Abstract
Tissue engineering scaffolds with complex geometries can provide an architecture that directs tissue formation. Drug delivery from these scaffolds to promote regeneration is often challenging due to the complex fabrication processes. Surface-mediated DNA delivery from multiple channel bridges was applied to deliver lipoplexes in vivo to the injured spinal cord. The surface properties of the polymer, DNA deposition with or without drying, and the presence of ECM components were investigated. In vitro studies revealed that fibronectin produced greater expression levels and immobilization efficiencies compared with collagen, laminin, and no coating. In addition, lipoplex incubation on ECM-coated PLG increased expression relative to either of the drying methods. Additionally, the incubation method had more homogeneously distributed lipoplexes and a higher number of transfected cells relative to the dried conditions. Translation to three dimensional bridges led to high levels of transgene expression in vitro. In vivo, lipoplexes immobilized to the bridge produced transgene expression levels in a rat spinal cord hemisection model that were 2-fold greater than naked plasmid. Additionally, expression with lipoplexes persisted for at least three weeks. Surface mediated delivery can be applied to scaffolds with complex geometries to promote transgene expression in vivo.
1. INTRODUCTION
The combination of gene therapy and tissue engineering scaffolds provides a versatile approach to create a conducive environment for constructing or regenerating tissues. The scaffolds function to create and maintain a space for tissue formation, and provide a support for cell adhesion and migration. Gene delivery from these scaffolds targets the host cells to serve as a bioreactor for the localized production of tissue inductive factors, which can direct cell function or influence cellular responses [1]. Non-viral vectors are attractive for their safety profile, and their ability to induce transient expression, as discontinuous therapies may enhance therapeutic efficacy [2]. However, a significant challenge remains to obtain efficient gene delivery due to decreases in vector activity or clearance from the implant site, and enhance the duration and levels of transgene expression for therapeutic effect after injury.
Non-viral vector delivery from scaffolds has been characterized as encapsulation for release, and substrate-mediated. Previous reports with vector encapsulation and release from biomaterial scaffolds, have demonstrated successful transgene expression in vivo for over 3 months [3, 4]. This encapsulation approach requires lyophilization of the vector, which may reduce activity and the vector must maintain its activity during polymer processing. An alternative to encapsulation that may retain vector activity is surface immobilization of the vector, in which the material is processes and then the vectors are immobilized. The advantage offered by this technique is its ability to being applied on scaffolds with complex geometries, or scaffolds fabricated by a wide variety of fabrication methods, such as processes involving high temperatures and organic solvents that would normally degrade or inactive the vectors. In addition, this approach has the opportunity to develop techniques to pattern DNA and thereby create controllable gradients of the inductive factors [5]. The surface-mediated DNA delivery technique has been successfully used in vitro and generally employs, relative to most controlled release strategies, DNA complexed with a transfection reagent [6]. Transfection reagents, such as cationic polymers and lipids, result in polyplexes and lipoplexes respectively, and have the ability to reduce the negative surface charge of DNA, provide protection against degradation, facilitate cellular trafficking, and support DNA immobilization through non-specific interactions (e.g., electrostatic, van der Waals, hydrophobic) and substrate-biomolecule interactions [7, 8]. On 3D scaffolds, surface-mediated delivery of polyplexes has transfected a large number of cells (> 60%) in vitro [9]. The surface properties of the material significantly impact gene delivery [10-12], as evidenced by extracellular matrix proteins (ECM) that are commonly immobilized to support cell adhesion, and have the ability to mediate vector binding [10]. Some ECM components associate with viral vectors as a means to co-localize the virus with cells and enhance cell association [13]. For polyplexes, fibronectin resulted in the highest levels of transgene expression relative to other ECM proteins [10].
This report investigates surface immobilization to deliver complexed DNA (lipoplexes) from a multiple channel bridge in order to promote transgene expression in the injured spinal cord using low quantities of DNA. Lipoplexes are generally non-toxic, however, their primary limitation for therapeutic use is their low expression levels, mainly due to the lipoplex instability upon injection in vivo [14, 15]. Local delivery of lipoplexes from a biomaterial may have the ability to maintain lipoplex stability, and therefore increase the number of transfected cells and transgene expression. After injury, spinal cord regeneration is limited by multiple barriers, including cell survival, scar tissue formation, and axonal elongation and guidance [16]. The versatility of gene delivery enables this approach to address these barriers by targeting a range of cellular processes. The spinal cord bridges used in this report contain multiple linear guidance channels and have been able to support cell infiltration and integrate effectively into the spinal cord, while the channels induced cell orientation along its major axis and supported and directed axons elongation across the channels [17, 18]. Lipoplexes were immobilized to the surface of the bridges using three strategies: i) incubation of DNA with ECM coated PLG surfaces (incubation), ii) drying of ECM onto PLG and then drying of DNA onto ECM (2-step drying), and iii) drying a mixture of DNA and ECM proteins onto PLG surfaces (1-step drying). A series of in vitro studies investigated the surface properties of the polymer, three ECM proteins, and the immobilization strategies for their ability to bind and stabilize the vector, and to transfect cells. In vivo studies were performed with a rat spinal cord lateral hemisection model using conditions identified from in vitro studies. Taken together, this combination of the bridge and gene delivery aim to combine physical and chemical guidance cues to promote spinal cord regeneration.
2. MATERIALS AND METHODS
2.1 Fabrication of PLG disks and multiple channel bridges
The fabrication methods for PLG disks and bridges have been adapted from previous reports [7, 17, 18]. Briefly, both are fabricated with high molecular weight PLG (75:25 mole ratio of D, L-lactide to glycolide, 0.76 dL/g, Lakeshore Biomaterials, Birmingham, AL). For 2D disks, PLG pellets were heated to 82 °C and pressed into a flat disk using a 5 kg weight. The temperature was incrementally decreased from 82 °C to 37 °C, after which the disks were incubated at 37 °C overnight [7]. After equilibration to room temperature, disks were cut out with a radius of 3.2 mm to fit in a 96 well plate.
