Abstract
We have initiated a series of experiments to analyze the biosynthesis and oligomerization of Cx43 in cells containing other connexins through the expression of site-directed mutants and chimeric connexin polypeptides. Here we report studies concerning a mutant of Cx43 (Cx43tr) that has been truncated after amino acid 251 to remove most of the Cx43 carboxyterminal region. In stably transfected HeLa cells, full length Cx43 localized primarily to appositional membranes while much more Cx43tr was observed in the cytoplasm. Both Cx43 and Cx43tr showed similar oligomerization profiles based on centrifugation through sucrose gradients. HeLaCx43tr cells showed limited transfer of microinjected Lucifer Yellow but did show electrical coupling. Co-expression of Cx43tr with Cx43 or Cx45 led to Cx43tr localization at appositional membranes and co-localization with the other connexins. Moreover, cells co-expressing Cx43tr with Cx43 or Cx45 showed extensive intercellular dye coupling. Thus, Cx43tr was able to oligomerize and form functional channels when expressed alone or with a compatible connexin, but it only formed plaques when co-expressed. These results suggest that the carboxyl tail of Cx43 is not important for oligomerization, but they implicate critical residues in the formation of gap junction plaques.
Keywords: Connexon, heteromeric-channels, homomeric channels
INTRODUCTION
Formation of a gap junction channel requires the oligomerization of six monomers (connexins) to form a hexameric hemi-channel (connexon) followed by the docking of two connexons. Oligomerization may involve all identical subunits (to form homomeric connexons) or various combinations of compatible connexins (to form heteromeric connexons). The cellular process of connexin oligomerization is beginning to be elucidated (1, 6, 8, 18). We and others have identified a number of compatible or incompatible partners for the formation of heteromeric connexons (3, 4, 5, 10, 11, 15). One of the most interesting compatible combinations is Cx43 and Cx45, since the permeability and regulatory properties of the mixed channels appear to be differently influenced by the two connexin components (15).
We have initiated a series of experiments to identify specific sequences within the connexin polypeptides that influence the ability of connexins to oligomerize with themselves or with other connexins by expressing site-directed mutants or chimeric molecules derived from Cx43 or other connexins in stably transfected cells (14). In the present manuscript, we describe our analysis of a Cx43-derived mutant (Cx43tr) that lacks virtually all of the carboxy terminal domain of Cx43 due to truncation after amino acid 251. We studied homo- and hetero-oligomerization of Cx43tr (as well as other aspects of gap junction biosynthesis and function) in HeLa cells that were stably transfected with Cx43tr alone or with Cx43tr and full length Cx43 or Cx45. Our data suggest that the carboxyl tail of Cx43 is not required for oligomerization, but they do suggest important roles in other aspects of gap junction biosynthesis.
MATERIALS AND METHODS
Culture and stable transfection of HeLa cells with Cx45 (HeLaCx45), Cx43 (HeLaCx43), or both Cx43 and Cx45 (HeLaCx43/Cx45) have been described previously (7, 15, 20). PCR was used to construct a variant of Cx43 truncated after amino acid 251 (Cx43tr) with the 9 amino acid influenza hemaglutinin (HA) epitope appended to its carboxyl terminus from the rat Cx43 cDNA. The parental HeLa cells or singly transfected cells were stably transfected with Cx43tr DNA to produce HeLaCx43tr, HeLaCx43/Cx43tr, and HeLaCx45/Cx43tr cells.
Immunofluorescence was performed as describe previously (15) using a rabbit polyclonal antibody directed against the HA tag (Zymed; 71-5500) to detect Cx43tr and mouse monoclonal antibodies to detect Cx43 (Chemicon; MAB 3068) or Cx45 (Chemicon; MAB 3100).
Oligomerization of connexins into connexons was assessed by immunoblotting fractions obtained after centrifuging cell lysates solubilized with 1% Triton X-100 through a continuous sucrose gradient (5–20%) (3, 20).
Intercellular coupling was examined after microinjection of the gap junction tracers, Lucifer Yellow (MW 457, net charge −2) and neurobiotin (MW 345, net charge +1) (15). The dual whole-cell voltage-camp technique was used to examine voltage-dependent gating and single-channel gap junction currents from cell pairs (7, 15).
