Abstract
Natural killer (NK) cells are lymphocytes generally recognized as sentinels of the innate immune system due to their inherent capacity to deal with diseased (stressed) cells, including malignant and infected. This ability to recognize many potentially pathogenic situations is due to the expression of a diverse panel of activation receptors. Because NK cell activation triggers an aggressive inflammatory response, it is important to have a means of throttling this response. Hence, NK cells also express a panel of inhibitory receptors that recognize ligands expressed by “normal” cells. Little or nothing is known about the endocytosis and trafficking of NK cell receptors, which are of great relevance to understanding how NK cells maintain the appropriate balance of activating and inhibitory receptors on their cell surface. In this review, we focus on the ITIM-containing inhibitory receptor CD94/NKG2A showing that it is endocytosed by a previously undescribed macropinocytic-like process that may be related to the maintenance of its surface expression.
Keywords: NK cells, Inhibitory/activating receptors, Endocytosis, Trafficking, CD94/NKG2A
Introduction
Natural killer (NK) cells are large granular lymphocytes with the inherent ability to kill target cells (e.g., tumor or virally-infected cells) and/or secrete cytokines [1]. Generally recognized as sentinels within the innate immune system, they express a large variety of activating receptors on their surface capable of recognizing target cells [2]. Due to the fact that the majority of normal cells express some of the ligands for these receptors, a mechanism to avoid autoimmune responses must exist. Consequently, NK cells also express inhibitory receptors whose signals are able to override basal activation signals [3, 4]. The ligands for the predominant inhibitory receptors are major histocompatibility complex (MHC) class I molecules that are expressed by most normal cells. Potential target cells must express sufficient levels of ligands for activating receptors relative to inhibitory ligand expression to override this inhibition in order to stimulate NK cell activation. In this light, any down-modulation of inhibitory receptor expression by NK cells would lower the threshold for NK cell activation possibly making normal cells vulnerable to attack.
In general, upon interaction with their specific ligands, receptors undergo down-modulation and are routed to lysosomes for degradation, a mechanism recognized as useful to dampen the intensity and duration of signaling to regulate activation responses [5]. This creates a conundrum of explaining how NK cells maintain surface expression of inhibitory receptors in a milieu of surrounding cells that under normal circumstances express inhibitory receptor ligands. Little is known regarding the endocytosis and trafficking of NK cell surface receptors. Here, we present a general overview on receptor trafficking routes emphasizing our description of a novel endocytic/trafficking pattern for the CD94/NKG2A inhibitory receptor [6].
NK cell receptors ligation and activation
Both inhibitory and activating NK cell receptors initiate intracellular signaling through specific amino acid sequence motifs contained in their cytoplasmic tails or their associated adapter proteins, respectively. Like many B and T cell antigen receptors, most NK cell activating receptors transduce an intracellular signal through assorted adapter proteins containing one or more immunoreceptor tyrosine-based activation motifs (ITAM) defined by the sequence Asp/Glu-x-x-Tyr-x-x-Leu/Ile, with x representing most other amino acids [7]. Depending on the receptor, these transmembrane-anchored adapter proteins can be DAP12, FcεRI-γ, or CD3-ζ. Receptor ligation leads to the phosphorylation of the Tyr residue within the ITAM motif, probably by Src family kinases. The subsequent recruitment of tyrosine kinases, such as Syk and/or Zap-70, via their SH2 domains leads to the propagation of a complex pattern of downstream signaling events that promote actin cytoskeleton reorganization, and induction of degranulation and/or the transcription of cytokine and chemokine genes [4, 8]. Unlike most activating receptors, such as CD16, CD94/NKG2C, KIR, NKp30, NKp44, NKp46 [4], NKG2D initiates signaling through its association with DAP10 that contains a YxxM–motif; phosphorylation of the Tyr residue activates the Syk-independent, PI3K/Grb-2 signaling pathway [9].
Recent research indicates that most NK cell effector functions are not mediated by a single activating receptor, but most probably from the ligation of a combination of receptors [10]. However, the low affinity Fc receptor for IgG CD16 (FcγRIII) [11], responsible for antibody-dependent cellular mediated cytotoxicity (ADCC) is apparently able to elicit NK effectors function without the simultaneous cross-linking of additional receptors.
The intracellular signaling of inhibitory receptors is mediated by immunoreceptor tyrosine-based inhibitory motifs (ITIM) that are characterized by the sequence Iso/Leu/Val/Ser-x-Tyr-x-x-Leu/Val with x representing most other amino acids [12]. Upon the interaction of inhibitory receptors with their ligands, ITIMs are tyrosine phosphorylated and act as docking sites for SHP-1, SHP-2, and/or SHIP-1 phosphatases. These tyrosine phosphatases are able to terminate NK cell effector function by dephosphorylating the protein substrates of the tyrosine kinases linked to activating NK receptors [13, 14]. A recent publication suggests that the binding of the phosphatase to the phosphorylated ITIM may be mediated by β-arrestin 2 [15]. In addition, a recent paper suggests that phosphorylated Ser residues outside the ITIM motif of KIR3DL1 may play a role in inhibitory receptor function [16].
