Abstract
The cause of reduced fecundity in women with endometriosis is unknown. Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) by both ectopic and eutopic endometrium reportedly has a role in the pathogenesis of endometriosis. We hypothesize that anomalous endometriotic TIMP protein synthesis, secretion, and localization also cause reproductive pathologies resulting in reduced fecundity. An established rat model for endometriosis (Endo) compared with nonendometriotic controls (Shams) was used to investigate reduced fecundity in endometriosis. Comparing Endo and Sham rats, Endo rats had altered ovarian dynamics, including fewer ovarian follicles and corpora lutea with luteinized unruptured follicles. Furthermore, in vivo anomalies in postovulatory oocyte structure and preimplantation embryo development, including misaligned chromosomes, nuclear and cytoplasmic fragmentation, and delayed or arrested cleavage, as well as spontaneous abortions, were found only in Endo rats. A causative role for TIMP1 in these phenomena is supported by our findings that Endo rats have more TIMP1 in their peritoneal fluid as detected by ELISA and more TIMP1 immunolocalization in the theca of antral follicles as measured by computer-assisted morphometric analysis. These data suggest that in endometriosis the accumulation of TIMP1 disrupts the normal MMP/TIMP enzymatic milieu in the peritoneal cavity and negatively affects ovarian dynamics, oocyte quality, and preimplantation embryo development, thereby decreasing fecundity. Most intriguingly, daughters of Endo rats that had no experimental interventions exhibited these same reproductive abnormalities. We predict that developmental exposure to endometriosis leads to permanent epigenetic changes in subsequent generations.
Keywords: embryo, endometriosis, infertility, ovary, TIMP1
Production and localization of endometriotic TIMP1 correlate with reduced ovarian function, anomalous oocyte structure and embryo development, and spontaneous pregnancy loss in female rats with surgically induced endometriosis and in their daughters.
INTRODUCTION
Endometriosis is a benign gynecological disorder in which shed endometrial tissues survive in the extrauterine environment, commonly implanting within the peritoneal cavity. The exact prevalence of endometriosis among the population is poorly defined because of the need to perform invasive procedures to determine the affected population. There is evidence demonstrating an association between endometriosis and infertility [1–14]. Up to 40% of women undergoing laparoscopic evaluation for infertility had evidence of peritoneal endometriosis [4].
Historically, endometriosis-associated infertility in women has been associated with significant but subtle abnormalities [1, 8, 12, 15–20]. These include ovarian anomalies such as the reduced rates of follicular growth, functional capacity of the preovulatory follicle, and early luteal function [1, 12, 16, 17]. Gamete and embryo anomalies include reduced rates of fertilization and defects in embryo development [12, 18, 20, 21]. Furthermore, endometriosis is associated with implantation failure and early pregnancy loss [19, 22]. Yet, to date a cause-and-effect relationship between endometriosis and reduced fecundity has not been established, to our knowledge.
Diverse assisted reproduction techniques have yielded conflicting outcomes on this issue. Investigations have shown that pregnancy outcome after in vitro fertilization (IVF) is similar in women with and without endometriosis [23]. However, other researchers have reported that fertilization and/or preimplantation embryo cleavage rates after IVF both in stimulated and nonstimulated cycles were significantly lower in women with endometriosis compared with women with tubal factor infertility and compared with couples with male factor-associated infertility [18, 24]. Fertilization and embryo cleavage rates did not improve in women with endometriosis after spermatozoa from their partners were substituted with spermatozoa from donors [18].
Investigations of early pregnancy loss in women with endometriosis are also inconclusive and are confounded by the unreliability of comparing retrospectively ascertained spontaneous abortion rates with those of prospective studies [19, 22, 25]. The incidence of first-trimester abortion for untreated women with endometriosis has been estimated to be as high as 52% [19] compared with the incidence in the general population, which is estimated to be between 5% and 20%. Some argue that most spontaneous abortions associated with endometriosis are not a direct result of the endometriosis [22]. Hence, further studies are needed to determine if endometriosis causes an increase in first-trimester pregnancy loss and to decipher whether ovarian, oocyte, and embryo abnormalities or anomalies in embryo implantation or placentation reduce fecundity.
Controlled studies of ovarian tissues from infertile women designed to test the consequence of endometriosis on fertility are ethically limited. Animal models for endometriosis provide an invaluable tool to study risk factors, prevalence, and the pathogenesis and pathophysiologies of endometriosis [26, 27]. Beyond the mere growth of endometrial implants in ectopic locations, rats with surgically induced endometriosis (Endo) display pathophysiologies similar to those of primates and humans with the disease, including pain and infertility [26–28]. An association between the presence of ectopic endometriotic implants in rats and reduced fecundity has been described [27, 29, 30]. However, the causative mechanisms for these phenomena were not investigated.
