine reduced Pin1 activity. Reduced Pin1 activity correlated with increased Pth mRNA levels in the PT glands of such animals. Inhibition of Pin1 activity with the inhibitor juglone increased Pth mRNA levels. The increase in Pth mRNA levels in juglone-treated PT was posttranscriptional, since nuclear run-on experiments revealed no changes in the transcription rate of Pth. In a surrogate cell line, HEK293 (a PT cell line is not available for transfection experiments), Pin1 overexpression accelerated Pth mRNA decay, whereas Pin1 knockdown with siRNA decreased Pth mRNA decay. Also, Pin1 overexpression was without effect on the half-life of a Pth transcript lacking the ARE-containing Pth 3′ UTR. Both the protein-interaction domain and the peptidyl-prolyl cis-trans isomerization domains of Pin1 were necessary for the effect of this protein on Pth mRNA stability. Interestingly, when the 63-nt Pth ARE was introduced into the 3′ UTR of a growth hormone reporter gene (GH63nt), Pin1 overexpression decreased chimeric GH mRNA levels. Treatment with juglone of cells transfected with such a construct decreased Pin1 activity and increased GH63-nt mRNA levels. Pin1 bound KSRP, an ARE-BP that increases the degradation of Pth mRNA. In cells transfected with a chimeric GH63-nt reporter containing the Pth 63-nt ARE, KSRP overexpression decreased GH63-nt mRNA levels. Conversely, KSRP depletion increased GH63-nt mRNA levels in the presence of Pin1 expression. In such cells, Pin1 inhibition prevented KSRP-mediated decreases in GH63-nt mRNA. The authors also demonstrated that inhibition of Pin1 by juglone in PT glands in vivo reduced KSRP binding to Pth mRNA, thus increasing Pth mRNA half-life. Nechama et al. showed that Pin1 prevents the phosphorylation of KSRP at serine residue 181 and that a mutant KSRP (S181A) that is incapable of being phosphorylated had increased activity. Pin1-knockout mice had increased PT gland PTH levels and circulating serum PTH concentrations without changes in serum Ca and Pi levels.
In summary, the data reported in this issue by Nechama et al. (46) are consistent with a biological role for Pin1 in the pathogenesis of secondary hyperparathyroidism in rat PT via regulation of the amount of active, nonphosphorylated KSRP in PT cells. Several additional areas of investigation remain to be explored. For example, it is unclear what triggers the reduction in Pin1 activity in the parathyroids in chronic renal failure and Ca deficiency. Future studies might be directed at identifying factors that regulate Pin1 activity and expression in the PT gland. Precise quantification of KSRP/AUF1 ratios in the PT gland in different conditions would also be of value. The development of PT gland–specific modulators of ARE-BPs might result in drugs that are effective for the control of secondary hyperparathyroidism and PT hyperplasia. Stay tuned for developments in this area.
Acknowledgments
The author’s laboratory is supported by NIH grants DK76829 and DK77669 and a grant from Genzyme (Genzyme Renal Innovations Program [GRIP]).
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 119:2887–2891 (2009). doi:10.1172/JCI40784
See the related article beginning on page 3102.
References
- 1.McKee A.E., Silver P.A. Systems perspectives on mRNA processing. Cell Res. . 2007;17:581–590. doi: 10.1038/cr.2007.54. [DOI] [PubMed] [Google Scholar]
- 2.Bentley D.L. Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr. Opin. Cell Biol. . 2005;17:251–256. doi: 10.1016/j.ceb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Blencowe B.J. Alternative splicing: new insights from global analyses. Cell. 2006;126:37–47. doi: 10.1016/j.cell.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Kornblihtt A.R., de la Mata M., Fededa J.P., Munoz M.J., Nogues G. Multiple links between transcription and splicing. RNA. 2004;10:1489–1498. doi: 10.1261/rna.7100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hieronymus H., Silver P.A. A systems view of mRNP biology. Genes Dev. . 2004;18:2845–2860. doi: 10.1101/gad.1256904. [DOI] [PubMed] [Google Scholar]
- 6.Moore M.J. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Diaz P., Penalva L.O. Post-transcription meets post-genomic: the saga of RNA binding proteins in a new era. RNA Biol. 2006;3:101–109. doi: 10.4161/rna.3.3.3373. [DOI] [PubMed] [Google Scholar]
- 8.Singh R., Valcarcel J. Building specificity with nonspecific RNA-binding proteins. Nat. Struct. Mol. Biol. . 2005;12:645–653. doi: 10.1038/nsmb961. [DOI] [PubMed] [Google Scholar]