RESULTS
Immunofluorescent Localization and Oligomerization of Cx43tr
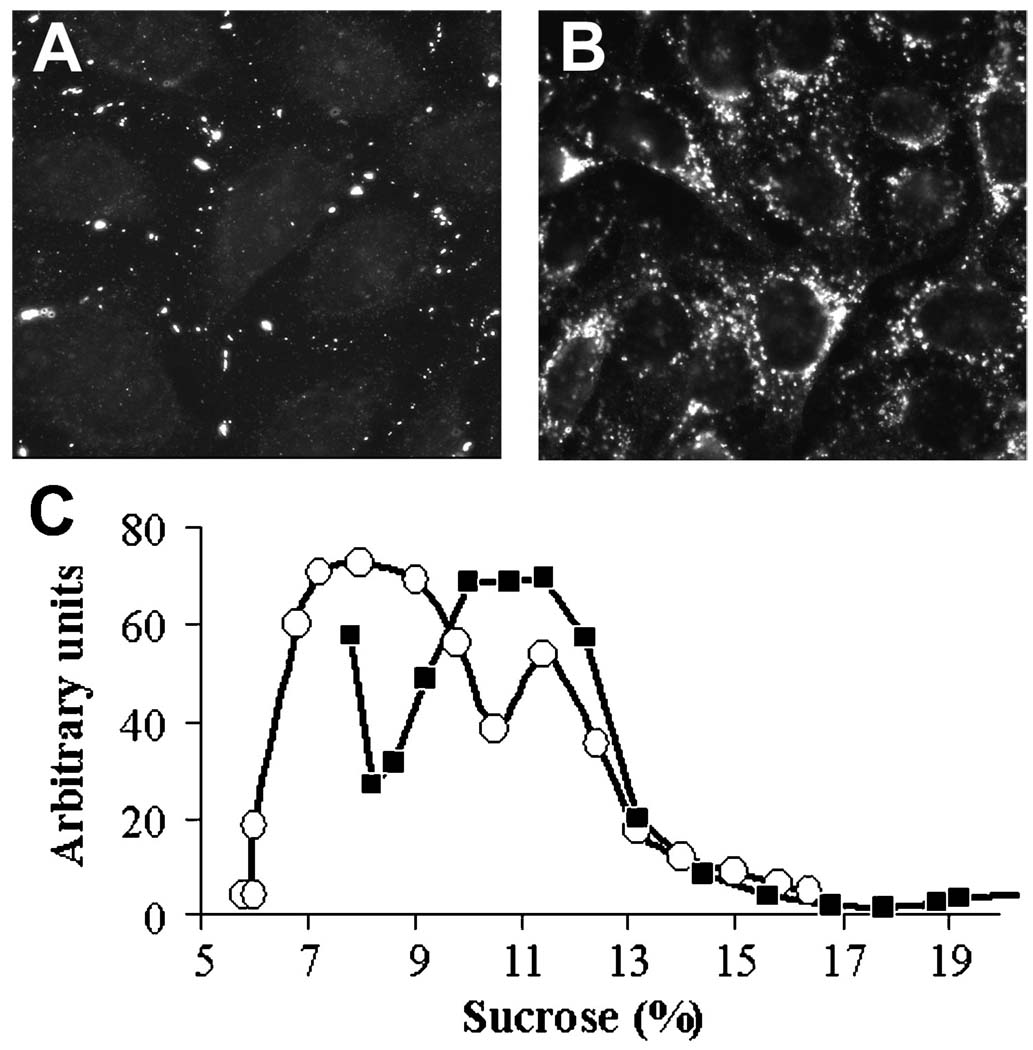
In HeLa cells stably transfected with full length Cx43, HeLaCx43 cells, immunoreactive Cx43 was abundantly localized in appositional membranes as expected for gap junction plaques (Figure 1A and previous studies [15, 20]). In contrast, the truncated Cx43 construct, Cx43tr, was mainly identified in a perinuclear region in HeLaCx43tr cells (Figure 1B) with little plasma membrane staining suggesting gap junction plaques. The cytoplasmic staining appeared to represent localization of Cx43tr within the Golgi apparatus based on double label immunostaining with antibodies to Golgi proteins (not shown).
Figure 1.
Cellular localization and oligomeric assembly of full length and truncated Cx43 in stably transfected HeLa cells. (A) Immunofluorescent detection of Cx43 in HeLaCx43 cells predominantly shows staining at plasma membrane appositions. (B) Immunofluorescent detection of Cx43tr in HeLaCx43tr cells primarily localizes the protein to a perinuclear compartment with little or no staining at appositional membranes. (C) Graph depicting the densitometric quantitation of the abundance of Cx43 (○) or Cx43tr (■) in sucrose gradient fractions prepared from HeLaCx43 and HeLaCx43tr cells, respectively. Both monomers and oligomers of Cx43 and Cx43tr were detected.