Matrix metalloproteinases (MMPs) are a multimember family of structurally related proteins that degrade extracellular matrix and basement membrane components [31]. Tissue inhibitors of metalloproteinases (TIMPs) inhibit MMPs to facilitate controlled proteolysis. Normal follicular development, ovulation, formation and regression of the corpora lutea (CL), embryo development, and embryo implantation require tightly coordinated remodeling of extracellular matrices by MMPs and TIMPs [32–43].
Significant evidence is present in the literature describing a role for anomalous MMP and TIMP production in the pathogenesis of endometriosis [44, 45]. We and others have shown that, compared with the expression of MMPs and TIMPs in normal endometrial remodeling, both human endometriotic lesions and rat endometriotic implants synthesize and secrete MMPs and TIMPs [46–50]. TIMP1 represents at least 10%–15% of the secretory proteins produced by both rat implants and human endometriotic lesions [46, 51]. Thus, the ovaries reside in a suboptimal environment of peritoneal fluid containing elevated levels of endometriotic TIMP1. We hypothesize that TIMP1 may disrupt the ovulatory process and cause luteinized unruptured follicle syndrome (LUFS), contribute to poor oocyte and embryo development, and/or increase the incidence of early pregnancy loss, thereby reducing fecundity in the setting of endometriosis. Therefore, in these studies we used an established animal model of endometriosis to evaluate differences in ovarian function, oocyte quality, preimplantation embryo development and pregnancy outcome, and a mechanistic role for TIMP1 in causing these anomalies.
MATERIALS AND METHODS
Animals
Mature female Sprague-Dawley rats (250 g; Harlan, Madison, WI) exhibiting regular 4-day to 5-day estrous cycles were housed in an environmentally controlled room with a 14L:10D cycle. All rats were allowed an acclimation period in the vivarium for 14 days (1 wk for acclimation and 1 wk to confirm reproductive cyclicity by evaluation of vaginal cytology) before any procedure was performed. The experiments were conducted with the approval of the University of Missouri Institutional Animal Care and Use Committee and in accord with the National Research Council's Guide for the Care and Use of Laboratory Animals (Washington, DC: National Academy Press; 1996).
Fluids in the Peritoneal Cavity Traverse the Reproductive Tract
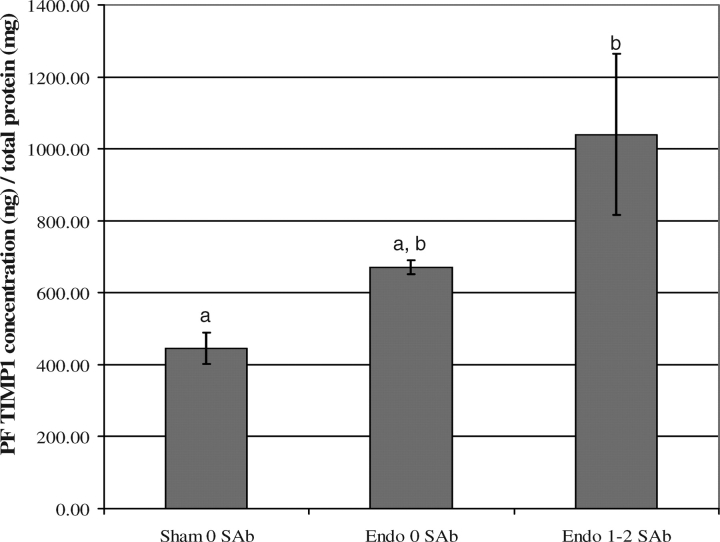
First, we validated the hypothesis that peritoneal fluid, including secretions from endometriotic lesions, can enter, traverse, and influence the whole reproductive tract. Methylene blue, a dye commonly used to evaluate tubal patency and endometrial architecture in women, was used to track the course of fluids in the peritoneal cavity through the rat oviduct and uterus. One milliliter of a sterile nontoxic methylene blue solution (1% in water) was injected into the peritoneal cavity of rats with normal reproductive cycles (n = 15) using a sterile 1-ml syringe with a sterile 26-gauge needle. Rats were then euthanized by routine CO2 overdose at time points of 6, 12, 24, 48, and 72 h. The reproductive tracts were collected and evaluated for oviductal and uterine endometrial blue staining. At 6 h, the exterior surfaces of most abdominal organs, including the reproductive tract, were stained blue by the dye. This effect diminished as time increased and was absent at 48 h. Between 12 and 24 h, the blue dye could be seen migrating into the uterotubal junction, traversing about one third the distance of the uterine horn. Between 24 and 48 h, the dye had migrated about two thirds the distance of the horn, and at 72 h the dye could be seen exiting the cervices. Hence, the results of this study showed that peritoneal fluid does in fact bathe the ovary, enter the oviducts, and traverse the reproductive tract of rats (Fig. 1).
