Abstract
Objectives
To assess the incidence of gastrointestinal haemorrhage associated with long term aspirin therapy and to determine the effect of dose reduction and formulation on the incidence of such haemorrhage.
Design
Meta-analysis of 24 randomised controlled trials (almost 66 000 participants).
Intervention
Aspirin compared with placebo or no treatment, for a minimum of one year.
Main outcome measures
Incidence of gastrointestinal haemorrhage.
Results
Gastrointestinal haemorrhage occurred in 2.47% of patients taking aspirin compared with 1.42% taking placebo (odds ratio 1.68; 95% confidence interval 1.51 to 1.88); the number needed to harm was 106 (82 to 140) based on an average of 28 months' therapy. At doses below 163 mg/day, gastrointestinal haemorrhage occurred in 2.30% of patients taking aspirin compared with 1.45% taking placebo (1.59; 1.40 to 1.81). Meta-regression showed no relation between gastrointestinal haemorrhage and dose. For modified release formulations of aspirin the odds ratio was 1.93 (1.15 to 3.23).
Conclusions
Long term therapy with aspirin is associated with a significant increase in the incidence of gastrointestinal haemorrhage. No evidence exists that reducing the dose or using modified release formulations would reduce the incidence of gastrointestinal haemorrhage.
Introduction
The use of aspirin in the prevention of cardiovascular disease is now well established; an estimated 50 million Americans have started taking aspirin over the past two decades.1 However, aspirin causes haemorrhagic complications. A systematic review in 1993 showed that the risk of gastrointestinal haemorrhage was significantly increased by long term aspirin.2 Only four of the 21 trials included in that review, however, used doses below 300 mg a day. Since then, new data have become available from eight studies involving 24 964 patients taking aspirin doses of 50-162.5 mg a day.
Recent trends towards the use of lower doses have been driven by the belief that these offer a better safety profile while retaining equivalent therapeutic efficacy. Is there evidence that the risk of gastrointestinal haemorrhage is substantially reduced at lower doses, and if so, by how much? Expensive “modified release” formulations have been developed in an attempt to reduce the likelihood of adverse gastrointestinal effects. What is the evidence that they do so?
We reviewed the safety of aspirin, studying the effect of dose and formulation and incorporating the new data from the eight studies mentioned above.
Methods
The review was conducted using a defined protocol.
Inclusion criteria
Studies—We included reports if they were full journal publications of randomised controlled trials of aspirin used as an antiplatelet agent. Trials were excluded if the term “randomised” was not specifically mentioned in the report or if the investigators clearly used non-random allocation, such as by date of birth. Abstracts, review articles, case reports, clinical observations, and unpublished data were not included. Trials with fewer than 50 patients in each arm were not included in the analysis because they were unlikely to be able to detect uncommon or rare adverse effects.3 We did not include studies designed to assess the effects of aspirin in special groups—such as pregnant women, children, and patients with pre-existing platelet disorders.
Intervention—We included trials in which patients in one treatment arm were allocated to oral aspirin alone and patients in the control arm to either placebo or no treatment, provided that the scheduled duration of treatment was at least 12 months. Crossover studies and those in which aspirin was used in conjunction with other antiplatelet agents or anticoagulants were excluded. Trials that compared aspirin at different doses or with other antiplatelet agents or anticoagulants, without a placebo or “no treatment” control arm, were also excluded.
Outcome measures—Only trials that provided numerical data on all gastrointestinal haemorrhages in both the treated and the control group were included. If specific terms were used to describe bleeding complications, we accepted data on patients with “haematemesis” or “melaena,” or with both of these, but not with “proctorrhagia.” We did not use data from trials that reported only on selected categories of gastrointestinal haemorrhage—for example, major bleeds and patients needing admission to hospital or blood transfusion—as trials varied considerably in their definition and reporting of such categories.4
Search strategy
The search was performed separately by each author. Relevant trials were identified through a combination of electronic searching and manual checking of reference lists from previous review papers and retrieved trials. We applied no language restrictions.
We concentrated specifically on selecting trials from a detailed list of over 200 antiplatelet studies identified in a systematic review that included trials published up to 1993.5 In view of the comprehensive search strategies used in that review, we chose not to repeat an electronic search for most of this period but carried out a search of Medline and Embase for 1990-9 to identify new trials and to provide a three year overlap. We found no discrepancy between the two lists of the trials identified in the overlap period.
