Abstract
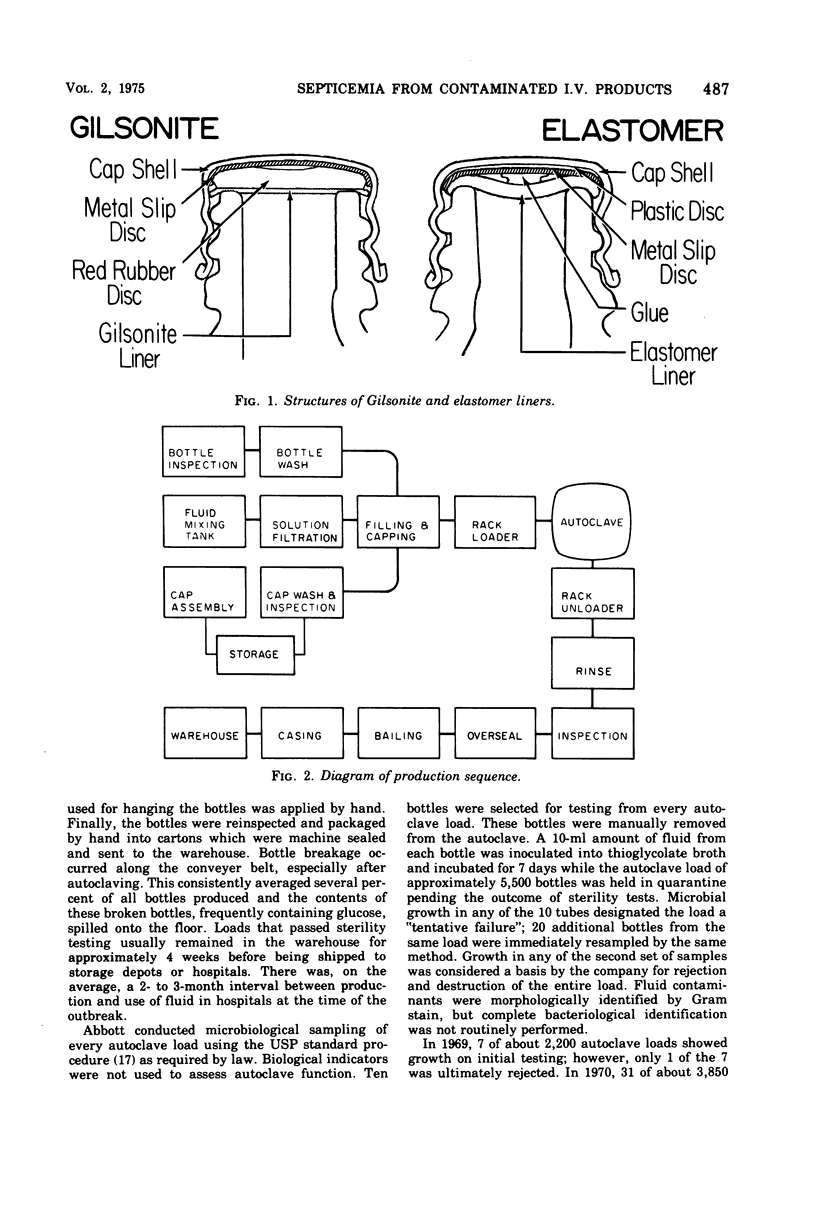
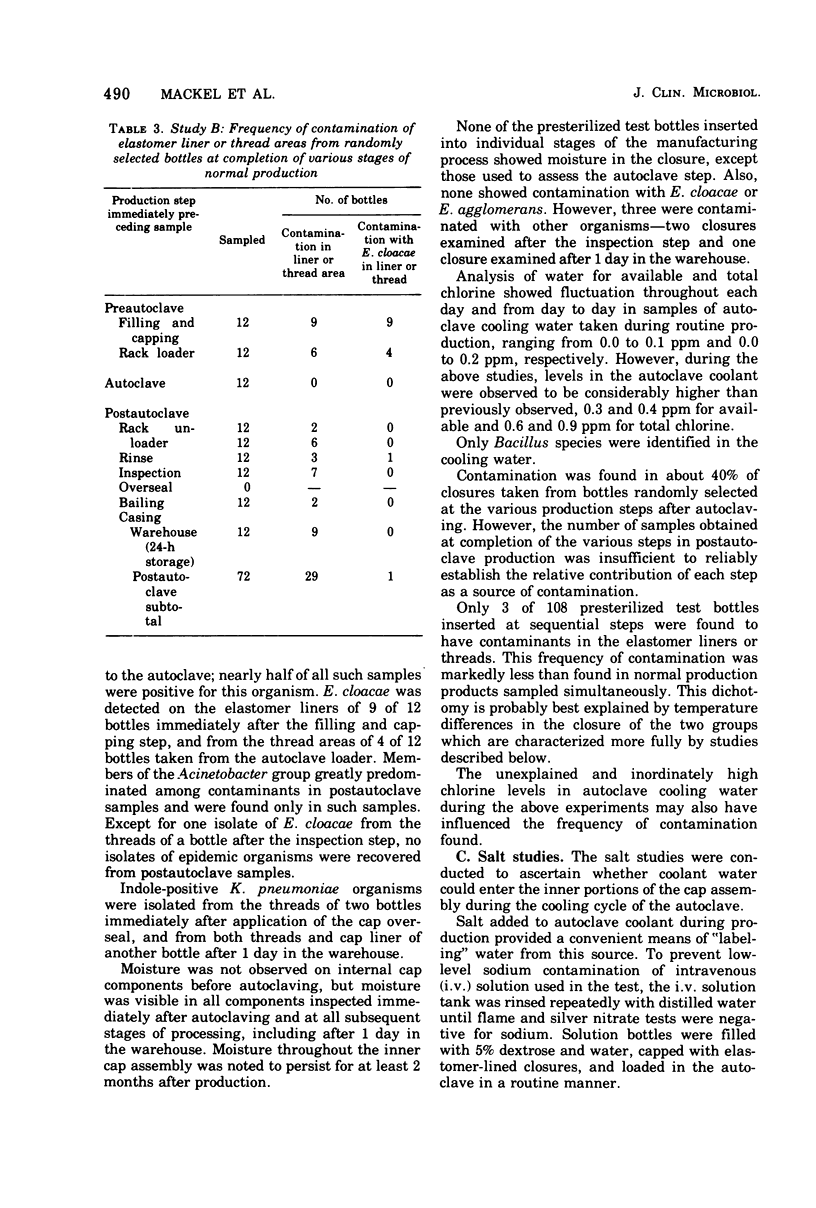
Between 1 July 1970 and April 1971, in many hospitals in this country, there were outbreaks of nosocomial septicemia caused by Enterobacter cloacae of E. agglomerans (formerly Erwinia, herbicola-lathyri). All of these hospitals used infusion products manufactured by one company, Abbott Laboratories, and all affected patients had onset of septicemia while receiving the company's infusion products. Septicemia was epidemiologically and microbiologically traced to intrinsic contamination of the company's screw-cap closure for infusion bottles which was sealed with a newly introduced elastomer liner. Epidemic organisms were isolated from these closures. Investigations both in the laboratory and in the manufacturing plant into the mechanism of contamination of these products revealed the following. (i) Epidemic strains were present in numerous areas throughout the manufacturing plants. (ii) Viable microorganisms gained access to the interior of screw-cap closures after the autoclave step of production. (iii) Cooling closures actively drew moisture through the thread interstices into the inner-most depths of the closure. (iv) Transfer of contaminants from closures to fluid was easily effected by simple manipulations duplicating normal in-hospital use. (v) The red-rubber liner used in the company's screw-cap closures before the introduction of elastomer contained a broad-spectrum antimicrobial inhibitor. The findings from this epidemic and the associated studies show that the screw-cap closure as it is now designed cannot be considered secure for products that must remain sterile.