FIG. 1.
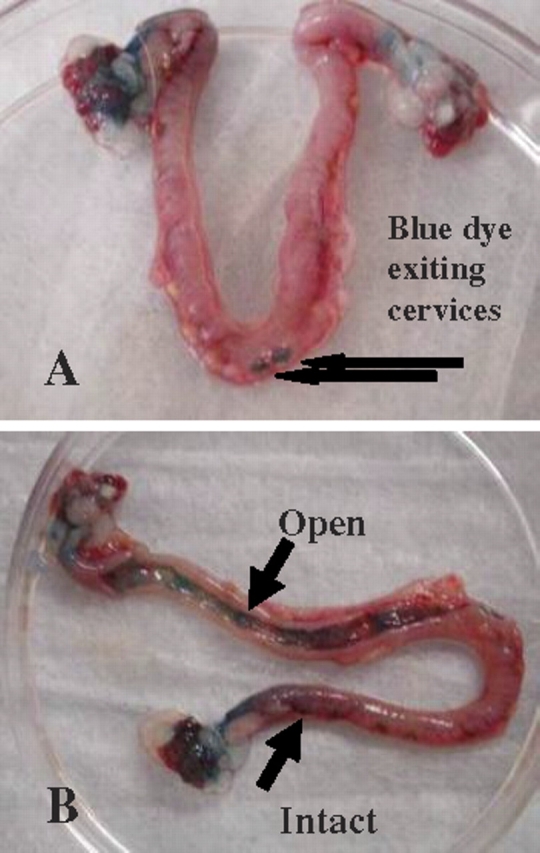
A) Intact uterine horns, dye exiting cervices. B) One uterine horn opened to show dye present throughout horn. Peritoneal fluid traverses the oviduct and uterine horn. In 72 h, blue dye injected into the peritoneal cavity moved through the oviduct and uterine horn and exited the duplex cervices, showing that products of the peritoneal fluid make contact with the complete reproductive tract. Black arrows indicate dye movement.
Surgical Induction of Endometriosis
Endometriosis was surgically induced in Endo rats as previously described by Vernon and Wilson [27] and as routinely performed in our laboratory [47–49, 51–53]. Animals were anesthetized with isoflurane (Butler Animal Health, St. Joseph, MO). Under aseptic conditions, the abdominal cavity was entered through a small 3- to 4-cm midventral incision initiated 2 cm above the urethral opening.
Briefly, unilateral hemihysterectomy and hemiovariectomy were performed. The right ovary and distal two thirds of the uterine horn were ligated with silk suture (American Cyanamid Co., Danbury, CT), surgically removed, and placed in warm (37°C) sterile PBS. The uterine tissue was trimmed of excess fat, bisected along its longitudinal axis, and cut into 2-mm squares. Four uterine squares (implants) were autotransplanted to the arterial cascades of the small intestine beginning at the cecum with 4–0 nonabsorbable nylon suture (Ethicon, Inc., Somerville, NJ). Sham-operated control rats (Shams) were subjected to unilateral hemihysterectomy and hemiovariectomy without the autotransplantation of uterine squares.
After completion of the surgical procedures, the abdominal cavity was rinsed with sterile PBS containing penicillin (100 IU/ml) and streptomycin (100 μg/ml) to hydrate the animal, lessen potential surgical adhesion formation, and help prevent infection. The muscle wall was closed with absorbable 3–0 suture (Ethicon, Inc.), and the skin was closed with 5-mm wound clips (MikRon; Becton Dickinson and Company, Sparks, MD). Rats received buprenorphine (Buprenex, 0.03 mg/kg; Rickitt & Coleman, Richmond, VA) subcutaneously for pain relief. One week after surgery, the wound clips were removed. Experimentation began after 4 wk, which were allotted for recuperation and development of endometriotic implants.