We used a sensitive search string for randomised controlled trials, based on that of the Cochrane Collaboration,6 in combination with the following free text terms: “aspirin” or “acetylsalicylic*” or “salicylic*”. This gave a yield of over 7000 hits on Medline and over 10 000 hits on Embase for 1990-9. We made no attempt to limit the yield by introducing terms for adverse effects or haemorrhage as we knew that this strategy would lead to the loss of relevant trials.7
Appraisal of study quality and data abstraction
We did not anonymise the reports before assessment. All potentially relevant studies were checked independently by both reviewers to determine eligibility for inclusion and to extract data. A list of trials which were excluded is available from the authors.
We sought information on participants, blinding, type of control, assessment of compliance, and duration of treatment and follow up, in addition to data on the numbers of participants with and without gastrointestinal haemorrhage. We also recorded the formulation of aspirin used—modified release or standard. Any discrepancies were resolved by discussion.
Statistical analysis
Pooled odds ratios and heterogeneity were analysed using the RevMan program, version 4.04, with Meta View 4.0 and MetaView 3.01. We used the Peto fixed effects model to calculate the pooled odds ratios as this is the most appropriate model for rare events.8 Closely similar results were obtained with the random effects model. Meta-regression is a technique used to assess statistically whether specific factors (covariates) influence the magnitude of effect across studies. Random effects meta-regression was performed with the STATA “metareg” command.9
The number needed to harm (with 95% confidence intervals) was calculated by applying the calculated odds ratio to the pooled control event rate. In our review the number needed to harm is the estimated number of patients who need to be treated with aspirin—rather than with placebo or no treatment—for one additional patient to be harmed by a gastrointestinal haemorrhage.
Results
We identified 24 randomised controlled trials of aspirin that fulfilled our inclusion criteria (table). (A further table and the references for the 24 trialsw1-w25 are on the BMJ's website.) The 65 987 participants were predominantly male (74%) and middle aged. Doses of aspirin used were 50-1500 mg/day for a mean duration of 28 months. Indications for aspirin extended from primary prevention in “healthy” individuals to secondary prophylaxis after stroke. In all trials patients were excluded if they had a history of peptic ulcer, previous gastrointestinal haemorrhage, or any other contraindication to aspirin.
All 24 trials were double blind and placebo controlled. Fourteen trials had sufficient data to suggest that adequate concealment of allocation had taken place; in the other trials this was unclear. The number of patients lost to follow up was reported in 16 trials, only one of which failed to achieve over 90% follow up.
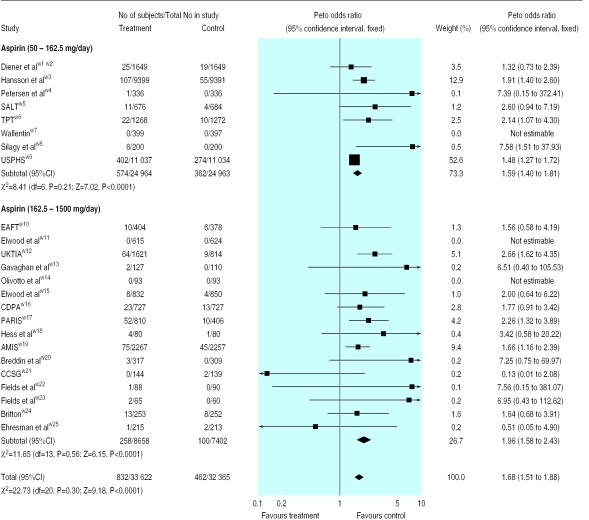
Figure 1 shows the results of our meta-analysis. Overall, gastrointestinal haemorrhage occurred in 2.47% of the patients taking aspirin compared with 1.42% of those taking placebo. The pooled odds ratio for gastrointestinal haemorrhage with aspirin was 1.68 (95% confidence interval 1.51 to 1.88; P<0.0001), and the number needed to harm based on an average of 28 months of aspirin was 106 (82 to 140). We found no evidence of significant heterogeneity between the trials.
Figure 1.