Because connexin oligomerization is an earlier biosynthetic step that precedes the observed lack of gap junction plaque formation, we studied the oligomeric status of connexins in HeLaCx43 or HeLaCx43tr by velocity centrifugation of Triton X-100-soluble cellular extracts through sucrose gradients. In extracts prepared from HeLaCx43tr cells, we detected two peaks of Cx43tr immunoreactivity at 7.5% and at 11% sucrose (Figure 1C). Peaks of Cx43 immunoreactivity were detected at similar positions in gradients prepared from HeLaCx43 cells (Figure 1C). Previous gradient calibration and detergent disruption experiments suggest that these two peaks correspond to connexin monomers and hexameric connexons (3).
Function of Cx43tr Channels
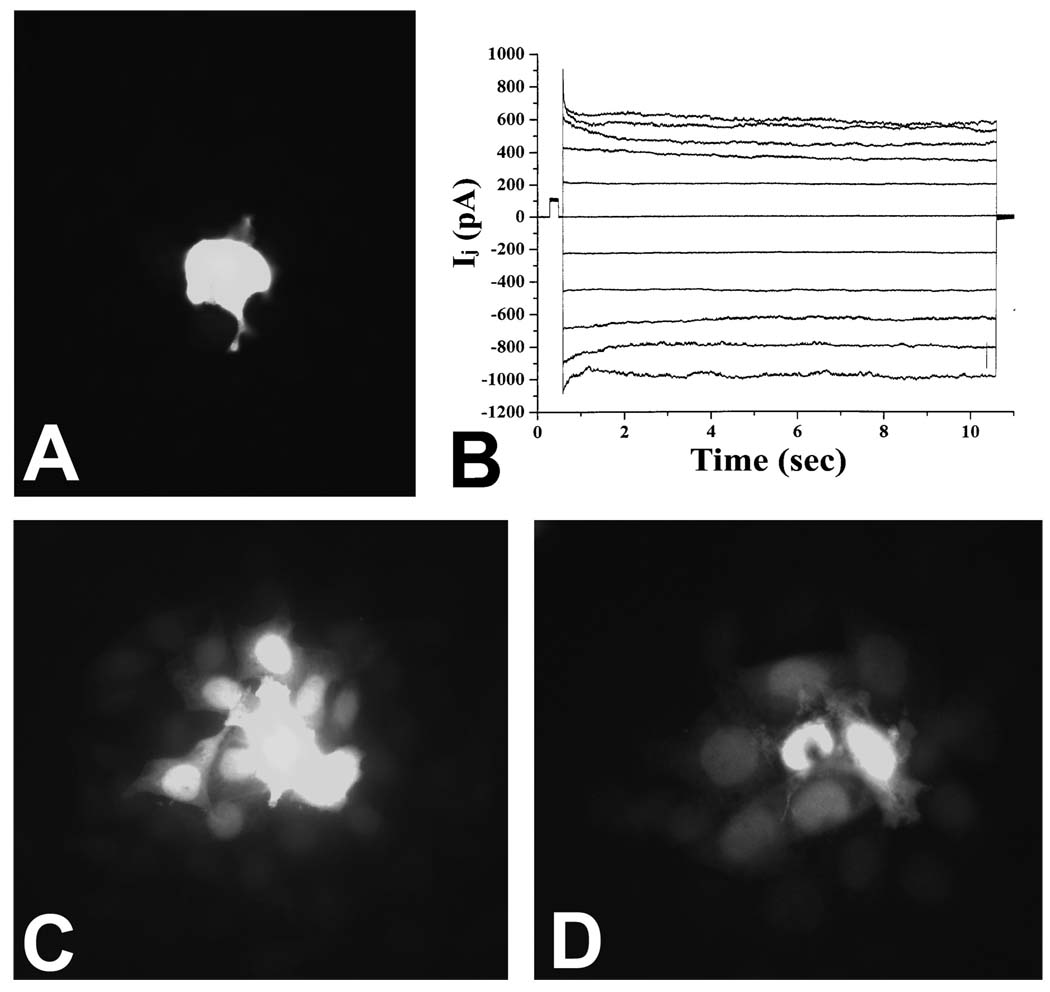
We tested the function of gap junctions in HeLaCx43tr cells by examining intercellular dye transfer and electrical coupling. In previous studies, we have shown that HeLaCx43 cells show extensive cellular coupling detected by Lucifer yellow microinjection (i.e., dye diffusion could be detected in more than 10 adjacent cells in addition to the injected cell (15). However, dye coupling was much more limited in HeLaCx43tr cells. In the example shown in Figure 2A, the Lucifer yellow remained restricted to the micro-injected cell, and in most injections, it diffused to no more than one or two adjacent cells.