Animal Husbandry
Four weeks after surgery, vaginal cytology was monitored daily in all rats as an index of reproductive cyclicity [52]. The first group of cycling Endo and Sham rats was euthanized in a CO2 chamber between 700 and 800 h on the morning of estrus to collect unfertilized oocytes following LH surge between 1700 and 1900 h the prior evening. Additional Endo and Sham rats were cocaged with proven breeder males overnight on the evening of proestrus. Mating was confirmed the next morning by the presence of a vaginal plug or the presence of spermatozoa in a vaginal lavage. Pregnant rats were euthanized the next morning (Day 1) to collect zygotes, on Day 5 to collect preimplantation embryos/blastocysts, or on Day 15 of gestation to evaluate pregnancy loss or were allowed to gestate to term (pups used in experiment 5).
Experiment 1: Effects of Endometriotic Implants on Peritoneal TIMP1 Concentrations
The concentration of TIMP1 in the peritoneal fluid of nonpregnant estrus-stage rats (five Endo and six Sham) and of Gestational Day 1 pregnant rats (five Endo and four Sham) was quantified. Rats were euthanized, 1 ml of warm PBS was injected into the peritoneal cavity, and the abdomen was gently massaged for 30 sec. The peritoneal fluid washings were then aspirated using a 1-ml syringe, centrifuged for 5 min at 4°C to remove residual cells, and stored in a −80°C freezer.
Peritoneal fluid TIMP1 concentrations were quantified using an ELISA for rat TIMP1 per the manufacturer's instructions (RayBiotech, Inc., Norcross, GA) and were normalized on a total protein basis using the DC Protein Assay according to the manufacturer's instructions (BioRad Laboratories, Hercules, CA). Results were reported as the ratio of TIMP1 (in nanograms per milliliters) to total protein (in milligrams per milliliter). Differences in peritoneal fluid TIMP1 concentrations between Endo and Sham rats were analyzed using two-way ANOVA as follows: model TIMP1 concentration = condition (Endo or Sham) × pregnancy status (estrus or Day 1) × interaction. Tukey post hoc testing was used for pairwise multiple comparison procedures.
Experiment 2: Effects of Endometriotic TIMP1 on Ovarian Function
Ovarian function during the estrus stage and at Day 1 of pregnancy was evaluated in additional Endo (n = 11) or Sham (n = 13) rats (experiment 2a). The remaining right ovary was excised, weighed, and cut in half, and one half was placed into 10% neutral buffered formalin for 24 h. The fixed ovary was then rinsed in PBS, routinely paraffin embedded, and serial sectioned at 8-μm intervals, placing three serial sections per slide. The first slide was stained with hematoxylin-eosin (H&E), and the next four slides were left unstained to evaluate TIMP1 protein localization by fluorescent immunohistochemistry. This pattern of sectioning and staining was repeated for the entire one half of the ovary. The other half of the ovary was snap frozen and stored at −80°C for future gene studies.
Differences in the numbers of follicles and CL and in the presence of LUFS between Endo rats (three estrus, three Day 5, and three Day 15) and Sham rats (five estrus, five Day 5, and three Day 15) were evaluated histologically and quantified morphometrically as described herein. Luteinization was confirmed by an increase in the cytoplasmic:nuclear ratio. Antral follicles and LUFS were counted in one section on every H&E-stained slide, whereas CL were counted on every sixth H&E slide. These morphometric calculations were based on size so that no antral follicle, CL, or LUF was counted more than once in each ovary [29]. All morphologic and morphometric analyses were performed by the same investigator blinded to the study group. Differences in the numbers of follicles and in the numbers of CL between Endo and Sham rats were analyzed using two-way ANOVA as follows: model follicular number, CL number, or LUFS = condition (Endo or Sham) × day (estrus, Day 5, or Day 15 of gestation) × interaction. Tukey post hoc testing was used for pairwise multiple comparison procedures.
Localization of TIMP1 protein in the ovaries of Day 1 pregnant Endo (n = 5) and Sham (n = 5) rats was quantified to detect potential TIMP1 involvement in ovarian dysfunction in endometriosis (experiment 2b). Briefly, tissues were deparaffinized with xylene and rehydrated through serial dilutions of ethanol from 100% to 70%. Nonspecific antibody binding was blocked by incubation of the tissues in 1% normal horse sera (Vector Laboratories, Burlingame, CA) in PBS (pH 7.4) for 20 min before incubation with the primary antibody. Polyclonal rabbit anti-rat TIMP1 (1:100, 5 ng/ml; Cell Applications, Inc., San Diego, CA) antibodies were diluted in PBS containing 0.1% bovine serum albumin (BSA; Sigma Chemical Company, St. Louis, MO) and 1% normal horse serum in PBS for 1 h. Tissues were then washed in PBS three times for 5 min.