Peto odds ratio for gastrointestinal haemorrhage with aspirin. SALT=Swedish aspirin low dose trial; TPT=thrombosis prevention trial; USPHS=US physicians health study; EAFT=European atrial fibrillation trial; UK-TIA=UK transient ischaemic attack aspirin trial; CDPA=coronary drug project aspirin study; PARIS=persantine-aspirin reinfarction study; AMIS=aspirin myocardial infarction study; CCSG=Canadian Cooperative Study Group
Current clinical practice favours the use of lower doses of aspirin, and to increase the relevance of our findings we analysed separately the eight trials that used doses of 50-162.5 mg/day in 49 927 participants. Gastrointestinal haemorrhage occurred in 2.30% of those taking aspirin compared with 1.45% taking placebo. Even at these lower doses, aspirin was associated with a significantly increased rate of gastrointestinal haemorrhage compared with placebo, with a pooled odds ratio of 1.59 (1.40 to 1.81; P<0.0001).
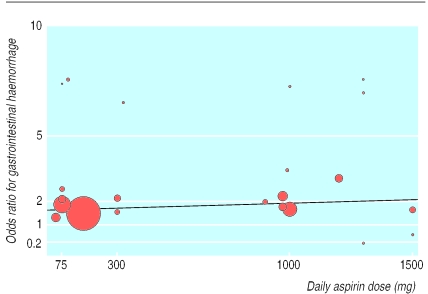
We performed meta-regression to test for a linear relation between daily dose of aspirin and risk of gastrointestinal haemorrhage (fig 2 ).The analysis gave a pooled odds ratio of 1.015 (0.984 to 1.047) per 100 mg dose reduction, with an estimated relative reduction in the incidence of gastrointestinal haemorrhage of 1.5% per 100 mg reduction of dose, but this was not significant (P=0.3).
What is already known on this topic
Aspirin is widely used to prevent and treat cardiovascular disease but carries an increased risk of gastrointestinal haemorrhage
Despite the lack of concrete evidence, lower doses and modified release formulations of aspirin have been used in the hope of reducing this risk
Data from several trials of low dose aspirin have become available recently
What this study adds
About 1 in 100 patients taking aspirin over a 28 month period will experience a gastrointestinal haemorrhage
No evidence exists that dose reduction or the use of modified release formulations significantly lowers the risk of gastrointestinal haemorrhage
Patients and doctors need to consider the trade-off between the benefits and harms of long term treatment with aspirin
Figure 2.
Meta-regression of Peto odds ratio for gastrointestinal haemorrhage against dose of aspirin (size of circle is proportional to size of trial)
Two of the trialsw3 w9 were much larger than the others, and to be sure that neither unduly influenced the results, we performed a sensitivity analysis. Omitting either or both trials from the meta-analysis did not significantly change our findings. Only five trials, with 4298 participants, specifically stated that modified release formulations of aspirin were used with daily doses of 75-1500 mg.w6 w14 w20 w24 w25 The odds ratio for gastrointestinal haemorrhage in these five trials was 1.93 (1.15 to 3.23).
Discussion
Long term aspirin therapy, even at a low dose, carries a risk of gastrointestinal haemorrhage, with a number needed to harm per year of 248. Although it would be preferable, for the purposes of comparison, to have a number needed to harm and a number needed to treat from the same trial or review, this may not always be feasible. It is possible, however, to obtain some idea of the trade-off between benefit and harm when using aspirin in patients with different cardiovascular risk levels by weighing up the pooled estimate of the number needed to harm against the number needed to treat from individual trials.
In the secondary prevention of stroke, for example, the number needed to treat per year with aspirin to prevent a further event was 106.w5 This means that if aspirin was used in patients with stroke who had similar baseline risks to those above, at least two recurrent strokes could be prevented at the cost of one gastrointestinal haemorrhage. The benefits of aspirin are less marked, however, in primary prevention of myocardial infarction—in the US physicians study, the number needed to treat per year was 555,w9 whereas the number needed to treat per year in hypertensive patients was 794.w3 Aspirin use in primary prevention could, depending on the baseline risks of the patients, cause two or three gastrointestinal haemorrhages for each myocardial infarction prevented.
As there are relatively few deaths after gastrointestinal haemorrhage (estimated death rate 12%10) compared with myocardial infarction, such a trade-off may be considered worth while. Doctors and patients involved in making decisions about aspirin therapy need to consider carefully whether the inconvenience of long term therapy and the associated risk of gastrointestinal haemorrhage are outweighed by the potential cardiovascular benefits. This is particularly so for those at low absolute risk of cardiovascular events (with correspondingly high numbers needed to treat), in whom the likelihood of harm is greater than that of therapeutic benefit (as shown in the example above for hypertensive patients).