The fact that NK inhibitory receptors recognize major histocompatibility complex (MHC) class I-type molecules that tend to be expressed by all normal cells suggests that this recognition process is important for controlling the potentially harmful lytic and inXammatory tendencies that NK cells possess toward all cells that they encounter [17]. In humans, the predominant NK inhibitory receptors interacting with MHC class I molecules are the heterodimeric CD94/NKG2A (and the alternative spliced form NKG2B), the killer cell Ig-like receptors (KIR) and the leukocyte immunoglobulin-like receptors (LILRs or ILTs). While the ligands for the type I transmembrane glycoproteins KIR and LILRs are mainly the classical human leukocyte antigen (HLA) class I molecules (HLA-A, B, and C), the ligand for CD94/NKG2A is the non-classical class I molecule HLA-E [18].
Routes for endocytosis and trafficking
As pointed out, NK cells must be finely regulated so that they aggressively respond to “abnormal” cells, but not to the extent that the resultant response becomes self-destructive. Maintenance of inhibitory receptor surface expression is critical for self-protection. In this light, it is important to understand the endocytic/trafficking process that functions to maintain constant inhibitory receptor expression.
Receptor endocytosis can be constitutive or ligand-induced [19]. Constitutive endocytosis occurs at a defined rate regardless of bound ligand, whereas ligand-induced endocytosis occurs upon receptor interaction with specific ligands. Receptors undergoing constitutive endocytosis are generally non-signaling ones that mediate the uptake of nutrients. The best example of this is the transferrin receptor (Tf-R). On the other hand as mentioned, receptors that “activate” cells tend to be down-regulated by endocytosis to prevent excessive responses, as exemplified by growth factor receptors like EGFR.
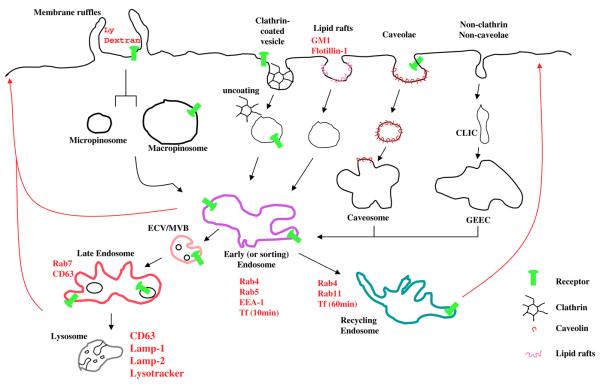
Two general types of endocytosis are recognized: phagocytosis that occurs in professional phagocytes and pinocytosis that occurs in most mammalian cell types. Currently the pinocytic pathway can be divided into the clathrin-dependent pathway and three clathrin-independent internalization pathways, namely, macro/micropinocytosis, caveolae-dependent internalization, and a pathway independent from both termed the GPI-enriched endosomal compartments (GEEC) pathway [20]. Internalization is just the beginning of the complex endocytic journey of receptors within the cell. In Fig. 1, we illustrate the potential endocytic routes and intracellular compartments available for receptor trafficking.
Fig. 1.
Schematic representation of the main endocytic pathways. The figure shows the main endocytic pathways and intracellular routes that a receptor can undergo. In the first step of endocytosis a receptor can undergo clathrin-dependent (clathrin-coated pit formation) or clathrin-independent internalization (micro- or macropinocytosis and caveolae-dependent internalization) or a pathway independent from both (GEEC). Following internalization, receptors carried by different vesicles can enter the early (or sorting) endosomes from where they are segregated into separate trafficking itineraries. A receptor can recycle back to the plasma membrane directly from this compartment or after being sorted into the recycling endosome or continue to traffic to the lysosome for the final degradation passing through the endosomal carrier vesicles/multivesicular bodies (ECV/MVBs) and late endosomes. Recycling can also occur at level of late endosomes and lysosomes. Specific markers for each compartment are indicated in red. Recycling pathways are indicated with red lines (The color version can be viewed online in the electronic version of the manuscript.)
Distinguishing endocytic routes
As mentioned, receptors can be endocytosed into the cell by a variety of pinocytic mechanisms [21, 22]. Of these, clathrin-mediated endocytosis is perhaps best understood. This process is thought to be initiated by adaptor proteins that localize to the plasma membrane through phosphatidylinositol-4, 5-bisphosphate-binding sites and that are also capable of recognizing sequence-specific motifs in the cytoplasmic tails of receptor proteins. The receptor-associated adaptor complex then recruits clathrin to the plasma membrane and promotes its assembly into a polygonal lattice leading to the formation of clathrin-coated transport vesicles [23, 24]. Although AP-2 is the major adaptor protein found in clathrin-coated pits, a number of accessory proteins can act together with AP-2 or act as alternative adaptors. Epsin, β-arrestin, AP180/CALM, Dab2, and Hip1 have been shown to interact not only with AP-2 but also with phosphatidylinositol-4, 5-bisphosphate and clathrin, which indicates that they may be able to bind to the plasma membrane and recruit clathrin independently of AP-2 [23, 24]. There is also evidence that each of these proteins can recognize specific internalization signals on the receptor cytoplasmic tail that diVer from those recognized by AP-2 [23].