Ovarian tissue sections were incubated for 30 min with secondary antibody anti-rabbit IgG conjugated with Alexa Fluor 488 (1 μg/ml in PBS; Invitrogen, Carlsbad, CA). Tissues were washed for 5 min with PBS before adding a coverslip with antifade mounting medium containing 4′,6′-diamidino-2-phenylindole (DAPI) (Vector Laboratories) to reduce photobleaching and to counterstain cell nuclei. To validate these results, additional ovarian sections were incubated with the same TIMP1 primary antibody as before and then incubated with a biotinylated secondary antibody anti-rabbit IgG (50 μl/10 ml in PBS with 0.1% BSA; Vector Laboratories) for 30 min, and tissues were again washed in PBS for 5 min. Tissues were then incubated with Texas Red-conjugated avidin D (15 μg/ml diluted in PBS; Vector Laboratories) for 30 min and mounted as already described. Immunostaining of both sets of tissues was examined using an Olympus inverted IX-71 microscope (Olympus, Melville, NY) equipped for fluorescence imaging.
Immunofluorescent localization of TIMP1 in the ovarian follicular theca and CL was quantified using computer-assisted image analysis (ImageJ; National Institute of Mental Health, Bethesda, MD). The morphometric parameter known as area fraction was used to quantify immunofluorescent intensity per area [54]. The perimeters of antral follicles and CL from the Endo and Sham rats were traced using the freehand selection tool. The area fraction for follicular theca and CL fluorescence was defined as the total area traced. All data were reported as mean intensity per area. Both follicular and CL TIMP1 area fractions of Endo and Sham rats were normally distributed and were compared using Student t-test.
Experiment 3: Effects of Endometriotic Implants on Oocyte Quality and Pre-Embryo Development
Oocyte quality and preimplantation embryo development were evaluated in oocytes on the morning of estrus (nine Endo and three Sham), Day 1 zygotes (10 Endo and four Sham), and Day 5 blastocysts (six Endo and three Sham) from additional Endo and Sham rats. The ovaries, oviducts, and uteri were excised en masse and placed in a 15-ml conical tube containing warm PBS for transportation (<5 min) to the microscopy laboratory.
The contents of each tube were emptied into a 35-mm culture dish. The cumulus masses containing oocytes or zygotes were located in the swollen ampulla of the oviduct using a Nikon SMZU stereoscope (Nikon Instruments Inc., Melville, NY) dissecting microscope. The ampulla was nicked with a 26-gauge needle so that the cumulus mass was extruded. The Day 5 preimplantation blastocysts were obtained by inserting a tuberculin syringe filled with PBS at 37°C into the oviduct and by flushing with the PBS through the uterine horn and out the cervix. Using a sterile Pasteur pipette, oocytes, zygotes, and blastocysts were transferred to a previously prepared dish of 37°C PBS.
To remove the cumuli oophori, the oocytes and embryos were repeatedly pipetted for 30 sec in a wash of serum-free Tyrode albumin lactate pyruvate (TALP)-Hepes (Hepes-buffered Tyrode-containing lactate, 0.2 mM pyruvate, and 3 mg/ml of BSA) with 0.5% w/v hyaluronidase (Sigma Chemical Company) and were then washed with TALP-Hepes. The zona pellucida was removed by a 5-min incubation in TALP-Hepes supplemented with 0.5% polyvinylpyrrolidone (molecular weight, 40 000; Sigma Chemical Company) and 0.5% pronase w/v (Sigma Chemical Company). Oocytes and embryos were washed in 37°C PBS. Formaldehyde (10%; Sigma Chemical Company) was added slowly to the PBS until reaching a final concentration of 2%. Embryos and oocytes were fixed at room temperature for 40 min. After fixation, embryos were washed and permeabilized with 0.1% Triton X-100 (Sigma Chemical Company) and then incubated with 1% normal goat serum to block nonspecific antibody binding before immunolabeling. Oocytes and embryos were pooled by gestational day (Day 1 or Day 5) within group (Endo or Sham).
The number, morphology, and quality of the oocytes and embryos were evaluated using epifluorescence microscopy combined with differential interference contrast (DIC) microscopy. Metaphase II oocytes collected from estrus rats at 6 h after ovulation were evaluated for quality by applying morphologic criteria. Day 1 zygotes were evaluated to determine the number of pronuclei and the presence of sperm flagellum in the ooplasm. A combination of DAPI staining to evaluate nuclei (Molecular Probes, Eugene, OR) and DIC microscopy to visualize ooplasm-incorporated sperm flagellum was applied to avoid false detection of parthenotes (one small peripherally located pronucleus, one polar body, and no flagellum in ooplasm) as fertilized ova (two centrally located pronuclei with incorporated flagellum). Pre-embryos collected on Day 5 were evaluated using the DNA stain DAPI combined with mouse monoclonal anti-tubulin antibody E7 (Developmental Studies Hybridoma Bank, Iowa City, IA) to evaluate chromosome alignment, spindle structure (mitotic blastomeres), and nuclear integrity.