For 3D multiple channel bridges, a solid mixture of PLG microspheres and NaCl particles in a 1:4 wt ratio was loaded into a mold layer by layer using the wet granulation method [4]. The mold consisted of an aluminum base, two Delrin pin guides with pre-drilled holes, and stainless steel pins to obtain multiple channels with a 250 μm diameter. Microspheres were produced by a primary oil in water (o/w) emulsion technique [4] and NaCl particles were sieved in a size range of 63-106 μm to function as porogen (WS Tyler, Mentor, OH). The molding was followed by compression molding and gas foaming using high pressure CO2 (800 psi, 16 hrs) [17, 18], with the microspheres fusing into an interconnected structure upon release (~20 psi/min) of the CO2. The construct was equilibrated at room temperature and atmospheric pressure for four hours before bridge removal from the mold. A porous multiple channel bridge was obtained by immersing the bridge in sterile water for 1 hour to leach the NaCl.
2.2 ECM coating of PLG disks and bridges
ECM components coated onto the surfaces were collagen I (BD Biosciences, San Jose, CA), fibronectin (Sigma-Aldrich, St. Louis, MO), and laminin I (Trevigen, Gaithersburg, MD). In case of the disks, PLG disks were deposited in the wells of a 96 well plate using sterile grease (Dow Corning high vacuum grease, Fisher, Pittsburgh, PA). To sterilize, they were incubated with 70% ethanol and rinsed with water. In some cases, the disks were hydrolyzed with 50 μL of 0.5 M NaOH for 1 minute and rinsed with water. A 50 μL solution of the ECM components was then applied onto the disk and allowed to evaporate overnight in a laminar flow hood. In case of the bridges, bridges were also sterilized with 70% ethanol and rinsed with water. They were dried briefly on a sterile gauze pad, placed on TCPS, after which the ECM solution was added. To adsorb a large quantity of the component, ECM was added in four consecutive steps. In case of the bridges, 6 μL of the ECM solution (2 μg/μL) was pipetted onto one side of the bridge 6 minutes after it was put onto a TCPS surface. Each consecutive adsorption step was done on a different surface of the bridge in 15 minute intervals. The bridges were then dried overnight.
2.3 Immobilization of DNA complexes to PLG disks
Plasmid encoding for firefly luciferase and β-galactosidase was complexed with Transfast in a wt/wt ratio of 1:0.5. Plasmid was purified from bacteria culture using Qiagen (Santa Clara, CA) reagent, and resuspended to obtain a high concentration in order to reduce the working volumes. DNA was complexed with the commercially available lipid, Transfast, which has been applied to neuronal cultures and has not demonstrated obvious neurotoxicity [19, 20]. Complexes were formed by adding Transfast to DNA, vortexing, and incubation for 15-30 minutes. Three surface-mediated DNA delivery techniques were performed: i) incubation: incubation of DNA with ECM coated PLG surfaces, ii) 2 steps drying: drying of DNA onto ECM coated PLG surfaces, and iii) 1 step drying: mixing of DNA with ECM and subsequent drying onto PLG surfaces. For incubation, H2O was added to the complexes to obtain a total volume of 100 μL, which was then applied onto the ECM coated disks, and incubated in a 5% CO2, 37 °C incubator for approximately 16 hrs. For 2-step drying, H2O was added to the complexes to obtain a total volume of 50 μL, which was then applied onto the ECM coated disks, and dried in a laminar flow hood overnight. For 1-step drying, 50 μL of ECM solution was added to the DNA complexes, applied onto the disks, and dried overnight in a laminar flow hood. For incubation of DNA onto ECM coated bridges, H2O was added to the complexes in small 500 μL centrifuge tubes to obtain total volumes of 50 μL. Each individual bridge was loaded into the tube, immersed in the solution, and similarly incubated for 16 hrs. For in vitro experiments, PLG surfaces were washed 2X with phosphate buffered saline (PBS) to remove unbound DNA before cell seeding.
2.4 Quantification of immobilized DNA and DNA release from PLG disks and bridges
The immobilization and release of DNA from PLG disks and bridges was monitored using DNA that was radiolabeled with α-32P dATP using a nick translation kit (Amersham Pharmacia Biotech, Piscataway, NJ) as previously reported [21]. The immobilization efficiency was reported as the amount of DNA on the surface after removing the incubation fluid (method i) and 2 washes with PBS. Stripwell microplates (Corning, Fisher) were used to analyze each well individually. The incubation fluid, two washes, and disks were immersed separately in scintillation cocktail (Biosafe II, Fisher) for measurement with a scintillation counter. A DNA release study from the bridges was performed in 500 μL Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco Invitrogen, Carlsbad, CA) in a 5% CO2, 37 °C incubator by transferring the bridges into fresh media, and measuring the amount of DNA released at each time point. At the end of the study, the bridges were immersed in scintillation cocktail to measure the amount of DNA left on the bridge. The cumulative release was reported as the total DNA released at each time point after washing divided by the total DNA released at the end of the study plus the DNA left on the bridge. The release profiles of complexed and uncomplexed DNA were compared.
2.5 Cell seeding onto PLG disks and bridges and quantification of cell proliferation
In vitro transfection experiments were performed with HEK293T cells cultured onto the PLG surfaces. For the disks, 20,000 cells were seeded per well, while the bridges were incubated in groups of three in 0.4 mL DMEM containing 106 cells on a shaking plate at 100 RPM for 4 hours in a 5% CO2, 37 °C incubator. After cell seeding, each bridge was transferred to an individual well in a 24 well plate containing 500 μL of DMEM. To quantify cell proliferation at a specific timepoint, a cell proliferation assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS), Promega, Madison, WI) was performed and analyzed using a 96-well plate reader at an absorbance of 490 nm.
2.6 Visualization of DNA immobilization and transfected cells
DNA complexes were fluorescently-tagged with Rhodamine (red) (DNA Rhodamine labeling kit, Mirus Bio, WI) to be visualized after immobilization to the PLG surfaces. In order to observe transfected cells onto the surfaces, complexes were formed with plasmid encoding for β-galactosidase, and an X-gal stain (blue) was performed after 2 days [22].