Figure 2.
Intercellular communication in cells expressing Cx43tr alone or co-expressing Cx43tr with Cx43 or Cx45. (A, C, D) Fluorescence micrographs obtained after micro-injection of Lucifer Yellow into a single cell within a confluent monolayer of HeLaCx43tr (A), HeLaCx43/Cx43tr (C), or HeLaCx45/Cx43tr (D) cells. While dye was seen only in the injected cell in the HeLaCx43tr cells, extensive dye transfer was seen in the co-expressing cells. (B) Gap junctional currents recorded from a pair of HeLaCx43tr cells.
Cellular coupling assessed using neurobiotin was also limited in HeLaCx43tr cells; intercellular diffusion of this molecule was only detected in an average of ~3 cells (data not shown). However, profuse electrical coupling was detected between pairs of HeLaCx43tr cells. The junctional currents showed substantial voltage dependence, but without any residual conductance or fast gating component (Figure 2B), similar to previous observations of expressed Cx43 truncated after residue 257 (2, 7, 16).
We detected single channel events with conductances around ~120 pS similar to previously recorded Cx43 gap junction channels. In some records, we detected small events that might result from endogenous expression of Cx45 in HeLa cells, and we detected channels consistent with heteromeric or heterotypic Cx43tr/Cx45 channels (data not shown).
Co-Localization of Cx43tr with Wild Type Cx43 or Cx45 in Gap Junction Plaques
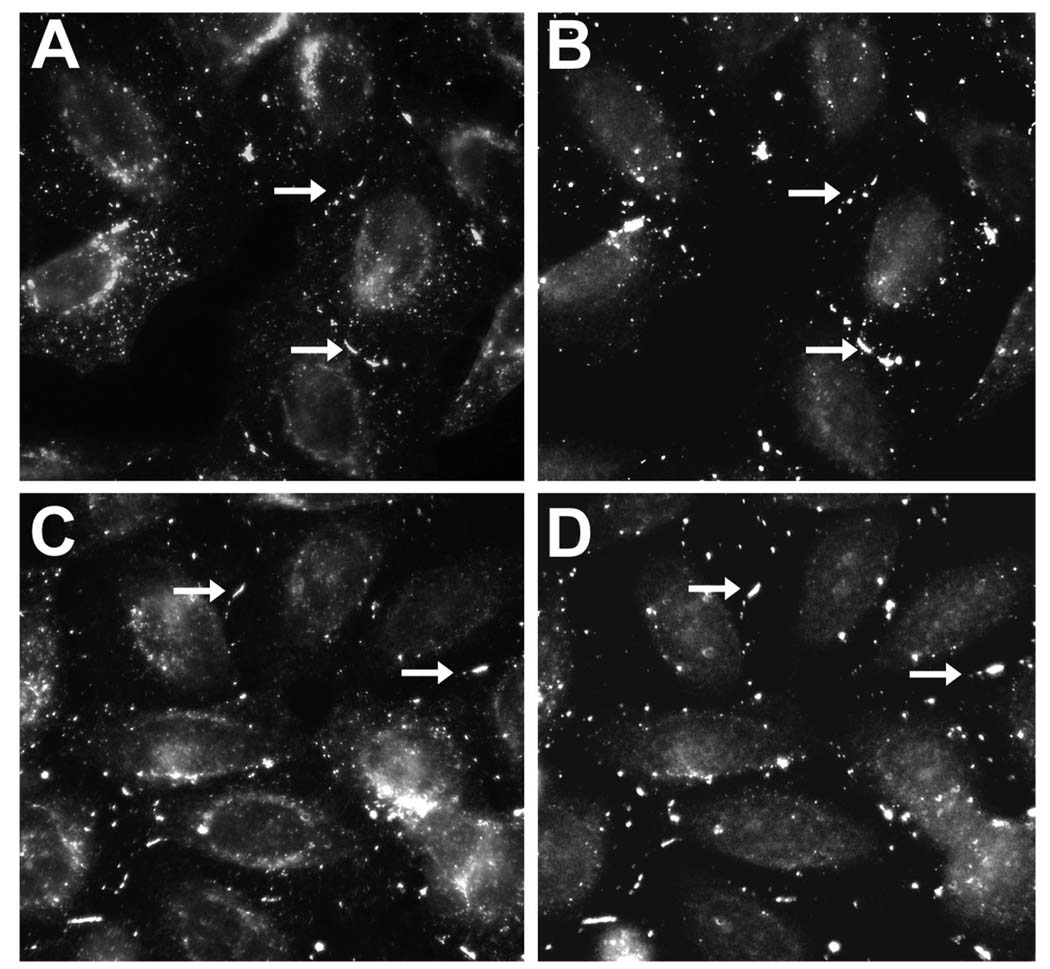
In HeLaCx43/Cx43tr and in HeLaCx45/Cx43tr cells, Cx43tr immunoreactivity localized at appositional membranes in essentially identical distributions with wild type Cx43 (Figures 3A, B) or Cx45 (Figures 3C, D), respectively. This co-localization suggests that both Cx43 and Cx45 can rescue the ability of Cx43tr to assemble into gap junction plaques.