Transferrin, through association with the Tf-R receptor, is specifically internalized via a clathrin-dependent mechanism and thus can be utilized as a marker for clathrin-dependent intracellular pathways. Hypertonic treatment of cells with 0.45 M sucrose is used to inhibit clathrin-mediated endocytosis [25, 26], but a more specific means is through treatment with specific siRNAs to deplete clathrin heavy chain (CHC) [6, 27] or the α-adaptin and μ2-subunit of the AP-2 adaptor complex [27]. The GTPase dynamin is targeted to coated pits through interactions with amphiphysin, which also binds AP-2 and clathrin [28, 29]. Dynamin facilitates the endocytic process by promoting membrane invagination and fission of cargo bearing clathrin-coated vesicles from the plasma membrane. Because of the process(es) it regulates, dynamin has been shown to be involved in a broad spectrum of endocytic events including caveolae/raft-mediated internalization, phagocytosis, and fluid-phase pinocytosis, as well as actin cytoskeleton reorganization [29-32].
Multiple cell surface receptors, such as GPI-anchored proteins, MHC class I molecules [33, 34], several types of G-protein-coupled receptors [35], and the γc-chain of IL-2 receptor [31] seem to require intact lipid rafts to be endocytosed [36]. It has been generally observed that rafts and raft-associated proteins are endocytosed by non-clathrin-mediated pathways [36-38], but exceptions to this have been reported [39].
Although many studies have demonstrated the existence of lipid rafts, a lot of controversy exists regarding their contents and distribution. Keeping this in mind, lipid rafts are generally characterized as domains present in the membrane usually rich in saturated lipids, cholesterol, and proteins involved in the signal transduction, as for example G proteins or glycosylphosphatidylinositol (GPI)-anchored proteins [40]. Different methods and markers are utilized to characterize them. One method used for their isolation consists of extracting detergent-resistant membrane (DRM) fraction with Triton X-100 [41]. The presence of receptors in this fraction indicates that they are raft-associated [6, 27]. Markers generally used to identify lipid domains are GM1 ganglioside [42] or Xotillin-1 [43]. Flotillin-1 appears to identify an endocytic pathway distinct from clathrin and caveolae endocytosis [43]; it is important to be aware that flotillin proteins can be found in most endocytic compartments and even in the nucleus [44].
Caveolae are special type of lipid rafts, whose main components are caveolin proteins 1, 2, and 3 that are abundant in specific cell types, such as fibroblasts, endothelial cells, and adipocytes but absent in lymphocytes. Caveolin-1 plays a pivotal role in the caveolae vesicle formation as demonstrated by the lack of caveolae in caveolin-1-knock out mice [45]. Dynamin is involved in the internalization step of caveolae [46]. And recent data have shown that caveolin-positive vesicles can interact with early endosomes in a Rab5-dependent process [47]. Cholera toxin and transforming growth factor β (TGF-β) receptor are examples of cargoes that are internalized via a caveolae-dependent process. Actually, TGF-β can be internalized by both the clathrin-dependent and caveolae-dependent pathways [48].
Most of the endocytic pathways described above are dynamin-dependent, but there are a growing number of descriptions of dynamin-independent endocytic routes for various cell surface proteins. These pathways are also clathrin and caveolin independent and are regulated by a variety of small GTPases [49]. For example, the Arf6 GTPase regulates the internalization of MHC Class I molecules [34]. Others have shown that the Rho family GTPase Cdc42 is responsible for the uptake of specific membrane components, such as GPI-anchored proteins and cholera toxin bound to GM1[49, 50], by a distinct pathway that does not require Arf6 function. Pinocytic vesicles devoid of clathrin and caveolin, termed clathrin- and dynamin- independent carriers (CLICs) [51], mediate the uptake and then fuse to form endosomal compartments termed GPI-anchored protein enriched early endosomal compartments (GEECs) [49, 52]. This pathway is also sensitive to cholesterol depletion and is inhibited by incubation with inhibitors of actin polymerization such as latrunculin A and cytochalasin D [49].
It is becoming clear that receptors are not tied to a single endocytic mechanism and that there may be cooperation among the various internalization pathways. A good example of this is the B cell receptor (BCR) which upon binding to antigen was observed to be internalized via lipid rafts or clathrin-coated pits by Putnam et al. [53]. A more recent study [54] showed that the most extensive BCR internalization occurs when clathrin cooperates with lipid rafts and the actin cytoskeleton. Ligated FcεRI is another example of a receptor that can apparently be internalized by either a clathrin-mediated [55] or lipid raft-mediated [27] mechanism.