2.7 Rat spinal cord hemisection model
In vivo transfection studies were performed using a rat spinal cord hemisection model [17]. Forty female Long-Evans rats (Charles River, 180-200 g) were treated according to ACUC guidelines at Northwestern University. The rats were pre-handled for two weeks pre-surgery and anaesthetized using an RC2 Rodent Anesthesia System (Colonial Medical Supply, Franconia, NH) with vaporized Isoflurane (Baxter, Deerfield, IL). To create a complete lateral hemisection, a laminectomy was performed at T9-10 and a 4 mm long spinal cord segment, lateral of the midline, was removed. The bridges were implanted in the injury space and covered with Gelfoam. The muscles were sutured together and the skin was stapled. Post-operative care consisted of the administration of baytril (Enrofloxacin 2.5 mg/kg s.c., once a day for 2 weeks), buprenorphine (0.01 mg/kg s.c., twice a day for 2 days), and lactate ringer solution (5 mL/100 g, once a day for 5 days). Bladders were expressed twice a day until bladder function recovered.
2.8 Quantification of transgene expression using a luciferase assay
The levels of transgene expression in vitro and in vivo were quantified using complexes formed with plasmid encoding for firefly luciferase. The amount of luciferase produced by the cells was measured using the Luciferase Assay System (Promega). For disks, 100 μL lysis buffer (1X Reporter Lysis Buffer, Promega) was added to the wells, while bridges were transferred to low retention microcentrifuge tubes containing 100 μL lysis buffer. Bridges were then cut in small pieces using microscissors. All surfaces were washed once with PBS before lysing.
For in vivo studies, rats were sacrificed and the spinal cord was retrieved. The injury site and 5 segments of 0.5 cm length rostral and caudal of the injury site were collected and stored on dry ice until transferred to -80 °C. Each tissue segment was cut using microscissors and refrozen before lysing with 100 μL lysis buffer (1X Cell Culture Lysis Reagent, Promega). The tissue segments were then vortexed within the lysis buffer and centrifuged at 14,000 RPM for 10 minutes. The supernatant was removed and used to measure luciferase activity using a luminometer. All luciferase readings were normalized with the total protein amount measured by the enhanced test tube protocol of the BCA (bicinchoninic acid) protein assay (Pierce, Rockford IL).
2.9 Statistical analysis
Statistical analyses were done using statistical package JMP (SAS, Cary, NC). For multiple pairs comparison, an ANOVA with post-hoc Tukey test was performed with a p-level of 0.05. A t-test was performed to analyze differences between individual pairs. Error bars represent standard deviations in all figures.
3. RESULTS
3.1 ECM coated PLG for surface-mediated DNA delivery
Multiple ECM components (fibronectin, collagen I, laminin I) and DNA deposition methods were analyzed for surface-mediated DNA delivery from PLG surfaces. Initial studies focused on surface modification of the polymer by hydrolysis, which has been reported to enhance binding of ECM components [23, 24]. The amount of DNA immobilized to each surface was 1 μg, and mixed and dried with 25 μg ECM. Expression was observed in all conditions, and surface hydrolysis did not enhance transgene expression for any of the surface coatings (Fig. 1A). For laminin, hydrolysis of the scaffolds significantly decreased expression (p<0.05); however, expression levels with laminin were relatively low. Given these transfection results with surface hydrolysis, all succeeding experiments were performed without pre-hydrolyzing the surfaces.
Figure 1.
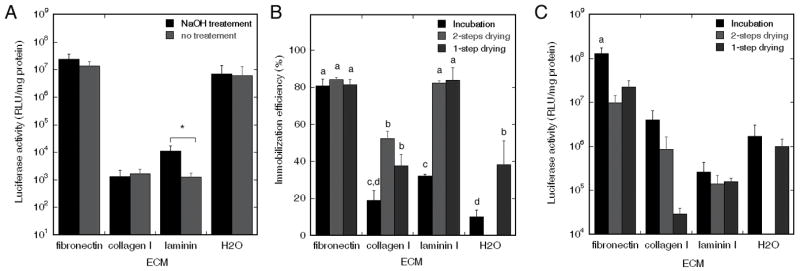
Transgene expression for surface treatment, and adsorption efficiency and expression levels for multiple deposition methods. (A) Transgene expression for PLG surfaces with and without pre-hydrolyzing treatment. DNA complexes were mixed with ECM (25 μg) and subsequently dried on PLG disks. Significant differences based on a t-test between surfaces with and without pre-hydrolyzing treatment are denoted by an asterisk (p<0.05). (B) Immobilization efficiency of DNA complexes onto ECM (25 μg) coated PLG disks using i) incubation, ii) 2-step drying, and iii) 1-step drying. (C) Transgene expression in vitro using the three deposition methods. Significant differences based on a Tukey multiple comparisons analysis are denoted by different letters (p<0.05).
The immobilization efficiency was subsequently investigated as a function of the ECM coating and the method of immobilization. The maximal immobilization efficiency was approximately 80%, and was achieved with fibronectin for all three coating methods, or with laminin using the 1- and 2-step drying methods (Fig. 1B). The drying methods enhanced immobilization for all surfaces with the exception of fibronectin. Both fibronectin and laminin I provided improved adsorption relative to uncoated surfaces.
In transfection studies, fibronectin-coated surfaces produced the highest levels of expression relative to other coatings (Fig. 1C). Additionally, incubation resulted in greater expression for all ECM coatings relative to the dried conditions, though the drying methods did retain the DNA activity. The highest expression levels achieved with fibronectin do correlate with the extent of DNA immobilized; however, the process of drying complexes in the presence of fibronectin does reduce expression levels by a factor of 6-to 13-fold. Drying the DNA in 1 or 2 steps using laminin, which resulted in similar levels of DNA binding as with fibronectin, resulted in expression that was decreased by 2 orders of magnitude relative to fibronectin. Most ECM coated PLG surfaces did not demonstrate a difference between the 1 or 2 steps drying techniques. Only for collagen I, drying the DNA complexes after collagen deposition was advantageous relative to 1-step drying. Based on these results, further surface-mediated DNA delivery studies were all performed using fibronectin coated PLG surfaces.