Figure 3.
Double label immunofluorescent localization of Cx43tr as compared to Cx43 or Cx45 in co-trasnfected cells. (A, B) Immunolocalization of Cx43tr (A) an Cx43 (B) in HeLaCx43/Cx43tr cells. (C, D) Immunoflourescent detection of Cx43tr (C) and Cx45 (D) in HeLaCx45/Cx43tr cells. The distributions of Cx43tr and Cx43 or Cx45 appeared very similar, with substantial co-localization in the gap junction plaques (arrows).
Effect of Cx43tr on Function of Co-Expressed Connexins
We examined the extent of intercellular communication in HeLaCx43/Cx43tr or HeLaCx45/Cx43tr cell cultures. We saw extensive transfer of microinjected Lucifer yellow to surrounding cells in both cell types (Figures 2C, D). Lucifer yellow transfer was identified in ≥8 HeLaCx43/Cx43tr or HeLaCx45/Cx43tr cells. In both co-transfected cell lines, micro-injected neurobiotin diffused to many (>25) adjacent cells per injection. Although Cx45 gap junction channels have low permeability to Lucifer Yellow, the extensive Lucifer yellow coupling of HeLaCx45/Cx43tr cells provides strong evidence that Cx43tr can co-oligomerize with Cx45 and produce channels with larger permeability than homomeric Cx43tr or Cx45.
DISCUSSION
Data presented in this manuscript have demonstrated that Cx43 truncated after amino acid 251 is capable of oligomerizing, forming functional channels by itself, and participating with full length Cx43 or Cx45 in formation of gap junction plaques and functional channels. These observations argue that residues 252–382 are not required for homo- or hetero-oligomerization nor are they required for formation of channels with at least some degree of function. The electrophysiological characteristics of Cx43tr channels appeared similar to those previously characterized for Cx43M257 channels (2, 7), emphasizing the role of the Cx43 carboxyl tail in fast gating and residual conductance.
Our data suggest that truncation of the Cx43 carboxy tail may affect channel permeability (in homomeric and heteromeric connexons). HeLaCx43tr cell pairs showed substantial junctional conductances despite limited transfer of Lucifer Yellow. Moreover, “rescue” of Cx43tr by co-expression with either Cx43 or Cx45 increased Lucifer Yellow transfer.
Cx43tr did not accumulate in large spots at appositional membranes (that likely represent gap junction plaques) when expressed by itself. This finding suggests that the carboxyl terminus of Cx43 must be important in formation of gap junction plaques or for the efficient delivery of the protein to the cell surface. Similarly, Martin and colleagues (13) have previously suggested that portions of the tail of Cx32 may be required for plaque formation. These data extend previous studies implicating the carboxyl tail in other functions including regulation by pH (17), phosphorylation (12), and interaction with other proteins (9). Previous studies have examined other truncated mutants of Cx43. Many studies have examined the effects of truncation after amino acid 257 on pH-dependence and other physiological functions (13, 16), and a mutant truncated at amino acid 263 was used for extensive structural studies (19). Both of these mutants make large gap junction plaques. Taken together with our new observations, these data imply that amino acids between 251 and 257 are critical for gap junction plaque formation.