Photomicrographs were taken with a Nikon Eclipse 800 microscope (Nikon Instruments Inc.) equipped with Cool Snap camera (Roper Scientific, Tucson, AZ) and MetaMorph software (Universal Imaging Corp., Downington, PA). Data were archived on CD-ROM compact disks, and color was merged using Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA). Differences in the number of oocytes and the number of zygotes between Endo and Sham rats were determined using Student t-test.
Experiment 4: Effect of Endometriotic Implants on Fecundity
The effects of endometriotic implants on fetal loss were evaluated on Gestational Day 15. Endo (n = 8) and Sham (n = 7) rats were euthanized in a CO2 chamber. Uteri were excised en masse and placed in a 100-mm Petri dish. The numbers of viable and nonviable fetal sacs were counted and photographed. Differences in the numbers of viable and nonviable fetuses (spontaneous fetal absorption/resorption sites [SAbs]) between Endo and Sham rats were determined using Student t-test and Mann-Whitney U rank sum test, respectively. The proportions of viable and SAb fetuses in Endo vs. Sham rats were evaluated using Chi-square test. Differences between the crown rump length of viable Endo and Sham rats were determined using Mann-Whitney U rank sum test. Peritoneal concentrations of TIMP1 were again measured by ELISA. Differences between peritoneal fluid TIMP1 concentrations of Endo with SAbs, Endo without SAbs, and Sham without SAbs were measured by one-way ANOVA and Tukey post hoc testing. Furthermore, peritoneal fluid concentrations of TIMP1 were correlated to the presence and number of SAbs using Spearman rank order correlation.
Experiment 5: Multigenerational Effects of Endometriosis on Oocyte Quality and Preimplantation Embryo Development
Possible inherited reproductive anomalies as a result of maternal exposure to endometriotic lesions were assessed. Female pups from experiment 1 (F1 generation) were allowed to sexually mature and were bred to a second group of proven breeder males. Oocytes, zygotes, and blastocysts were collected from the F1 daughters of Endo (n = 9) and Sham (n = 9) rats and were evaluated as described for their mothers, the F0 generation. Furthermore, F1 daughters of Endo (n = 3) and Sham (n = 3) mothers were allowed to sexually mature, were bred, and were allowed to gestate to Day 15. The numbers of implantation sites and SAbs were counted and photographed.
Statistical Analysis
All statistical tests described for each experiment were performed using the Sigma Stat package (Systat Software, Inc., Point Richmond, CA). P < 0.05 was considered significant. The data were normally distributed and were reported as the mean ± SD except where noted.
RESULTS
Experiment 1: Effects of Endometriotic Implants on Peritoneal TIMP1 Concentrations
Peritoneal fluid TIMP1 concentrations did not differ between pregnant and not pregnant rats; therefore, the data were pooled within Endo rats and within Sham rats for analysis. Significantly more TIMP1 was measured in the peritoneal fluid of Endo rats compared with Sham rats (mean ± SD, 126.2 ± 24.1 ng/ml vs. 62.6 ± 23.9 ng/ml; P < 0.001).
Experiment 2: Effects of Endometriotic TIMP1 on Ovarian Function
Significantly fewer antral follicles and fewer CL were present in Endo rat ovaries than in Sham rat ovaries (Table 1). Luteinized unruptured follicles were observed in 50% of the ovaries from Endo rats, whereas 0% of the Sham had LUFS. Ovaries from Sham rats had healthy follicles and CL. LUFS was identified by the presence of intact oocytes within mature antral follicles, where granulosa cells were in the process of luteal transformation. Luteinized unruptured follicles could be distinguished from atretic follicles because the granulosa cells of atretic follicles did not transform into luteal cells but contained numerous pyknotic nuclei, vacuoles, and intraluminal leukocytes, with an absence of mitotic figures.
TABLE 1.
Follicles, CL, and LUFS in Endo versus Sham rats.