3.2 Transfected cells and DNA complexes on fibronectin coated PLG
Incubation of DNA complexes resulted in an increased number of transfected cells and less aggregation of the complexes (Fig. 2A and B) relative to the drying methods. The amount of fibronectin dried onto the disks was 25 μg, with 1 μg of DNA incubated to each surface. For the incubation method, DNA complexes were homogeneously distributed on the surface, and aggregation of the complexes was not readily apparent. Transfected cells were present in large numbers on the surface and were also homogeneously distributed across the surface. Complexes deposited by 1-step or 2-step drying exhibited some aggregation (Fig. 2, D and F), and were less homogeneously distributed on the surface relative to incubation. Many transfected cells were observed with the dried complexes (Fig. 2, C and E), though the numbers were less than with incubation and the transfected cells were preferentially observed at the edge of the polymer than in the center.
Figure 2.
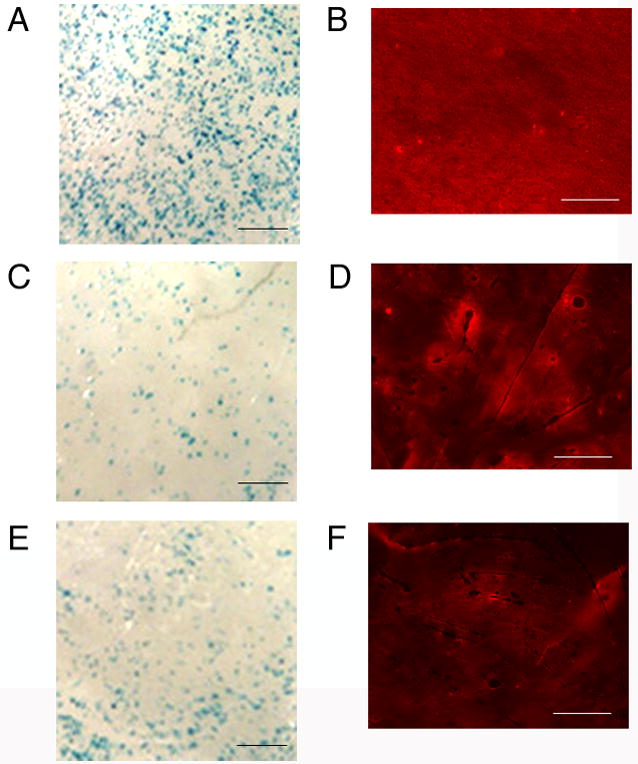
Distribution of transfected cells and immobilized complexes. Transfected cells (blue: A,C,E) and immobilized lipoplexes (red: B,D,F) on fibronectin (25 μg) coated disks. Complexes were deposited by incubation (A,B), 2-step drying (C,D), or 1-step drying (E,F). Transfected cells: 10X magnification, scale bar: 200 μm; fluorescently labeled DNA: scale bar: 400 μm.
3.3 Cell proliferation and transfection for varying amounts of fibronectin
The dose response for fibronectin was subsequently investigated, with characterization of both cell proliferation and expression levels. PLG disks were coated with 0 μg to 50 μg of fibronectin. Cell proliferation was maximal for a fibronectin coating of 25 μg fibronectin per disk, though the presence of fibronectin at all densities enhanced proliferation above the control (Fig. 3A). At the maximal condition, the number of viable cells present on fibronectin coated disks after 2 days was 50 percent greater than on control disks.
Figure 3.
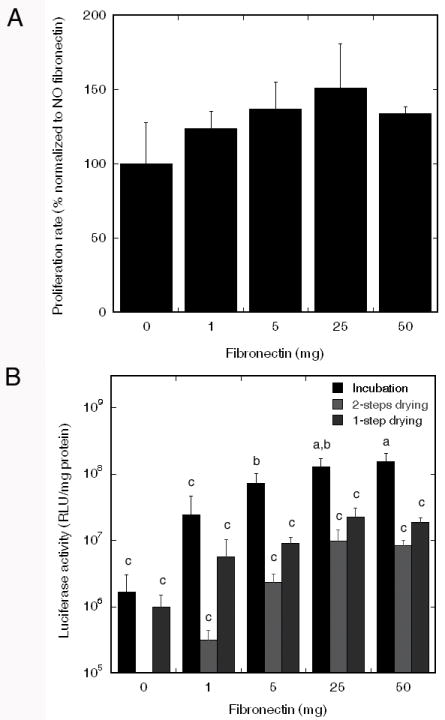
Fibronectin density and transfection. (A) Cell proliferation on PLG surfaces coated with multiple fibronectin densities. (B) Transgene expression on PLG disks coated with varied fibronectin density. Significant differences based on a Tukey multiple comparisons analysis are denoted by different letters (p<0.05).
Transfection studies indicated that complexes incubated on disks containing 25 or 50 μg fibronectin had significantly higher levels of transgene expression (> 1.2*108) compared with control disks and 1 μg fibronectin (Fig. 3B). For control disks and 1 μg fibronectin, incubation did not significantly enhance transgene expression compared with the 1 and 2 steps drying methods. Based on these results, subsequent studies were performed using PLG coated with 25 μg fibronectin, and lipoplexes were deposited by incubation.
3.4 DNA Dose Response
We next investigated the quantity of DNA to maximize transgene expression. Six doses of DNA (0, 1, 3, 5, 10, and 20 μg) were incubated on PLG disks coated with 25 μg of fibronectin. Increasing the DNA dose produced increasing levels of transgene expression through a dose of 3 μg, but subsequent increases in DNA amounts did not alter transgene expression (Fig. 4).
Figure 4.
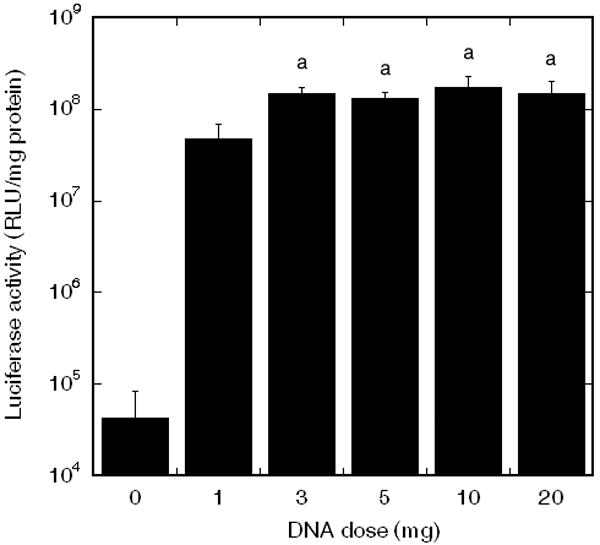
Transgene expression on fibronectin coated PLG disks incubated with multiple doses of DNA complexes. Significant differences based on a Tukey multiple comparisons analysis are denoted by different letters (p<0.05).