REFERENCES
- 1.Ahmad S, Diez JA, George CH, Evans WH. Synthesis and assembly of connexins in vitro into homomeric and heteromeric functional gap junction hemichannels. Biochem J. 1999;339:247–253. [PMC free article] [PubMed] [Google Scholar]
- 2.Anumonwo JMB, Taffet SM, Gu H, Chanson M, Moreno AP, Delmar M. The carboxyl terminal domain regulates the unitary conductance and voltage dependence of connexin40 gap junction channels. Circ Res. 2001;88:666–673. doi: 10.1161/hh0701.088833. [DOI] [PubMed] [Google Scholar]
- 3.Berthoud VM, Montegna EA, Atal N, Aithal NH, Brink PR, Beyer EC. Heteromeric connexons formed by the lens connexins, connexin43 and connexin56. Eur J Cell Biol. 2001;80:11–19. doi: 10.1078/0171-9335-00132. [DOI] [PubMed] [Google Scholar]
- 4.Bevans CG, Kordel M, Rhee SK, Harris AL. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem. 1998;273:2808–2816. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- 5.Brink PR, Cronin K, Banach K, Peterson E, Westphale EM, Seul KH, Ramanan SV, Beyer EC. Evidence for heteromeric gap junction channels formed from rat connexin43 and human connexin37. Am J Physiol (Cell Physiol) 1997;273:C1386–C1396. doi: 10.1152/ajpcell.1997.273.4.C1386. [DOI] [PubMed] [Google Scholar]
- 6.Das Sarma J, Wang F, Koval M. Targeted gap junction protein constructs reveal connexin-specific differences in oligomerization. J Biol Chem. 2002;277:20911–20918. doi: 10.1074/jbc.M111498200. [DOI] [PubMed] [Google Scholar]
- 7.Elenes S, Martinez AD, Delmar M, Beyer EC, Moreno AP. Heterotypic docking of cx43 and cx45 connexons blocks fast voltage gating of cx43. Biophys J. 2001;81:1406–1418. doi: 10.1016/S0006-3495(01)75796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falk MM, Buehler LK, Kumar NM, Gilula NB. Cell-free synthesis and assembly of connexins into functional gap junction membrane channels. EMBO J. 1997;16:2703–2716. doi: 10.1093/emboj/16.10.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 10.Jiang JX, Goodenough DA. Heteromeric connexons in lens gap junction channels. Proc Natl Acad Sci USA. 1996;93:1287–1291. doi: 10.1073/pnas.93.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagree V, Brunschwig K, Lopez P, Gilula NB, Richard G, Falk MM. Specific amino-acid residues in the N-terminus and TM3 implicated in channel function and oligomerization compatibility of connexin43. J Cell Sci. 2003;116:3189–3201. doi: 10.1242/jcs.00604. [DOI] [PubMed] [Google Scholar]
- 12.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 13.Martin PE, Steggles J, Wilson C, Ahmad S, Evans WH. Targeting motifs and functional parameters governing the assembly of connexins into gap junctions. Biochem J. 2000;349:281–287. doi: 10.1042/0264-6021:3490281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez AD, Gemel J, Beyer E. Coexpression of Cx43 with Cx26 or chimeric constructs hows critical differences in heteromeric oligomerization and trafficking of connexins. Mol Biol Cell. 2002;13:353a. [Google Scholar]
- 15.Martinez AD, Hayrapetyan V, Moreno AP, Beyer EC. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ Res. 2002;90:1100–1107. doi: 10.1161/01.res.0000019580.64013.31. [DOI] [PubMed] [Google Scholar]
- 16.Moreno AP, Chanson M, Anumonwo J, Scerri I, Gu H, Taffet SM, Delmar M. Role of the carboxyl terminal of connexin43 in transjunctional fast voltage gating. Circ Res. 2002;90:450–457. doi: 10.1161/hh0402.105667. [DOI] [PubMed] [Google Scholar]
- 17.Morley GE, Ek-Vitorin JF, Taffet SM, Delmar M. Structure of connexin43 and its regulation by pHi. J Cardiovasc Electrophysiol. 1997;8:939–951. doi: 10.1111/j.1540-8167.1997.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 18.Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- 19.Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- 20.Valiunas V, Gemel J, Brink PR, Beyer EC. Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol (Heart Circ Physiol) 2001;281:H1675–H1689. doi: 10.1152/ajpheart.2001.281.4.H1675. [DOI] [PubMed] [Google Scholar]