Although doctors have hoped that changes in the dose or formulation of aspirin might reduce the problem of gastrointestinal haemorrhage, the results of this meta-analysis do not suggest that either approach offers clear benefits. Our findings are supported by those of two recent case-control studies, in which aspirin increased the risk of gastrointestinal haemorrhage, despite the use of low dose or modified release formulations.11,12 The results of our meta-regression are compatible with those of a large Dutch trial of transient ischaemic attack in which no significant difference in the rate of gastrointestinal haemorrhage was found between two different doses of aspirin.13 In this study, aspirin was efficacious at a dose of 30 mg a day, but a threshold dose for either the therapeutic or adverse effects of aspirin has yet to be established, and further attempts at dosage reduction might compromise therapeutic efficacy before adverse effects are eliminated completely.
Insufficient evidence exists to support the view that modified release formulations are safer, in terms of gastrointestinal haemorrhage, than standard formulations. Here we have studied the effect of dose and formulation on the incidence of gastrointestinal haemorrhage only; it may be that other symptomatic gastrointestinal adverse effects, such as nausea and epigastric pain, can be significantly reduced.13
The incidence of gastrointestinal haemorrhage with aspirin is relatively low, and to avoid factors that could have led us to underestimate the risk, we set inclusion and exclusion criteria such that only trials of a certain quality, with adequate numbers and follow up, would be selected. Although there is some asymmetry in the funnel plot (see figure on BMJ's website), suggesting the possibility of selection bias, adjustment for the likely effect of bias using “trim and fill” gave a pooled odds ratio of 1.62, which is only a slight change from our estimate of 1.68.14 Our meta-analysis seems reasonably robust to the asymmetry observed in the funnel plot.
We believe that the findings of our study are relevant to everyday practice. No significant heterogeneity was found, even though the studies we analysed encompassed a broad selection of patients with varying clinical indications. All the trials excluded patients at increased risk of gastrointestinal haemorrhage or with aspirin intolerance, but this is consistent with current advice on the use of aspirin and does not invalidate the relevance of our findings. Nevertheless, aspirin is available over the counter, and the risk of gastrointestinal haemorrhage could be higher in patients who take it without consulting a doctor.
Supplementary Material
Table.
Characteristics of studies included in meta-analysis
| Study | Year | Aspirin formulation | Dose (mg/day) | Average treatment duration (months) | Indication for aspirin therapy |
|---|---|---|---|---|---|
| Diener et alw1 w2 | 1996 | Unspecified | 50 | 24 | Transient ischaemic attack or stroke |
| Hansson et alw3 | 1998 | Standard | 75 | 45 | Hypertension |
| Petersen et alw4 | 1989 | Unspecified | 75 | 24 | Atrial fibrillation |
| SALTw5 | 1991 | Standard | 75 | 32 | Transient ischaemic attack or stroke |
| TPTw6 | 1998 | Modified release | 75 | 81 | Cardiovascular risk factors |
| Wallentinw7 | 1991 | Unspecified | 75 | 12 | Unstable angina |
| Silagy et alw8 | 1993 | Standard | 100 | 12 | Primary prevention |
| USPHSw9 | 1989 | Standard | 162.5 | 60 | Primary prevention |
| EAFTw10 | 1993 | Unspecified | 300 | 28 | Transient ischaemic attack or stroke |
| Elwood et alw11 | 1974 | Standard | 300 | 12 | Myocardial infarction |
| UK-TIAw12 | 1991 | Various | 300, 1200 | 48 | Transient ischaemic attack |
| Gavaghan et alw13 | 1991 | Standard | 324 | 12 | Coronary artery bypass grafting |
| Olivotto et alw14 | 1996 | Modified release | 325 | 12 | Breast cancer |
| Elwood et alw15 | 1979 | Standard | 900 | 12 | Myocardial infarction |
| CDPAw16 | 1976 | Unspecified | 972 | 22 | Myocardial infarction |
| PARISw17 | 1980 | Unspecified | 972 | 41 | Myocardial infarction |
| Hess et alw18 | 1985 | Unspecified | 990 | 24 | Peripheral vascular disease |
| AMISw19 | 1980 | Standard | 1000 | 36 | Myocardial infarction |
| Breddin et alw20 | 1980 | Modified release | 1000 | 24 | Myocardial infarction |
| CCSGw21 | 1978 | Standard | 1300 | 26 | Transient ischaemic attack |
| Fields et alw22 | 1977 | Standard | 1300 | 24 | Transient ischaemic attack |
| Fields et alw23 | 1978 | Standard | 1300 | 24 | Transient ischaemic attack |
| Brittonw24 | 1987 | Modified release | 1500 | 24 | Stroke |
| Ehresman et alw25 | 1977 | Modified release | 1500 | 12 | Peripheral vascular disease |
SALT=Swedish aspirin low dose trial; TPT=thrombosis prevention trial; USPHS=US physicians health study; EAFT=European atrial fibrillation trial; UK-TIA=UK transient ischaemic attack aspirin trial; CDPA=coronary drug project aspirin study; PARIS=persantine-aspirin reinfarction study; AMIS=aspirin myocardial infarction study; CCSG=Canadian Cooperative Study Group.