3.5 Substrate-mediated DNA delivery from 3D multiple channel bridges in vitro
The immobilization strategy obtained on 2D PLG surfaces was next translated to the 3D PLG multiple channel spinal cord bridges. Bridges were coated with a total of 24 μg fibronectin that was applied in 4 steps, each at a concentration of 2 μg/μL. A Sirius red stain indicated the presence of fibronectin on the outside and in the center of the bridge, with control bridges not stained (Fig. 5A). Applying the incubation technique, fluorescently tagged DNA complexes bound throughout the bridge (Fig. 5B). Approximately 15% of the DNA immobilized to the bridge was lost during washes. For the DNA still immobilized on the bridge after washing, lipoplexes were released more slowly from the bridge relative to naked plasmid (Fig. 5C), with the cumulative release of naked plasmid and lipoplexes being significantly different (p<0.02) for all timepoints after the two hour timepoint (p<0.04). During the first 11 hours, 69.3 ± 3.7% of the lipoplexes had been released from the bridges, compared to 94.7 ± 1.0% for naked plasmid. After 21 days, most of the lipoplexes had been released, with 11.6 ± 1.1% remaining on the surface. Fibronectin coated bridges incubated with varying doses of lipoplexes resulted in higher expression levels with increasing doses of DNA (Fig. 5D). The maximum amount of DNA that could be incubated with the bridge was 32 μg, which was based on the volume of the lipoplexes and the volume of the bridge. Transgene expression levels were 2.3*107 RLU/mg protein at this maximal dose.
Figure 5.
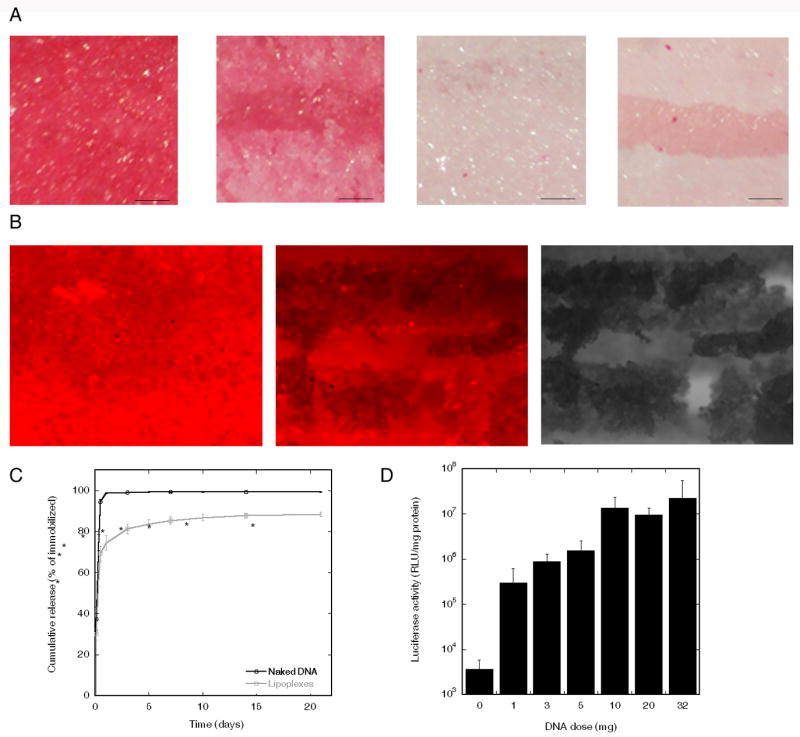
ECM and complex deposition and transfection on bridges. (A) Sirius red stain of fibronectin coated multiple channel bridges. From left to right: top view and cross section of bridge coated with 24 μg fibronectin, and top view and cross-section of uncoated bridge. Scale bar: 150 μm. (B) Bridge coated with 24 μg fibronectin and incubated with 1 μg fluorescently tagged DNA (red). From left to right: top view and cross section of bridge imaged with fluorescence microscopy, and cross section bridge imaged with phase contrast microscopy, Scale bar: 200 μm. (C) Release of uncomplexed and complexed DNA. Significant differences based on a t-test are denoted by an asterisk (p<0.05). (D) Transgene expression in vitro for different doses of DNA incubated with fibronectin coated bridges.
3.6 Gene expression from lipoplex loaded bridges implanted in spinal cord
Transgene expression in the spinal cord was subsequently investigated by implantation of the multiple channel bridges into a rat spinal cord lateral hemisection model. Bridges were coated with 24 μg fibronectin and incubated with 16 μg DNA, either naked or complexed with lipid. Bridges with immobilized lipoplexes had transgene expression that was substantially greater after 1 week compared with uncomplexed DNA, demonstrating 2-fold higher levels of transgene expression at the injury site. Interestingly, expression levels 0.5 cm caudal of the injury site were increased 350-fold for lipoplexes relative to naked plasmid, whereas segments 0.5 cm rostral and 1 cm caudal had 85-fold increased expression (Fig. 6A). The duration of transgene expression after implantation of lipoplex loaded bridge after spinal cord injury was subsequently investigated, with implants retrieved after 3 days, 1 week, 2 weeks, and 3 weeks (Fig. 6B). After 3 days, the highest level was observed at the injury site, while after 1, 2, and 3 weeks, the highest expression was located in the tissue immediately adjacent to the injury site. At two weeks the levels were decreased by a factor of 25, while expression levels decreased further after 3 weeks. Doubling the dose of lipoplexes incubated with the bridges did not enhance the expression levels (n=4, data not shown).
Figure 6.
