Abstract
The role of B cells and autoimmunity as contributing factors to poor neurological outcomes following spinal cord injury (SCI) is poorly understood. The study by Ankeny et al., in this issue of the JCI, identifies a new immunopathological mechanism arising after SCI in mice (see the related article beginning on page 2990). The study shows that B cells produce pathogenic antibodies that impair lesion repair, resulting in worse neurological outcome. This new understanding of SCI disease pathogenesis, if confirmed in humans, reveals potential avenues for the development of novel neuroprotective immunotherapies.
Primary trauma to the CNS initiates a series of interrelated responses, including edema, excitotoxicity, and inflammation, that lead to secondary injury, resulting in further expansion of the initial lesion and additional loss of neurological function. The treatment of neuroinflammation in the context of both traumatic brain injury and spinal cord injury (SCI) still lacks a standard, universally accepted therapy that leads to improved neurological outcomes. There exists a clear, unmet medical need for an effective antiinflammatory treatment for the acute and chronic stages of traumatic brain injury and SCI arising in general civilian as well in injured military personnel populations. Currently, an important area of SCI research focuses on understanding the dual nature of post-SCI neuroinflammation. It can lead to increased damage to the neural tissue, yet it is an essential part of the wound healing process (1, 2). It is becoming increasingly clear that injury to the CNS results in alterations to the systemic immune system that in turn affect the competing pathogenic and wound healing processes evolving at the site of injury (3–5). The study reported by Ankeny et al. in this issue of the JCI (6) offers a new perspective on a poorly understood aspect of CNS inflammation in response to traumatic injury in mice, namely the impact of B cells and autoimmunity on neurological outcomes. This new understanding of SCI disease pathogenesis, if confirmed in humans, reveals avenues for the development of novel neuroprotective immunotherapies.
B cells in the normal and diseased CNS
B lymphocytes are a key component of the adaptive immune system. They arise from bone marrow hematopoietic stem cells that differentiate in an antigen-independent manner to the immature B cell stage. In the presence of antigen, further differentiation leads to mature and activated B cells. Activated B cells convert into short-lived, antibody-secreting plasma cells or into memory B cells and then into long-lived, antibody-secreting plasma cells (7, 8). Activated B cells and long-lived plasma cells migrate not only to the bone marrow and secondary lymphoid organs but to the CNS via normal homeostatic processes (7). This B cell recruitment mechanism is upregulated during CNS autoimmune diseases, such as MS (7, 9). There are several B cell–specific factors and receptor interactions that are critical to B cell function and are potential therapeutic targets. B cell–activating factor (BAFF), lymphotoxin-β, and a proliferation-inducing ligand (APRIL) have roles important to B cell survival, differentiation, germinal center formation, and antibody synthesis (7, 8). These factors are secreted by macrophages and, within the CNS, by astrocytes (7, 8). Thus, B cells have an established mechanism that allows them to traffic to and be supported in the CNS. The normally assumed role of B cells is to produce antibodies, but it is now clear that B cells can serve as potent regulatory and antigen-presenting cells (8, 10). It is well known that under normal circumstances human cerebrospinal fluid (CSF) harbors low levels of antibody, produced by long-lived plasma cells, some of which are autoreactive (11). The role of B cells in various CNS autoimmune conditions is also well known. However, until recently the role of B cells was deemed secondary to that of T cells in disease pathogenesis. There is now clear evidence that B cells and associated autoantibodies can play an important primary role in CNS autoimmune disease (8, 12).