Macro- and micropinosome formation resembles the ruffling involved in lamellipodia formation [56]. The engulfment of extracellular fluid-phase markers, such as fluorophore-conjugated dextrans and lucifer yellow, are usually used as markers to identify fluid-phase pinocytic processes. Macro- and micropinosome are morphologically distinguished by their size, greater or lesser than 0.2 μm [56]. It is not yet clear how many types of pinocytic vesicles exist and how many pathways are involved in their endocytosis [57]; however, it is clear that distinguishing these pathways by the size of the endocytic vesicle utilized is somewhat arbitrary [56].
The formation of macropinocytic vesicles have been shown to involve actin polymerization [56, 58] and small GTPases, such as ADP-ribosylation factor 6 (Arf6) [59, 60] and Rac1 [61]. Moreover, the process is regulated by PI3K [58, 62] and, consequently, macropinocytosis can be transiently stimulated by phorbol esters, like PMA, that activate the protein kinase C [56]. The use of actin-disrupting drugs, such as latrunculin A and cytochalasin D or aluminum Xuoride (AlF4−), that perturbs Arf6 function [60] or the PI3 kinase inhibitor wortmannin [62] are tools generally used to study a macropinocytic process. Also, the macropinocytic mechanism is sensitive to inhibitors of Na+/H+ exchange, such as amiloride [63-65]. Thus, beside the size of the pinocytic vesicles, micropinocytosis is distinguished from macropinocytosis by its PI3K and actin independency [66], and resistance to AlF4− and amiloride exposure.
Ubiquitination is a post-translational and reversible modification in which a single (mono-) or a chain (poly-) of ubiquitin molecules (Ub) is added to a substrate protein. Such modifications can not only regulate protein degradation and signaling processes, but also protein endocytosis [67-69]. For example the epidermal growth factor receptor (EGFR) undergoes a clathrin or caveolae-dependent endocytosis based on the levels of extracellular ligand and ubiquitination [70, 71]. At high doses of EGF, receptors undergo monoubiquiti-nation mediated by the E3 ubiquitin ligase Cbl and are mainly internalized via caveolae. At low (physiological) ligand doses, EGFR internalizes through a clathrin-dependent pathway and it is not ubiquitinated.
In the end, the characterization of endocytic machinery is an evolving process [72] that will probably lead to continued refinement of our understanding of the mechanisms involved.
Intracellular trafficking
Generally, after endocytosis, receptors are first delivered to early endosomes localized toward the cell periphery and characterized by mildly acidic pH that generally promotes receptor dissociation from its ligand. From the early endosomes, also named the sorting endosomes, the internalized receptors are either recycled to the plasma membrane or delivered to the lysosomes for degradation [5]. In the former case, the receptors are either directly recycled to the plasma membrane or sorted through the recycling endosome. In the latter case, the receptors are translocated to the more acidic and spherical compartments, the so-called endosomal carrier vesicles or multivesicular bodies (ECV/MVBs). These endosomes that share a diameter of 400–500 nm translocate the majority of the receptors along microtubules to the late endosomes with which the ECV/MVB fuse. Late endosomes share the presence of cisternal, tubular, and multivesicular regions and their shape is highly pleiomorphic. Receptors destined for degradation are then transferred from late endosomes into the lysosomes located closer to the nucleus; however, some of them can still recycle back to the cell surface from these locations [73].
The traffic of internalized cargo proteins within the various endosomal compartments can be followed using specific markers. A well-established marker for endocytic compartments is the early endosomal antigen 1 (EEA1) that marks early endosomes. Several small GTPases of the Rab family regulate transport among these organelles and speciffically mark different endocytic compartments [74]. Rab4 and Rab11 are specific markers of the recycling endosome and modulate the recycling from this compartment to the plasma membrane. At times, Rab4 can also be found within early endosomes presumably related to the delivery of the receptors into the recycling compartment [75]. Rab5 is a specific marker of early endosomes and Rab7 is a specific marker of late endosomes. Lysotracker and lysosome-associated membrane protein (Lamp)-1 and -2 identify lysosomes and late endosomes, and CD63 identifies all the late endosomes including multivesicular bodies. Since the trafficking pattern of the Tf-R has been well characterized, whether or not a receptor co-localizes with internalized Tf-R can provide valuable information about the trafficking of that receptor. Tf upon association with Tf-R, depending on the cell type and the time of treatment, 10 min or 1 h, is generally used to mark the early endosomes or the recycling endosome, respectively [76].
Receptor trafficking can vary depending on various factors, such as post-translational modifications or the nature of bound ligands. A common post-translational modification of trafficking proteins is ubiquitination that generally targets proteins for degradation by proteasome or by lysosomes [67, 77]. The endocytic process can also be regulated by differential binding of the receptors to its multiple ligands. As an example of ligand-mediated trafficking, it has been shown that the keratinocyte growth factor receptor/fibroblast growth factor receptor 2b (KGFR/FGFR2b) can be activated by two different ligands, the keratinocyte growth factor (KGF)/fibroblast growth factor (FGF) 7 and the FGF10/KGF2. Both ligands induce KGFR/FGFR2b clathrin-dependent internalization. In the case of KGF/FGF7, the receptor is ubiquitinated by c-Cbl and degraded at level of the lysosomes, as demonstrated by the co-localization of KGFR with both CD63- and lysotracker-positive vesicles. KGFR ligated with FGF10/KGF2 is not ubiquitinated and is delivered to the recycling endosome, as shown by the co-localization with Tf and absence of co-localization with either CD63 or lysotracker [76].