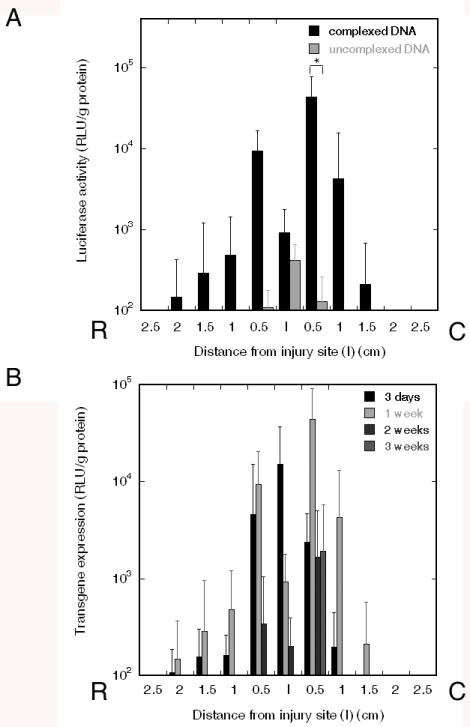
In vivo expression with spinal cord bridges. (A) Transgene expression in rat spinal cord hemisection model after 1 week using either 16 μg complexed (n=8) or naked plasmid (n=4) incubated with fibronectin coated bridges. Significant differences based on an ANOVA with posthoc Wilcoxon 1-way test are denoted by an asterisk (p<0.05). (B) Transgene expression at multiple time points after implantation of fibronectin coated bridges incubated with 16 μg lipoplexes: 3 days (n=10), 1 week (n=8), 2 weeks (n=10), 3 weeks (n=4).
4. DISCUSSION
This report investigated the deposition method of lipoplexes onto PLG scaffolds and translated surface-mediated DNA delivery techniques to achieve in vivo transgene expression in the spinal cord. Lipoplexes were delivered from PLG by immobilization in the presence of ECM components and also with and without drying. These results revealed that incubating DNA led to higher levels of transgene expression compared with drying the DNA onto an ECM coated surface (2 steps drying), or drying the DNA mixed with ECM (1 step drying). DNA complexes were less aggregated with incubation compared with drying, resulting in a significantly greater number of transfected cells. This result is not surprising as lipid/DNA complexes typically have reduced activity following dehydration, though stabilizers may be able to minimize the reduction in activity [25], [26]. The incubation method was subsequently applied to multiple channel bridges, which are porous to support cell infiltration, and have channels to organize cells and orient axonal elongation [17]. Fibronectin and lipoplexes were distributed throughout the bridge, and led to transfection in vitro, indicating that surface-mediated DNA delivery can be adapted to three-dimensional structures with complex geometry. In vivo, transgene expression was observed for small quantities of DNA lipoplexes immobilized to the bridge. Relative to naked plasmid, transgene expression was significantly increased and persisted for up to 3 weeks with the highest levels observed adjacent to the injury site.
The incubation method for lipoplex deposition on fibronectin significantly enhanced transgene expression relative to collagen I, laminin I, and uncoated PLG. These results were in agreement with trends observed when DNA/PEI complexes were incubated on ECM coated tissue culture polystyrene (TCPS) surfaces [10], which revealed that both collagen I and fibronectin yielded in the highest DNA internalization by the cells, but that fibronectin led to the highest levels of expression. The specific mechanism by which fibronectin enhances transgene expression remains unclear; however, several reports suggest that multiple aspects of the transfection process may be affected. Coating of surfaces with proteins has enhanced the number of transfected cells with immobilized lipoplexes, though fibronectin led to greater increases in expression for polyplexes [27]. Fibronectin and other immobilized proteins can reduce the hydrophobicity of PLG surfaces, which can reduce aggregation of DNA complexes and facilitate transfection in vitro [9, 27]. Immobilized fibronectin can increase the contact area between cells and the surface, which may increase the quantity of DNA that associates with cells. Fibronectin may also influence cellular processes that enhance gene transfer. Proliferation is greater on fibronectin relative to uncoated PLG, and proliferation has been linked to enhanced gene transfer [28]. Fibronectin has been suggested to target endocytosis through caveolae [10], or enhance internalization due to the cells’ actin filaments, causing mechanical stress on the cell membrane and nucleus, which may enhance DNA uptake [29]. Interestingly, the density of fibronectin that maximized gene transfer in this report (0.78 μg/mm2 density), is significantly greater than that reported for culture on TCPS (0.025 μg/mm2) [10]. This result may be due to different interactions between DNA/PEI polyplexes and lipoplexes with fibronectin coated surfaces or the binding efficiency of fibronectin to different surface chemistries [27].
The surface-mediated DNA delivery technique was translated from 2D PLG surfaces to 3D multiple channel spinal cord bridges. Spinal cord bridges have been fabricated from a range of materials, with the objective to stabilize the injury area, and generally provide a favorable environment for spinal cord regeneration [30-39]. The bridge structure, used in this report was composed of PLG, which has been implanted previously in the spinal cord [17, 40], and has been extensively used to fabricate scaffolds for localized gene transfer by encapsulation and release of plasmid and polyplexes [3, 4, 17, 22, 41]. The spinal cord, however, is a challenging site for gene delivery due to the constant exchange of cerebrospinal fluid, which may lead to faster clearance of DNA relative to other sites. The ability to promote transgene expression in vivo by vector delivery from polymers will also depend on the implantation site due to a variety of physiological conditions and cell types present [3, 42, 43]. Gene therapy approaches inside the spinal cord usually inject viral DNA intrathecally in the cerebral spinal fluid, which is located in between the layers of the meninges, or implant viral genetically modified cells (e.g. fibroblasts, Schwann cells) [44-46]. Challenges associated with these methods include the initiation of inflammatory responses toward the viral DNA, and cell migration and death of transplanted cells. As an alternative, non-viral DNA has been delivered after spinal cord injury by local injection of lipoplexes but resulted in limited transgene expression [47].