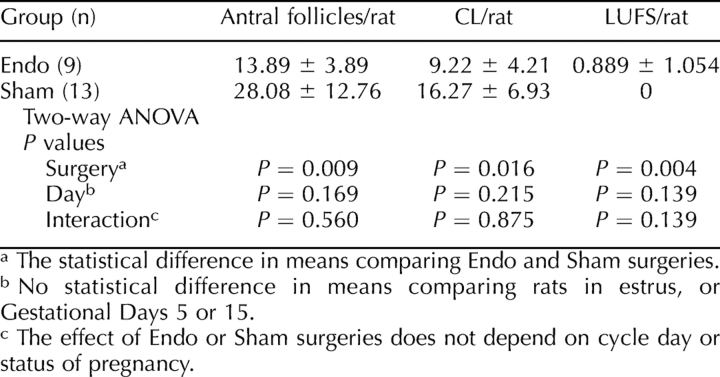
To provide insight into possible mechanism(s) by which surgically induced endometriosis elicited ovarian dysfunction and LUFS, the ovaries from Endo and Sham rats were examined for the presence and localization of TIMP1 protein. Significantly more TIMP1 (Fig. 2 and Table 2) localized in the ovarian theca of antral follicles and, to a lesser extent, in the CL from Endo rats compared with Sham rats.
FIG. 2.
TIMP1 protein localization in follicular theca of Endo rats. Immunohistochemistry showed that Endo rats (A) had more (P < 0.001) TIMP1 localization (Texas Red, arrows) in the thecal cells of antral follicles than Sham rats (B). An excess of TIMP1 in the thecal cells may block the function of MMPs necessary for ovulation. Original magnification ×200.
TABLE 2.
TIMP1 localization in follicular theca and CL.
Experiment 3: Effects of Endometriotic Implants on Oocyte Quality and Pre-Embryo Development
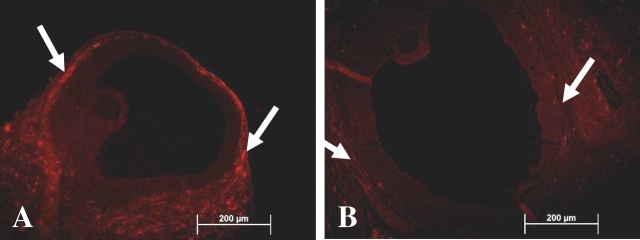
Endo rats had significantly fewer oocytes compared with Sham rats (mean ± SD, 11.0 ± 2.2 vs. 15.0 ± 1.7, P = 0.016). Oocytes from Sham rats had normal metaphase II spindle formation. Metaphase II oocytes from Endo rats frequently displayed scattered chromosomes, cytoplasmic fragmentation, spontaneous oocyte activation, and formation of pseudopronuclei or karyomeres surrounded by a de novo-formed nuclear envelope with nuclear pore complexes (Figs. 3 and 4B).
FIG. 3.
Abnormal morphology of ova and embryos from Endo rats compared with those from Sham rats. A) Sham ova demonstrating normal morphology, round shape, clear cytoplasm, and polar body. B) Endo ova showing dark irregular-shaped ooplasm. C) Sham Day 3 embryos show eight distinct even-sized cells free of defects, with nuclei foretelling cleavage to the 16-cell stage. D) Endo rat Day 3 embryos show fewer, uneven, degenerated, and fragmented cells. Nuclei were present only in some cells, and an intact sperm tail was still visible within some embryos. Black arrow indicates sperm tail; yellow arrows, degenerating cells. Original magnification ×60.
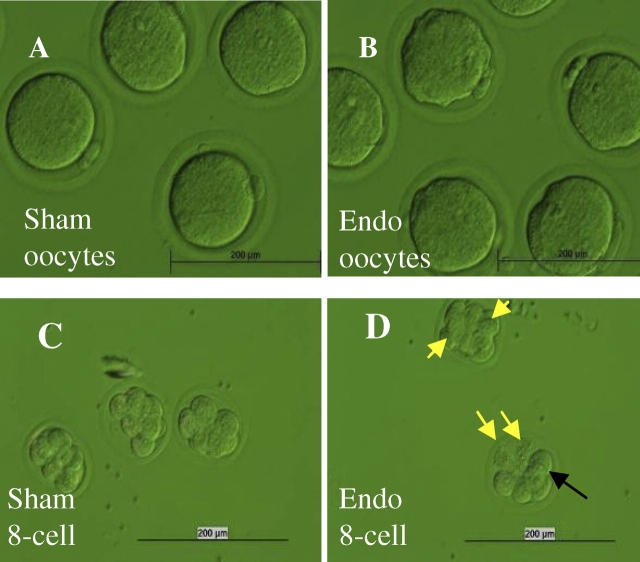
FIG. 4.