SCI leads to pathogenic autoantibody production
The results presented in this issue by Ankeny et al. (6) clearly demonstrate that, in a mouse model of SCI, trauma of moderate severity at thoracic level 9 (T9) leads to a surprisingly robust B cell response that produces pathogenic antibodies. This important conclusion is supported by experiments demonstrating that spontaneous neurological recovery after injury was greatly improved in B cell–knockout mice compared with WT mice. Following SCI, coordinated stepping involving all four limbs was achieved in 88% of B cell–knockout mice but in only 35% of WT mice at the end of the nine-week observation period. Consistent with this improved functional recovery, the neuropathology observed in the B cell–knockout mice was also markedly less pronounced compared with WT animals. This suggests that, in WT mice that received an SCI, B cells play a role in the evolving inflammatory response that impedes neurological recovery. Importantly, passive transfer (injection) of purified pathogenic antibody into the spinal cord of WT mice under sterile conditions induced a similar type of neurotoxicity to that observed in mice with SCI. This confirmed that neurotoxic product of the SCI-induced B cell activation was likely pathogenic antibodies.
This article (6) raises the question as to why B cells produce pathogenic antibodies when the SCI is in the lower half of the spinal cord (T9–T10). Yet, these same authors previously reported that when the SCI occurs at a higher level (T4–T5) profound immune suppression occurs, including that of B cell function (13). A likely explanation may lie in the fact that a high SCI disrupts the cholinergic antiinflammatory pathway by removing the sympathetic contribution (via the splenic nerves) due to injury to the intermediolateral column sympathetic fibers. This pathway plays a key role in regulating systemic inflammation (4). Further investigation is required to understand the underlying pathological mechanisms created by the loss of sympathetic control that results in immune suppression and whether this is a permanent feature of SCI located above T4/T5. Alternatively, the appearance of autoimmune neuropathogenic antibodies may only be delayed.
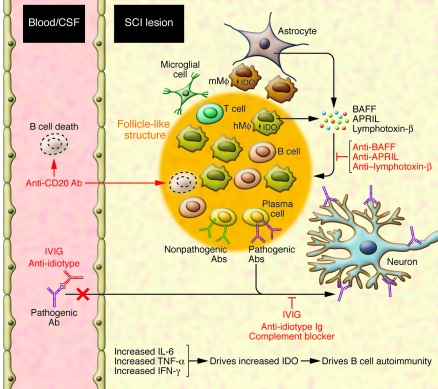
The presence of pathogenic antibodies in the spinal lesion is in part derived from systemic sources via a compromised blood–spinal cord barrier after injury. However, evidence was presented by Ankeny et al. (6) that B cell “follicle-like” structures were present in the lesion area, suggesting that local antibody production may be critical to the pathogenic outcome. Both activated B cells and plasma cells are present in these structures. The presence of B cell follicle-like structures in the diseased CNS is not unique to SCI but has been described in other CNS autoimmune diseases, including MS (9). The local SCI inflammatory environment likely would support the development of B cell follicle-like structures in two ways. Infiltrating monocyte/macrophages as well as the large number of proliferating astrocytes responding to the SCI likely could supply sufficient BAFF and APRIL to support B cell survival, proliferation, differentiation as well as follicle development (Figure 1). Secondly, SCI-induced neuroinflammation dysregulates an important immune regulatory mechanism known as the tryptophan–kynurenine pathway (14–17). The critical component of this tryptophan catabolic pathway is the rate-limiting enzyme, indoleamine 2,3-dioxygenase (IDO), which is primarily induced by IFN-γ but can be superinduced in the presence of TNF-α (14, 15). Both cytokines are present following SCI. IDO is a key regulator of tolerance and a preventer of autoimmunity (18, 19). IDO is expressed by activated microglia and infiltrating leukocytes (14). T cells, but not B cells, are particularly sensitive to the effects of IDO. The tryptophan metabolites generated by IDO, such as quinolinic acid, are well known neurotoxins and also render T cells, but not B cells, highly susceptible to apoptosis and other aspects of IDO-mediated regulation (4, 14, 17, 19). This alters the local cytokine environment to one that favors B cell autoimmunity and antibody production (17, 19) (Figure 1). Thus, within the SCI lesion, the local environment is conducive to the support of B cells and antibody production.
Figure 1. B cells and an autoimmune response contribute to poor neurological outcome following SCI.