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bourne H. G., Yee H. T., Seferian S. The toxicity of rubber additives. Findings from a survey of 140 plants in Ohio. Arch Environ Health. 1968 May;16(5):700–706. doi: 10.1080/00039896.1968.10665130. [DOI] [PubMed] [Google Scholar]
- Duma R. J., Warner J. F., Dalton H. P. Septicemia from intravenous infusions. N Engl J Med. 1971 Feb 4;284(5):257–260. doi: 10.1056/NEJM197102042840508. [DOI] [PubMed] [Google Scholar]
- GARVAN J. M., GUNNER B. W. THE HARMFUL EFFECTS OF PARTICLES IN INTRAVENOUS FLUIDS. Med J Aust. 1964 Jul 4;2:1–6. doi: 10.5694/j.1326-5377.1964.tb114892.x. [DOI] [PubMed] [Google Scholar]
- Maki D. G., Martin W. T. Nationwide epidemic of septicemia caused by contaminated infusion products. IV. Growth of microbial pathogens in fluids for intravenous infusions. J Infect Dis. 1975 Mar;131(3):267–272. doi: 10.1093/infdis/131.3.267. [DOI] [PubMed] [Google Scholar]
- Phillips I., Eykyn S., Laker M. Outbreak of hospital infection caused by contaminated autoclaved fluids. Lancet. 1972 Jun 10;1(7763):1258–1260. doi: 10.1016/s0140-6736(72)90981-6. [DOI] [PubMed] [Google Scholar]
- Sack R. A. Epidemic of gram-negative organism septicemia subsequent to elective operation. Am J Obstet Gynecol. 1970 Jun 1;107(3):394–399. doi: 10.1016/0002-9378(70)90565-x. [DOI] [PubMed] [Google Scholar]