Lipoplexes, immobilized to the surface of the spinal cord bridge resulted in the highest levels of trangene expression in vitro with 10 μg of DNA or more incubated onto fibronectin coated bridges. In vivo, lipoplexes increased transgene expression levels by two orders of magnitude relative to similar quantities of plasmid delivered, with 16 μg of DNA incubated. The immobilization of lipoplexes to the bridge may have the ability to better maintain the lipoplexes at the implant site and subsequently increase local transgene expression. Increasing the exposure time of lipoplexes to cells has demonstrated to enhance the number of plasmid copies inside the cell, leading to a higher number of cells expressing the gene [48]. In addition, the delivery of lipoplexes may enhance the activity of the DNA after its release from the bridge, which was indicated by the significantly different levels of transgene expression between plasmid and lipoplexes, and the high levels of transgene expression in the spinal cord segments adjacent to the bridge in case of lipoplex immobilization. An alternative approach to maintaining effective concentrations locally is to encapsulate DNA within the bridge and provide a sustained release in which the DNA lost to clearance can be replaced by release from the bridge. In a previous report with these multiple channel bridges, we investigated the encapsulation and release of 800 μg of plasmid, which resulted in transgene expression for up to two weeks, with decreasing levels further away from the injury site [17]. In this report, transfection with immobilized DNA was performed with a 50-fold lower amount of DNA, resulting in overall higher levels of transgene expression after spinal cord implantation, and an extended duration of expression, as higher levels of transgene expression were observed after 2 and 3 weeks compared with encapsulation and release of naked plasmid [17].
5. CONCLUSION
This report indicates that lipoplexes can be immobilized to ECM coated PLG, with fibronectin maximizing gene transfer. Incubation of lipoplexes onto fibronectin coated PLG led to high expression levels in vitro and allowed for lipoplex immobilization to pre-fabricated scaffolds with a complex geometry. In vivo, multiple channel bridges, immobilized with lipoplexes were implanted in the spinal cord after injury and resulted in higher levels of transgene expression compared with naked plasmid. Delivery of lipoplexes from spinal cord bridges was demonstrated to be an efficient DNA delivery system after injury, as a small amount of DNA was sufficient to induce transgene expression up to 3 weeks. Surface-mediated delivery of lipoplexes from a fibronectin coated multiple channel bridge is a promising technique that combines tissue engineering and gene therapy for spinal cord regeneration.
Acknowledgments
The authors are grateful to Jennifer Cruz Rea for technical assistance. Financial support for this research was provided by the NIH (RO1 EB005678, R21 EB006520, RO1 EB 003806).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillery A. Continuous v. discontinuous therapy. Pharmaceutical Science & Technology Today. 1998;1:6–7. [Google Scholar]
- 3.Huang YC, Riddle K, Rice KG, Mooney DJ. Long-term in vivo gene expression via delivery of PEI-DNA condensates from porous polymer scaffolds. Hum Gene Ther. 2005;16:609–17. doi: 10.1089/hum.2005.16.609. [DOI] [PubMed] [Google Scholar]
- 4.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: transgene expression and cellular transfection. Mol Ther. 2005;12:475–83. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houchin-Ray T, Whittlesey KJ, Shea LD. Spatially patterned gene delivery for localized neuron survival and neurite extension. Mol Ther. 2007;15:705–12. doi: 10.1038/mt.sj.6300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Laporte L, Cruz Rea J, Shea LD. Design of modular non-viral gene therapy vectors. Biomaterials. 2006;27:947–54. doi: 10.1016/j.biomaterials.2005.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houchin-Ray T, Swift LA, Jang JH, Shea LD. Patterned PLG substrates for localized DNA delivery and directed neurite extension. Biomaterials. 2007;28:2603–11. doi: 10.1016/j.biomaterials.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segura T, Chung PH, Shea LD. DNA delivery from hyaluronic acid-collagen hydrogels via a substrate-mediated approach. Biomaterials. 2005;26:1575–84. doi: 10.1016/j.biomaterials.2004.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang JH, Bengali Z, Houchin TL, Shea LD. Surface adsorption of DNA to tissue engineering scaffolds for efficient gene delivery. J Biomed Mater Res A. 2006;77:50–8. doi: 10.1002/jbm.a.30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bengali Z, Rea JC, Shea LD. Gene expression and internalization following vector adsorption to immobilized proteins: dependence on protein identity and density. J Gene Med. 2007;9:668–78. doi: 10.1002/jgm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pannier AK, Anderson BC, Shea LD. Substrate-mediated delivery from self-assembled monolayers: effect of surface ionization, hydrophilicity, and patterning. Acta Biomater. 2005;1:511–22. doi: 10.1016/j.actbio.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pannier AK, Wieland JA, Shea LD. Surface polyethylene glycol enhances substrate-mediated gene delivery by nonspecifically immobilized complexes. Acta Biomater. 2008;4:26–39. doi: 10.1016/j.actbio.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajaj B, Lei P, Andreadis ST. High efficiencies of gene transfer with immobilized recombinant retrovirus: kinetics and optimization. Biotechnol Prog. 2001;17:587–96. doi: 10.1021/bp010039n. [DOI] [PubMed] [Google Scholar]
- 14.Wyatt SK, Giorgio TD. DNA delivery to cells in culture using cationic liposomes. Methods Mol Biol. 2004;245:83–94. doi: 10.1385/1-59259-649-5:83. [DOI] [PubMed] [Google Scholar]
- 15.Patil SD, Rhodes DG, Burgess DJ. DNA-based therapeutics and DNA delivery systems: a comprehensive review. Aaps J. 2005;7:E61–77. doi: 10.1208/aapsj070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 17.De Laporte L, Yang Y, Zelivyanskaya ML, Cummings BJ, Anderson AJ, Shea LD. Plasmid releasing multiple channel bridges for transgene expression after spinal cord injury. Mol Ther. 2008 doi: 10.1038/mt.2008.252. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, De Laporte L, Zelivyanskaya M, Whittlesey KJ, Anderson AJ, Shea LD. Multiple channel bridges for spinal cord injury: cellular characterization of host response. J Biomed Mater Res. 2008 doi: 10.1089/ten.tea.2009.0081. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ango F, Albani-Torregrossa S, Joly C, Robbe D, Michel JM, Pin JP, et al. A simple method to transfer plasmid DNA into neuronal primary cultures: functional expression of the mGlu5 receptor in cerebellar granule cells. Neuropharmacology. 1999;38:793–803. doi: 10.1016/s0028-3908(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 20.Ango F, Fagni L. DNA transfer to neuronal primary cultures using TransFast™ transfection reagent. Applications in neuroscience. 2000;1:14–17. [Google Scholar]