A unique trafficking route maintains CD94/NKG2A surface expression
CD94/NKG2A is an inhibitory receptor and in humans its ligand is HLA-E [18, 78]. It is expressed by NK cells, certain populations of CD8 T cells, and, at times, CD4 T cells. HLA-E is expressed by most normal cells thereby protecting themselves from NK cell aggression by its interaction with CD94/NKG2A. HLA-E, along with other MHC Class I molecules, tends to be down-regulated by virally infected and tumor cells, presumably as a mechanism to avoid recognition by cytotoxic T cells (CTL); however, such cells may no longer have sufficient inhibitory ligands to protect them from NK cell attack [79, 80].
Ligation of CD94/NKG2A by HLA-E leads to the phosphorylation of Tyr within the ITIM motifs present in the NKG2A intracellular tail that now can bind SHP-1 and/or SHP-2 [13, 80]. Activated phosphatases lead to the dephosphorylation of the Vav and ezrin/radixin/moesin (ERM) proteins that leads to the disruption of the actin cytoskeleton. This disrupts the signaling potential of activation receptor-bearing lipid rafts at the site of NK cell/target cell contact [79, 81, 82]. Because of its vital role in suppressing the tendency of NK cells to attack normal cells, CD94/NKG2A cell surface expression needs to be maintained while being constantly exposed to ligand expressed by surrounding cells. In agreement with this hypothesis we showed that CD94/NKG2A is not down-regulated upon interaction with the ligand, but it is long lived and continuously recycles back to the cell surface, and traffics intracellularly through different compartments than the Tf-R and the CD94/NKG2C activating receptor [83]. This is in marked contrast to the NK cell NKG2D activating receptor, like CD94/NKG2A a member of the NKG2 C-type lectin family, which is down-regulated upon ligation [84-87] apparently by a clathrin-mediated process [87] that seems to be actin dependent [6].
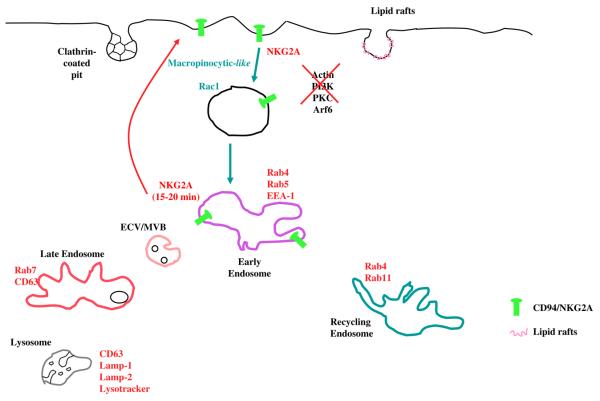
As previously pointed out, to avoid the auto-reactive tendency of NK cells, it is important to understand the endocytic and trafficking processes that cooperate to maintain constant inhibitory receptor expression. Thus, we decided to study the trafficking process that supports the resilient surface expression of CD94/NKG2A [6]. Our data indicate that the endocytosis of CD94/NKG2A is clearly a pinocytic process as it is co-endocytosed with fluid-phase markers. The fact that CD94/NKG2A endocytic vesicles seems to be much greater in diameter (0.5–1.5 μm) than micropinosomes (<0.2 μm) characterizes them as macropinosomes [72]. The fact that internalized CD94/NKG2A co-localizes with dextran and lucifer yellow, and that its endocytosis is amiloride sensitive and Rac1 dependent supported this conclusion [63, 88]. In human umbilical vein endothelial cells, the clustered cell adhesion molecules ICAM-1 and platelet endothelial cell adhesion molecule-1 are internalized by a macropinocytic mechanism that is independent of clathrin, caveolin, and PI3K activity; however, this internalization process requires actin and dynamin activity [63]. The use of RNA interference to suppress the expression of clathrin heavy chain (ch1 and ch2) and hypertonic treatment of cells did not affect CD94/NKG2A endocytosis demonstrating that the receptor is not internalized with a clathrin-dependent mechanism [6]. Moreover, Rac-1 activity is required for CD94/NKG2A internalization. Evidence that CD94/NKG2A internalization does not require actin, dynamin, or Arf6 and is insensitive to PI3K inhibition and PKC stimulation clearly distinguishes it from previously described macropinocytic mechanisms. On the other hand, despite the fact that CD94/NKG2A-containing endocytic vesicles are too large to fit the definition of a micropinosomes, they do share, in addition to PI3K independence, the notable feature of actin independency with the micropinocytic process. Keeping this in mind, considering the “plasticity” of endocytic process that can take place, we arbitrarily chose to term CD94/NKG2A endocytosis as macropinocytic-like realizing that it is clearly biochemically distinguishable from previously described macropinocytic mechanisms. This novel endocytic process is coupled to an abbreviated intracellular trafficking pattern in which endocytosed CD94/NKG2A enters the early endosomal compartment, but does not enter late endosomes nor does it fully enter the recycling compartment. Co-localization was in fact observed with EEA1 and Rab5, only partially with Rab4 and never with Rab7, Rab11, or Lamp-1 and 2. Figure 2 shows a schematic model of the CD94/NKG2A endocytic route. In the end, the macropinocytic-like pathway seems to be a process utilized by NK cells for its function and homeostasis. If this pathway turns out to be unique to CD94/NKG2A, it would indicate how crucial this protein is for NK cell function. Moreover, if the macropinocytic-like pathway seems to be utilized by others inhibitory receptors in general, it would emphasize the importance, efficiency, and plasticity of cells to adopt such energy saving tactics for its functional homeostasis.