After SCI, B cells, together with other leukocytes such as monocyte/macrophages (hMϕ) and T cells, infiltrate the lesion. Resident activated microglia, microglia-derived macrophages (mMϕ), and astrocytes, also surround and/or infiltrate the lesion. Infiltrating macrophages as well as proliferating astrocytes respond to the SCI by producing BAFF, lymphotoxin-β, and APRIL, which support B cell survival, proliferation, and differentiation as well as B cell follicle development. Follicle-like structures appear at the lesion area and house activated B cells and plasma cells. Additionally, the high levels of IFN-γ and TNF-α present at the SCI lesion will induce IDO expression, a key regulator of tolerance and a preventer of autoimmunity. IDO is expressed by activated microglia and infiltrating leukocytes. The toxic tryptophan metabolites generated by IDO render T cells but not B cells highly susceptible to apoptosis. This creates a cytokine environment that favors B cell autoimmunity and antibody production. Thus, within the SCI lesion, the local environment is conducive to the support of B cells and antibody production. Therapeutics such as blockers of BAFF, APRIL, or lymphotoxin-β; anti-CD20 monoclonal antibodies; and IVIG could prevent follicle-germinal center development, deplete B cells (but not plasma cells), and block the pathogenic activity of autoantibodies to neural antigens at the site of the SCI. These therapeutic agents could be used singly or in combination in order to obtain maximal benefit.
Potential new immunotherapies for SCI
The results reported by Ankeny et al. (6) suggest opportunities for the development of new immunotherapies for SCI. Therapies that target the B cell or block the affects of pathogenic antibodies have demonstrated considerable promise in early phase I/II clinical trials (7, 10, 20). However, there are no FDA-approved, licensed anti–B cell products yet available for CNS autoimmune indications. Plasmapheresis is one approach that might improve neurological outcomes in SCI patients, by removing pathogenic autoantibodies at crucial times in the early stages of recovery, leading to a potential neuroprotective benefit. Whether plasmapheresis would lead to sustained improved neurological outcomes for SCI patients needs to be determined. Intravenous immunoglobulin (IVIG) administration has the potential for significant neuroprotection. IVIG is known to block, via an anti-idiotype mechanism, the binding of pathogenic antibodies to their targets, and IVIG also blocks the complement system (20) (Figure 1). The results of Ankeny et al. (6) support the testing of IVIG for SCI treatment, as both autoantibody binding to spinal tissue and complement were demonstrated to be involved in SCI-associated autoantibody-induced pathology. IVIG therapy is generally well tolerated with few adverse side effects (20).
B cell depletion as a neuroprotective therapy is another viable option for SCI (Figure 1). Clinical trial results testing the beneficial affects of B cell depletion in various CNS autoimmune conditions support the approach, but with caution (7, 10, 12). Anti-CD20 antibody–mediated depletion of B cells is fast, effective, long-lasting, and largely free of adverse events in short-term applications (7, 10). Anti-CD20 does not block anamnestic antibody-mediated immune responses and is not immunosuppressive in that context (8). Long-term application of anti-CD20 therapy is still a concern (8) especially since SCI individuals are already immunosuppressed (3). For SCI, the real issue is whether anti-CD20 antibody–mediated B cell depletion will lead to improved outcomes. CD20 is expressed on early, mature, and activated B cells but not on antibody-secreting plasma cells (8). Consequently, long-term B cell depletion only has small to moderate affects on reducing plasma antibody levels and is not effective in eliminating B cells in follicles (8, 12). Pre-existing plasma cells that secret potentially pathogenic antibodies will not be eliminated by anti-CD20 therapy. It may be affective in preventing the appearance of de novo–created short-lived plasma cells by elimination of their precursors (7, 8). If antigen presentation or other B cell functions are critical to the induction of the SCI-induced pathogenic autoantibodies, then anti-CD20 therapy will likely be effective. These are important issues that need to be resolved. Alternatively, there are monoclonal antibody therapies being developed to block the actions of BAFF, lymphotoxin-β, and APRIL (8). Providing sufficient levels of neutralizing antibody could be delivered to target areas, preventing the development of B cell follicles in the SCI lesion area and in secondary lymphoid tissues may reduce the levels of autoantibody, such that the pathogenic consequences are minimal.