- 21.Segura T, Volk MJ, Shea LD. Substrate-mediated DNA delivery: role of the cationic polymer structure and extent of modification. J Control Release. 2003;93:69–84. doi: 10.1016/j.jconrel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–4. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 23.Park GE, Pattison MA, Park K, Webster TJ. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials. 2005;26:3075–82. doi: 10.1016/j.biomaterials.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Salvay DM, Rives CB, Zhang X, Chen F, Kaufman DB, Lowe WL, et al. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation. 2008;85:1456–1464. doi: 10.1097/TP.0b013e31816fc0ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anchordoquy TJ, Carpenter JF, Kroll DJ. Maintenance of transfection rates and physical characterization of lipid/DNA complexes after freeze-drying and rehydration. Arch Biochem Biophys. 1997;348:199–206. doi: 10.1006/abbi.1997.0385. [DOI] [PubMed] [Google Scholar]
- 26.Chiantia S, Kahya N, Schwille P. Dehydration damage of domain-exhibiting supported bilayers: an AFM study on the protective effects of disaccharides and other stabilizing substances. Langmuir. 2005;21:6317–23. doi: 10.1021/la050115m. [DOI] [PubMed] [Google Scholar]
- 27.Bengali Z, Pannier AK, Segura T, Anderson BC, Jang JH, Mustoe TA, et al. Gene delivery through cell culture substrate adsorbed DNA complexes. Biotechnol Bioeng. 2005;90:290–302. doi: 10.1002/bit.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng WC, Haselton FR, Giorgio TD. Mitosis enhances transgene expression of plasmid delivered by cationic liposomes. Biochim Biophys Acta. 1999;1445:53–64. doi: 10.1016/s0167-4781(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 29.Yoshikawa T, Uchimura E, Kishi M, Funeriu DP, Miyake M, Miyake J. Transfection microarray of human mesenchymal stem cells and on-chip siRNA gene knockdown. J Control Release. 2004;96:227–32. doi: 10.1016/j.jconrel.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 30.Stokols S, Sakamoto J, Breckon C, Holt T, Weiss J, Tuszynski MH. Templated Agarose Scaffolds Support Linear Axonal Regeneration. Tissue Eng. 2006 doi: 10.1089/ten.2006.12.2777. [DOI] [PubMed] [Google Scholar]
- 31.Stokols S, Tuszynski MH. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials. 2006;27:443–51. doi: 10.1016/j.biomaterials.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 32.Geller HM, Fawcett JW. Building a bridge: engineering spinal cord repair. Exp Neurol. 2002;174:125–36. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- 33.Houweling DA, Lankhorst AJ, Gispen WH, Bar PR, Joosten EA. Collagen containing neurotrophin-3 (NT-3) attracts regrowing injured corticospinal axons in the adult rat spinal cord and promotes partial functional recovery. Exp Neurol. 1998;153:49–59. doi: 10.1006/exnr.1998.6867. [DOI] [PubMed] [Google Scholar]
- 34.King VR, Phillips JB, Hunt-Grubbe H, Brown R, Priestley JV. Characterization of non-neuronal elements within fibronectin mats implanted into the damaged adult rat spinal cord. Biomaterials. 2006;27:485–96. doi: 10.1016/j.biomaterials.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Moore K, MacSween M, Shoichet M. Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng. 2006;12:267–78. doi: 10.1089/ten.2006.12.267. [DOI] [PubMed] [Google Scholar]
- 36.Moore MJ, Friedman JA, Lewellyn EB, Mantila SM, Krych AJ, Ameenuddin S, et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27:419–29. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 37.Oudega M, Gautier SE, Chapon P, Fragoso M, Bates ML, Parel JM, et al. Axonal regeneration into Schwann cell grafts within resorbable poly(alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials. 2001;22:1125–36. doi: 10.1016/s0142-9612(00)00346-x. [DOI] [PubMed] [Google Scholar]
- 38.Prang P, Muller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27:3560–9. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, De Laporte L, Rives CB, Jang JH, Lin WC, Shull KR, et al. Neurotrophin releasing single and multiple lumen nerve conduits. J Control Release. 2005;104:433–46. doi: 10.1016/j.jconrel.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99:3024–9. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12:418–26. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 42.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1:363–9. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 43.Rives CB, des Rieux A, Zelivyanskaya M, Stock SR, Lowe WL, Shea LD. Layered PLG Scaffolds for In Vivo Plasmid Delivery. Biomaterials. 2008 doi: 10.1016/j.biomaterials.2008.09.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber AB, Ehrengruber MU, Schwab ME, Brosamle C. Adenoviral gene transfer to the injured spinal cord of the adult rat. Eur J Neurosci. 2000;12:3437–42. doi: 10.1046/j.1460-9568.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- 45.Mannes AJ, Caudle RM, O’Connell BC, Iadarola MJ. Adenoviral gene transfer to spinal-cord neurons: intrathecal vs. intraparenchymal administration. Brain Res. 1998;793:1–6. doi: 10.1016/s0006-8993(97)01422-4. [DOI] [PubMed] [Google Scholar]
- 46.Nakahara Y, Gage FH, Tuszynski MH. Grafts of fibroblasts genetically modified to secrete NGF, BDNF, NT-3, or basic FGF elicit differential responses in the adult spinal cord. Cell Transplant. 1996;5:191–204. doi: 10.1177/096368979600500209. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi K, Schwarz E, Ljubetic C, Murray M, Tessler A, Saavedra RA. DNA plasmid that codes for human Bcl-2 gene preserves axotomized Clarke’s nucleus neurons and reduces atrophy after spinal cord hemisection in adult rats. J Comp Neurol. 1999;404:159–71. doi: 10.1002/(sici)1096-9861(19990208)404:2<159::aid-cne2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 48.Tseng WC, Haselton FR, Giorgio TD. Transfection by cationic liposomes using simultaneous single cell measurements of plasmid delivery and transgene expression. J Biol Chem. 1997;272:25641–7. doi: 10.1074/jbc.272.41.25641. [DOI] [PubMed] [Google Scholar]