- 9.Barreau C., Paillard L., Osborne H.B. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 2005;33:7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misquitta C.M., Chen T., Grover A.K. Control of protein expression through mRNA stability in calcium signalling. Cell Calcium. 2006;40:329–346. doi: 10.1016/j.ceca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Schiavi S.C., Belasco J.G., Greenberg M.E. Regulation of proto-oncogene mRNA stability. Biochim. Biophys. Acta. . 1992;1114:95–106. doi: 10.1016/0304-419x(92)90009-n. [DOI] [PubMed] [Google Scholar]
- 12.Schiavi S.C., et al. Multiple elements in the c-fos protein-coding region facilitate mRNA deadenylation and decay by a mechanism coupled to translation. J. Biol. Chem. 1994;269:3441–3448. [PubMed] [Google Scholar]
- 13.Chen C.Y., Shyu A.B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 1995;20:465–470. doi: 10.1016/S0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 14.Lebreton A., Tomecki R., Dziembowski A., Seraphin B. Endonucleolytic RNA cleavage by a eukaryotic exosome. Nature. 2008;456:993–996. doi: 10.1038/nature07480. [DOI] [PubMed] [Google Scholar]
- 15.McPheeters D.S., et al. A complex gene regulatory mechanism that operates at the nexus of multiple RNA processing decisions. Nat. Struct. Mol. Biol. . 2009;16:255–264. doi: 10.1038/nsmb.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schilders G., Pruijn G.J. Biochemical studies of the mammalian exosome with intact cells. Methods Enzymol. 2008;448:211–226. doi: 10.1016/S0076-6879(08)02611-6. [DOI] [PubMed] [Google Scholar]
- 17.Habener J.F., Rosenblatt M., Potts J.T., Jr. Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol. Rev. 1984;64:985–1053. doi: 10.1152/physrev.1984.64.3.985. [DOI] [PubMed] [Google Scholar]
- 18.Potts J.T. Parathyroid hormone: past and present. J. Endocrinol. . 2005;187:311–325. doi: 10.1677/joe.1.06057. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R. Metabolism of 1,25-dihydroxyvitamin D3. Physiol. Rev. 1984;64:478–504. doi: 10.1152/physrev.1984.64.2.478. [DOI] [PubMed] [Google Scholar]
- 20.DeLuca H.F., Schnoes H.K. Vitamin D: recent advances. Annu. Rev. Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- 21.Gesek F.A., Friedman P.A. On the mechanism of parathyroid hormone stimulation of calcium uptake by mouse distal convoluted tubule cells. J. Clin. Invest. 1992;90:749–758. doi: 10.1172/JCI115947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofer A.M., Brown E.M. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. . 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 23.Fox J., Heath H., 3rd. The “calcium clamp”: effect of constant hypocalcemia on parathyroid hormone secretion. Am. J. Physiol. 1981;240:E649–E655. doi: 10.1152/ajpendo.1981.240.6.E649. [DOI] [PubMed] [Google Scholar]
- 24.Naveh-Many T., Silver J. Regulation of parathyroid hormone gene expression by hypocalcemia, hypercalcemia, and vitamin D in the rat. . J. Clin. Invest. 1990;86:1313–1319. doi: 10.1172/JCI114840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naveh-Many T., Friedlaender M.M., Mayer H., Silver J. Calcium regulates parathyroid hormone messenger ribonucleic acid (mRNA), but not calcitonin mRNA in vivo in the rat. Dominant role of 1,25-dihydroxyvitamin D. Endocrinology. 1989;125:275–280. doi: 10.1210/endo-125-1-275. [DOI] [PubMed] [Google Scholar]
- 26.Audran M., Gross M., Kumar R. The physiology of the vitamin D endocrine system. Semin. Nephrol. 1986;6:4–20. [PubMed] [Google Scholar]
- 27.Audran M., Kumar R. The physiology and pathophysiology of vitamin D. Mayo Clin. Proc. 1985;60:851–866. doi: 10.1016/s0025-6196(12)64791-0. [DOI] [PubMed] [Google Scholar]
- 28.Cozzolino M., et al. Pathogenesis of parathyroid hyperplasia in renal failure. J. Nephrol. 2005;18:5–8. [PubMed] [Google Scholar]
- 29.Dusso A.S., et al. Molecular basis of parathyroid hyperplasia. J. Ren. Nutr. 2007;17:45–47. doi: 10.1053/j.jrn.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Dusso A.S., et al. Pathogenic mechanisms for parathyroid hyperplasia. Kidney Int. Suppl. . 2006;102:S8–S11. doi: 10.1038/sj.ki.5001595. [DOI] [PubMed] [Google Scholar]