Fig. 2.
CD94/NKG2A intracellular trafficking. The endocytic route of CD94/NKG2A is schematically represented. We have no direct evidence of receptor association with membrane ruffles. The CD94/NKG2A positive vesicles that are formed near the plasma membrane are morphologically similar to macropinosomes in size. However the CD94/NKG2A internalization process does not require actin, dynamin, or Arf6; it is insensitive to PI3K inhibition and PKC stimulation and requires the involvement of Rac1. Thus we arbitrarily termed it a macropinocytic-like process. Upon 15–20 min of endocytosis, the receptor colocalizes with EEA1 and Rab5 and only minimally with Rab4. Co-localization with Rab7 or Rab11 and Lamp-1 or Lamp-2 is never observed indicating that the receptor is recycled back to the plasma membrane without entering the Rab7+ or Rab11+ compartments (red line) (The color version can be viewed online in the electronic version of the manuscript.)
Our current aim is to investigate whether other inhibitory ITIM-bearing receptors expressed on NK cells that function similarly to CD94/NKG2A are also endocytosed via an actin-independent process and whether they traffic similarly to CD94/NKG2A. Our work is currently focused on the study of the intracellular trafficking of the inhibitory leukocyte-associated Ig-like receptror-1 (LAIR-1) receptor, expressed by most lymphocytes [89].
Conclusions and future directions
Knowledge endocytosis and trafficking of activating and inhibitory receptors on NK cells and other lymphocytes is important for understanding how regulation of receptor expression within the endocytic compartments relates to the functional status of these cells. Our recent identification of a novel endocytic route and trafficking pattern for CD94/NKG2A leads us to hypothesize that inhibitory receptors may have a unique trafficking pathway. We plan to test this hypothesis by studying the bioprocessing of other ITIM-bearing inhibitory receptors expressed by lymphocytes, such as LAIR-1 (CD305) and CD300a. We also plan to look at ITIM–bearing inhibitory receptors on other cell-types, e.g., CD300lf on myeloid cells. Continued research on the trafficking pathways of NK cell receptors may enable us to experimentally alter these pathways in disease conditions that could result in better treatment strategies for cancer and autoimmune diseases.
Acknowledgment
This work was supported by the intramural program of the NIAID/NIH.
References
- 1.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moretta L, Biassoni R, Bottino C, Cantoni C, Pende D, Mingari MC, et al. Human NK cells and their receptors. Microbes Infect. 2002;4:1539–44. doi: 10.1016/s1286-4579(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 3.Hallett WH, Murphy WJ. Positive and negative regulation of natural killer cells: therapeutic implications. Semin Cancer Biol. 2006;16:367–82. doi: 10.1016/j.semcancer.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 6.Masilamani M, Narayanan S, Prieto M, Borrego F, Coligan JE. Uncommon endocytic and trafficking pathway of the natural killer cell CD94/NKG2A inhibitory receptor. Traffic. 2008;9:1019–34. doi: 10.1111/j.1600-0854.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 7.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–4. [PubMed] [Google Scholar]
- 8.McVicar DW, Taylor LS, Gosselin P, Willette-Brown J, Mikhael AI, Geahlen RL, et al. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J Biol Chem. 1998;273:32934–42. doi: 10.1074/jbc.273.49.32934. [DOI] [PubMed] [Google Scholar]
- 9.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4:557–64. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 10.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–66. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, et al. Impaired IgG-dependent anaphylaxis and arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–8. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 12.Daeron M, Jaeger S, Du Pasquier L, Vivier M. Immunoreceptor tyrosine-based inhition motifs: a quest in the past and future. Immunol Rev. 2008;244:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 13.Kabat J, Borrego F, Brooks A, Coligan JE. Role that each NKG2A immunoreceptor tyrosine-based inhibitory motif plays in mediating the human CD94/NKG2A inhibitory signal. J Immunol. 2002;169:1948–58. doi: 10.4049/jimmunol.169.4.1948. [DOI] [PubMed] [Google Scholar]
- 14.Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP–1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 2003;23:6291–9. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu MC, Su LL, Zou L, Liu Y, Wu N, Kong L, et al. An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat Immunol. 2008;9:898–907. doi: 10.1038/ni.1635. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Arias DA, Campbell KS. Protein kinase C regulates expression and function of inhibitory killer cell Ig-like receptors in NK cells. J Immunol. 2007;179:5281–90. doi: 10.4049/jimmunol.179.8.5281. [DOI] [PubMed] [Google Scholar]