Acknowledgments
We thank Jon Deeks for encouragement and statistical support, particularly with the meta-regression; Alex Sutton for helping with the funnel plot; and Jeff Aronson for help with the manuscript.
Editorial by Tramèr
Footnotes
Funding: SD was supported by a grant from the Sir Jules Thorne Trust.
Competing interests: None declared.
A further table and figure plus the references for the included studies appear on the BMJ's website
References
- 1.Bayer Pharmaceuticals. Facts about aspirin. www.wonderdrug.com/press/factsheets/aspirin_fact_sheet.pdf (accessed 28 July 2000).
- 2.Roderick PJ, Wilkes HC, Meade TW. The gastrointestinal toxicity of aspirin: an overview of randomised controlled trials. Br J Clin Pharmacol. 1993;35:219–226. doi: 10.1111/j.1365-2125.1993.tb05689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eypasch E, Lefering R, Kum CK, Troidl H. Probability of adverse events that have not yet occurred: a statistical reminder. BMJ. 1995;311:619–620. doi: 10.1136/bmj.311.7005.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanchetti A, Hansson L. Risk of major gastrointestinal bleeding with aspirin. Lancet. 1999;353:148–150. doi: 10.1016/S0140-6736(05)76185-7. [DOI] [PubMed] [Google Scholar]
- 5.Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke M, Oxman AD, editors. Review Manager (RevMan) [computer program]. Version 4.0. Oxford: Cochrane Collaboration; 1999. Optimal search strategies for RCTs. Cochrane reviewers' handbook 4.0 (appendix 5c) [Google Scholar]
- 7.Loke YK, Edwards J, Derry S. Conventional search strategies cannot easily identify those trials of drug therapy which provide quantitative adverse effects data [abstract]. Proceedings of the VII Cochrane Colloquium, Rome 1999. www.clinpharm.ox.ac.uk/SearchStrategy.htm (accessed 28 July 2000).
- 8.Deeks JJ, Bradburn MJ, Localio R, Berlin J. Much ado about nothing: meta-analysis for rare events [abstract]. Proceedings of 2nd symposium on systematic reviews: beyond the basics, Oxford 1999. www.ihs.ox.ac.uk/csm/talks.html#p23 (accessed 28 July 2000).
- 9.Sharp S. she 23: meta-analysis regression. Stata Technical Bulletin. 1998;42:16–22. [Google Scholar]
- 10.Tramer MR, Moore RA, Reynolds DJM, McQuay HJ. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain. 2000;85:169–182. doi: 10.1016/s0304-3959(99)00267-5. [DOI] [PubMed] [Google Scholar]
- 11.Weil J, Colin-Jones D, Langman M, Lawson D, Logan R, Murphy M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ. 1995;310:827–830. doi: 10.1136/bmj.310.6983.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper-gastrointestinal bleeding with enteric-coated or buffered product. Lancet. 1996;348:1413–1416. doi: 10.1016/S0140-6736(96)01254-8. [DOI] [PubMed] [Google Scholar]
- 13.Dutch TIA Trial Study Group. A comparison of two doses of aspirin (30 mg vs 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. N Engl J Med. 1991;325:1261–1266. doi: 10.1056/NEJM199110313251801. [DOI] [PubMed] [Google Scholar]
- 14.Duval S, Tweedie R. Nonparametric “trim and fill” method for accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.