It remains to be determined whether pathogenic autoantibodies are a primary cause of human secondary SCI. However, there is now sufficient evidence to support the development of strategies, in appropriate animal models, to test whether potential immunotherapies restricting B cell responses following SCI warrant translation to the treatment of human SCI.
Acknowledgments
We thank Lynne C. Weaver for her critical review of the literature and Ilda Moniz for her assistance.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 119:2881–2884 (2009). doi:10.1172/JCI40839
See the related article beginning on page 2990.
References
- 1.Rossignol S., Schwab M., Schwartz M., Fehlings M.G. Spinal cord injury: time to move? J. Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popovich P.G., Longbrake E.E. Can the immune system be harnessed to repair the CNS? Nat. Rev. Neurosci. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- 3.Riegger T., et al. Immune depression syndrome following human spinal cord injury (SCI): a pilot study. Neuroscience. 2009;158:1194–1199. doi: 10.1016/j.neuroscience.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Rosas-Ballina M., Tracey K.J. Cholinergic control of inflammation. J. Intern. Med. 2009;265:663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J., et al. Systemic inflammatory response following acute traumatic brain injury. Front. Biosci. 2009;14:3795–3813. doi: 10.2741/3489. [DOI] [PubMed] [Google Scholar]
- 6.Ankeny D.P., Guan Z., Popovich P.G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalakas M.C. B cells as therapeutic targets in autoimmune neurological disorders. Nat. Clin. Pract. Neurol. 2008;4:557–567. doi: 10.1038/ncpneuro0901. [DOI] [PubMed] [Google Scholar]
- 8.Dalakas M.C. Invited article: inhibition of B cell functions: implications for neurology. Neurology. 2008;70:2252–2260. doi: 10.1212/01.wnl.0000313840.27060.bf. [DOI] [PubMed] [Google Scholar]
- 9.Corcione A., et al. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11064–11069. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waubant E. Spotlight on anti-CD20. Int. MS J. 2008;15:19–25. [PubMed] [Google Scholar]
- 11.Elkon K., Casali P. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 2008;4:491–498. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsushita T., Tedder T.F. B-lymphocyte depletion for the treatment of multiple sclerosis: now things really get interesting. Expert Rev. Neurother. 2009;9:309–312. doi: 10.1586/14737175.9.3.309. [DOI] [PubMed] [Google Scholar]
- 13.Lucin K.M., Sanders V.M., Jones T.B., Malarkey W.B., Popovich P.G. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp. Neurol. 2007;207:75–84. doi: 10.1016/j.expneurol.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwidzinski E., Bechmann I. IDO expression in the brain: a double-edged sword. J. Mol. Med. 2007;85:1351–1359. doi: 10.1007/s00109-007-0229-7. [DOI] [PubMed] [Google Scholar]
- 15.Oxenkrug G.F. Genetic and hormonal regulation of tryptophan kynurenine metabolism: implications for vascular cognitive impairment, major depressive disorder, and aging. Ann. N. Y. Acad. Sci. 2007;1122:35–49. doi: 10.1196/annals.1403.003. [DOI] [PubMed] [Google Scholar]
- 16.Kwidzinski E., et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 17.Xu H., Zhang G.X., Ciric B., Rostami A. IDO: a double-edged sword for T(H)1/T(H)2 regulation. Immunol. Lett. 2008;121:1–6. doi: 10.1016/j.imlet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellor A.L., Munn D.H. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat. Rev. Immunol. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 19.Scott G.N., et al. The immunoregulatory enzyme IDO paradoxically drives B cell-mediated autoimmunity. J. Immunol. 2009;182:7509–7517. doi: 10.4049/jimmunol.0804328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold R., Stangel M., Dalakas M.C. Drug insight: the use of intravenous immunoglobulin in neurology--therapeutic considerations and practical issues. Nat. Clin. Pract. Neurol. 2007;3:36–44. doi: 10.1038/ncpneuro0376. [DOI] [PubMed] [Google Scholar]