- 17.Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, et al. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637–60. doi: 10.1016/s0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 18.Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998;187:813–8. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benmerah A, Lamaze C. Clathrin-coated pits: vive la difference? Traffic. 2007;8:970–82. doi: 10.1111/j.1600-0854.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 20.Gong Q, Huntsman C, Ma D. Clathrin-independent internalization and recycling. J Cell Mol Med. 2008;12:126–44. doi: 10.1111/j.1582-4934.2007.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 22.Sorkin A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr Opin Cell Biol. 2004;16:392–9. doi: 10.1016/j.ceb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 24.Traub LM. Sorting it out: AP–2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–8. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacquier V, Prummer M, Segura JM, Pick H, Vogel H. Visualizing odorant receptor trafficking in living cells down to the single-molecule level. Proc Natl Acad Sci U S A. 2006;103:14325–30. doi: 10.1073/pnas.0603942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 2006;7:28. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fattakhova G, Masilamani M, Borrego F, Gilfillan AM, Metcalfe DD, Coligan JE. The high-affinity immunoglobulin-E receptor (FcepsilonRI) is endocytosed by an AP–2/clathrin-independent, dynamin-dependent mechanism. Traffic. 2006;7:673–85. doi: 10.1111/j.1600-0854.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 28.Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol. 2004;5:133–47. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 29.Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7:661–71. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- 31.Sauvonnet N, Dujeancourt A, Dautry-Varsat A. Cortactin and dynamin are required for the clathrin-independent endocytosis of gammac cytokine receptor. J Cell Biol. 2005;168:155–63. doi: 10.1083/jcb.200406174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold ES, Underhill DM, Morrissette NS, Guo J, McNiven MA, Aderem A. Dynamin 2 is required for phagocytosis in macrophages. J Exp Med. 1999;190:1849–56. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naslavsky N, Weigert R, Donaldson JG. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell. 2003;14:417–31. doi: 10.1091/mbc.02-04-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–52. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fourgeaud L, Bessis AS, Rossignol F, Pin JP, Olivo-Marin JC, Hemar A. The metabotropic glutamate receptor mGluR5 is endocytosed by a clathrin-independent pathway. J Biol Chem. 2003;278:12222–30. doi: 10.1074/jbc.M205663200. [DOI] [PubMed] [Google Scholar]
- 36.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–26. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 37.Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003;116:4707–14. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- 38.Sato SB, Ishii K, Makino A, Iwabuchi K, Yamaji-Hasegawa A, Senoh Y, et al. Distribution and transport of cholesterol-rich membrane domains monitored by a membrane-impermeant fluorescent polyethylene glycol-derivatized cholesterol. J Biol Chem. 2004;279:23790–6. doi: 10.1074/jbc.M313568200. [DOI] [PubMed] [Google Scholar]
- 39.Cuitino L, Matute R, Retamal C, Bu G, Inestrosa NC, Marzolo MP. ApoER2 is endocytosed by a clathrin-mediated process involving the adaptor protein Dab2 independent of its rafts' association. Traffic. 2005;6:820–38. doi: 10.1111/j.1600-0854.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- 40.Laude AJ, Prior IA. Plasma membrane microdomains: organization, function and trafficking. Mol Membr Biol. 2004;21:193–205. doi: 10.1080/09687680410001700517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–94. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 42.Nichols BJ, Kenworthy AK, Polishchuk RS, Lodge R, Roberts TH, Hirschberg K, et al. Rapid cycling of lipid raft markers between the cell surface and golgi complex. J Cell Biol. 2001;153:529–41. doi: 10.1083/jcb.153.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glebov OO, Bright NA, Nichols BJ. Flotillin–1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 44.Payne CK, Jones SA, Chen C, Zhuang X. Internalization and trafficking of cell surface proteoglycans and proteoglycan-binding ligands. Traffic. 2007;8:389–401. doi: 10.1111/j.1600-0854.2007.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin–1 gene-disrupted mice. Science. 2001;293:2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 46.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven Wssion from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–14. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–80. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Di Guglielm GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 49.Chadda R, Howes MT, Plowman SJ, Hancock JF, Parton RG, Mayor S. Cholesterol-sensitive Cdc42 activation regulates actin polymerization for endocytosis via the GEEC pathway. Traffic. 2007;8:702–17. doi: 10.1111/j.1600-0854.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gauthier NC, Monzo P, Kaddai V, Doye A, Ricci V, Boquet P. Helicobacter pylori VacA cytotoxin. A probe for a clathrin-independent and Cdc42-dependent pinocytic pathway routed to late endosomes. Mol Biol Cell. 2005;16:4852–66. doi: 10.1091/mbc.E05-05-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirkham M, Fujita A, Chadda R, Nixon SJ, Kurzchalia TV, Sharma DK, et al. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168:465–76. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–23. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 53.Putnam MA, Moquin AE, Merrihew M, Outcalt C, Sorge E, Caballero A, et al. Lipid raft-independent B cell receptor-mediated antigen internalization and intracellular trafficking. J Immunol. 2003;170:905–12. doi: 10.4049/jimmunol.170.2.905. [DOI] [PubMed] [Google Scholar]