- 31.Danese M.D., et al. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am. J. Kidney Dis. 2006;47:149–156. doi: 10.1053/j.ajkd.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Jadoul M., et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. . 2006;70:1358–1366. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 33.Rudser K.D., de Boer I.H., Dooley A., Young B., Kestenbaum B. Fracture risk after parathyroidectomy among chronic hemodialysis patients. J. Am. Soc. Nephrol. 2007;18:2401–2407. doi: 10.1681/ASN.2007010022. [DOI] [PubMed] [Google Scholar]
- 34.Block G.A., et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 35.Block G.A., et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N. Engl. J. Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 36.Drueke T.B. Calcimimetics versus vitamin D: what are their relative roles? Blood Purif. 2004;22:38–43. doi: 10.1159/000074922. [DOI] [PubMed] [Google Scholar]
- 37.Locatelli F., et al. Management of disturbances of calcium and phosphate metabolism in chronic renal insufficiency, with emphasis on the control of hyperphosphataemia. Nephrol. Dial. Transplant. 2002;17:723–731. doi: 10.1093/ndt/17.5.723. [DOI] [PubMed] [Google Scholar]
- 38.Moe S.M., Drueke T.B. Management of secondary hyperparathyroidism: the importance and the challenge of controlling parathyroid hormone levels without elevating calcium, phosphorus, and calcium-phosphorus product. Am. J. Nephrol. 2003;23:369–379. doi: 10.1159/000073945. [DOI] [PubMed] [Google Scholar]
- 39.Moallem E., Kilav R., Silver J., Naveh-Many T. RNA-Protein binding and post-transcriptional regulation of parathyroid hormone gene expression by calcium and phosphate. J. Biol. Chem. 1998;273:5253–5259. doi: 10.1074/jbc.273.9.5253. [DOI] [PubMed] [Google Scholar]
- 40.Levi R., et al. Increased parathyroid hormone gene expression in secondary hyperparathyroidism of experimental uremia is reversed by calcimimetics: correlation with posttranslational modification of the trans acting factor AUF1. J. Am. Soc. Nephrol. 2006;17:107–112. doi: 10.1681/ASN.2005070679. [DOI] [PubMed] [Google Scholar]
- 41.Naveh-Many T., Sela-Brown A., Silver J. Protein-RNA interactions in the regulation of PTH gene expression by calcium and phosphate. Nephrol. Dial. Transplant. 1999;14:811–813. doi: 10.1093/ndt/14.4.811. [DOI] [PubMed] [Google Scholar]
- 42.Kilav R., Bell O., Le S.Y., Silver J., Naveh-Many T. The parathyroid hormone mRNA 3’-untranslated region AU-rich element is an unstructured functional element. J. Biol. Chem. 2004;279:2109–2116. doi: 10.1074/jbc.M305302200. [DOI] [PubMed] [Google Scholar]
- 43.Naveh-Many T., Bell O., Silver J., Kilav R. Cis and trans acting factors in the regulation of parathyroid hormone (PTH) mRNA stability by calcium and phosphate. FEBS Lett. 2002;529:60–64. doi: 10.1016/S0014-5793(02)03259-3. [DOI] [PubMed] [Google Scholar]
- 44.Nechama M., Ben-Dov I.Z., Briata P., Gherzi R., Naveh-Many T. The mRNA decay promoting factor K-homology splicing regulator protein post-transcriptionally determines parathyroid hormone mRNA levels. FASEB J. 2008;22:3458–3468. doi: 10.1096/fj.08-107250. [DOI] [PubMed] [Google Scholar]
- 45.Nechama M., Ben-Dov I.Z., Silver J., Naveh-Many T. Regulation of PTH mRNA stability by the calcimimetic R568 and the phosphorus binder lanthanum carbonate in CKD. Am. J. Physiol. Renal Physiol. 2009;296:F795–F800. doi: 10.1152/ajprenal.90625.2008. [DOI] [PubMed] [Google Scholar]
- 46.Nechama M., Uchida T., Mor Yosef-Levi I., Silver J., Naveh-Many T. The peptidyl-prolyl isomerase Pin1 determines parathyroid hormone mRNA levels and stability in rat models of secondary hyperparathyroidism. J. Clin. Invest. 2009;119:3102–3114. doi: 10.1172/JCI39522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu K.P., Liou Y.C., Zhou X.Z. Pinning down proline-directed phosphorylation signaling. Trends Cell Biol. 2002;12:164–172. doi: 10.1016/S0962-8924(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 48.Lu K.P., Zhou X.Z. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat. Rev. Mol. Cell Biol. . 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 49.Shen Z.J., Esnault S., Malter J.S. The peptidyl-prolyl isomerase Pin1 regulates the stability of granulocyte-macrophage colony-stimulating factor mRNA in activated eosinophils. Nat. Immunol. 2005;6:1280–1287. doi: 10.1038/ni1266. [DOI] [PubMed] [Google Scholar]
- 50.Shen Z.J., et al. Pin1 regulates TGF-beta1 production by activated human and murine eosinophils and contributes to allergic lung fibrosis. . J. Clin. Invest. 2008;118:479–490. doi: 10.1172/JCI32789. [DOI] [PMC free article] [PubMed] [Google Scholar]