- 54.Stoddart A, Jackson AP, Brodsky FM. Plasticity of B cell receptor internalization upon conditional depletion of clathrin. Mol Biol Cell. 2005;16:2339–48. doi: 10.1091/mbc.E05-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson BS, Steinberg SL, Liederman K, Pfeiffer JR, Surviladze Z, Zhang J, et al. Markers for detergent-resistant lipid rafts occupy distinct and dynamic domains in native membranes. Mol Biol Cell. 2004;15:2580–92. doi: 10.1091/mbc.E03-08-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–8. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- 57.Jones AT. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J Cell Mol Med. 2007;11:670–84. doi: 10.1111/j.1582-4934.2007.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, et al. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol. 2002;291:487–94. doi: 10.1078/1438-4221-00157. [DOI] [PubMed] [Google Scholar]
- 59.Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4, 5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–17. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–9. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Lindmo K, Stenmark H. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci. 2006;119:605–14. doi: 10.1242/jcs.02855. [DOI] [PubMed] [Google Scholar]
- 63.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, et al. A novel endocytic pathway induced by clustering endothelial ICAM–1 or PECAM-1. J Cell Sci. 2003;116:1599–609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 64.West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109:2731–9. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.von Delwi A, Hilkens CM, Altmann DM, Holmdahl R, Isaacs JD, Harding CV, et al. Inhibition of macropinocytosis blocks antigen presentation of type II collagen in vitro and in vivo in HLA-DR1 transgenic mice. Arthritis Res Ther. 2006;8:R93. doi: 10.1186/ar1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–60. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu YC, Penninger J, Karin M. Immunity by ubiquitylation: a reversible process of modification. Nat Rev Immunol. 2005;5:941–52. doi: 10.1038/nri1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haglund K, Dikic I. Ubiquitylation and cell signaling. EMBO J. 2005;24:3353–9. doi: 10.1038/sj.emboj.7600808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–5. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 70.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102:2760–5. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen H, De Camilli P. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc Natl Acad Sci U S A. 2005;102:2766–71. doi: 10.1073/pnas.0409719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–12. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–23. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 74.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 75.Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–14. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belleudi F, Leone L, Nobili V, Raffa S, Francescangeli F, Maggio M, et al. Keratinocyte growth factor receptor ligands target the receptor to different intracellular pathways. Traffic. 2007;8:1854–72. doi: 10.1111/j.1600-0854.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- 77.Elsasser S, Finley D. Delivery of ubiquitinated substrates to protein-unfolding machines. Nat Cell Biol. 2005;7:742–9. doi: 10.1038/ncb0805-742. [DOI] [PubMed] [Google Scholar]
- 78.Brooks AG, Posch PE, Scorzelli CJ, Borrego F, Coligan JE. NKG2A complexed with CD94 defines a novel inhibitory natural killer cell receptor. J Exp Med. 1997;185:795–800. doi: 10.1084/jem.185.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borrego F, Masilamani M, Kabat J, Sanni TB, Coligan JE. The cell biology of the human natural killer cell CD94/NKG2A inhibitory receptor. Mol Immunol. 2005;42:485–8. doi: 10.1016/j.molimm.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 80.Borrego F, Masilamani M, Marusina AI, Tang X, Coligan JE. The CD94/NKG2 family of receptors from molecules and cells to clinical relevance. Immunol Res. 2006;35:263–78. doi: 10.1385/IR:35:3:263. [DOI] [PubMed] [Google Scholar]
- 81.Sanni TB, Masilamani M, Kabat J, Coligan JE, Borrego F. Exclusion of lipid rafts and decreased mobility of CD94/NKG2A receptors at the inhibitory NK cell synapse. Mol Biol Cell. 2004;15:3210–23. doi: 10.1091/mbc.E03-11-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masilamani M, Nguyen C, Kabat J, Borrego F, Coligan JE. CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J Immunol. 2006;177:3590–6. doi: 10.4049/jimmunol.177.6.3590. [DOI] [PubMed] [Google Scholar]
- 83.Borrego F, Kabat J, Sanni TB, Coligan JE. NK cell CD94/NKG2A inhibitory receptors are internalized and recycle independently of inhibitory signaling processes. J Immunol. 2002;169:6102–11. doi: 10.4049/jimmunol.169.11.6102. [DOI] [PubMed] [Google Scholar]
- 84.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 85.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–40. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 86.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249–58. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 87.Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 88.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 89.Meyaard L. The inhibitory collagen receptor LAIR–1 (CD305) J Leukoc Biol. 2008;83:799–803. doi: 10.1189/jlb.0907609. [DOI] [PubMed] [Google Scholar]