Endo rats and their F1 daughters without surgery have developmental anomalies in their oocytes and embryos, as seen on immunohistochemistry: A) Normal metaphase II spindle (green) in F1 Sham offspring (no surgery and born of an F0 Sham). F0 Sham founders were identical to the F1-generation Sham daughters and are not shown. B) Oocyte fragmentation and formation of micronuclei enclosed by a nuclear envelope with nuclear pore complexes (green) in F0 Endo rat. C) Metaphase II oocytes with scattered chromosomes (blue) in F1 Endo offspring. D) Zygote with two normal pronuclei in F1 Sham offspring. E) Failed fertilization of an ovum from a mated F0 Endo rat. F) Reduced pronuclear size, failed pronuclear apposition, and cytoplasm fragmentation in F1 Endo offspring. G) Normal Day 5 blastocyst from F1 Sham offspring. Microtubules were labeled with anti-tubulin antibody (green). H) Day 5 embryo from an F0 Endo female failed to develop beyond the eight-cell stage. The sperm tail remnant spans two adjacent blastomeres at the point of midbody formation (arrow). I) Enlargement of H showing the presence of midbodies (green). Sperm tails should normally be gone at this stage; because they seem to dictate where the midbody forms, they may be preventing these embryos from cleaving again. In summary, meiotic spindle and chromosome architecture in oocytes and embryos, which are features paramount to the success of early embryo development, are anomalous in this model of endometriosis. See experiment 3 for additional details. Original magnification ×600 (A–H) and ×1000 (I).
Endo rats also had significantly fewer zygotes compared with Sham rats (mean ± SD, 10.4 ± 3.6 vs. 15.8 ± 0.5; P = 0.015). Sham rat zygotes displayed normal apposition of two large pronuclei. Endo rat zygotes had delayed or anomalous pronuclear development, reduced pronuclear size, failed pronuclear apposition, misaligned chromosomes, and nuclear fragmentation (Fig. 4E). Embryos from Endo rats also showed cytoplasmic fragmentation and delayed or arrested cleavage between the first mitosis through blastocyst formation, not observed in Sham embryos. Blastocyst embryos from Sham rats had a normal-appearing blastocoel, and cytoplasmic microtubule networks were present that were indicative of normal cytoskeletal organization. Only 8–16 cell embryos were recovered from Endo rats on Day 5 of gestation, and their cytoplasmic microtubule networks were absent or disorganized (Fig. 4, H and I).
Experiment 4: Effect of Endometriotic Implants on Fecundity
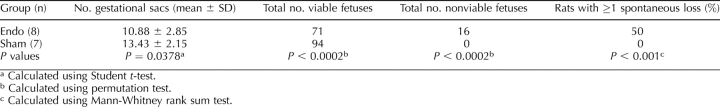
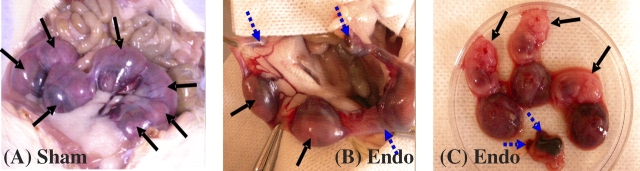
Endometriosis significantly affected fecundity. SAbs were observed in 50% of Endo rats, whereas 0% of Sham rats had SAbs (Table 3 and Fig. 5). Fewer viable fetuses per rat were found in Endo rats compared with Sham rats. The crown rump length of the viable fetuses did not vary between Endo rats (median, 10.98; 25%/75%, 9.15/13.71 mm) and Sham rats (median, 11.76; 25%/75%, 10.93/11.82 mm) (P = 0.867). Concentrations of peritoneal TIMP1 were statistically greater in Endo rats with SAbs compared with Sham rats without SAbs (Fig. 6).
TABLE 3.
Effects of endometriosis on fecundity at Gestational Day 15 in rats with surgically-induced endometriosis compared to sham-operated controls.
FIG. 5.
Pregnancy loss in Endo rats at Day 15. A) Control rats demonstrating normal viable fetuses and no losses. B) Endo rats showing ≥1 loss per rat. C) Viable fetus crown rump length was similar in Endo and Sham rats (P = 0.867); resorbed fetal placental units were smaller in Endo rats. The male:female ratios of viable Endo and Sham fetuses were not different (P = 0.414). Black arrows indicate normal fetus; dashed blue arrows, fetal loss. The incidence of fetal loss in Endo rats but not Sham rats provides evidence of the link between endometriosis and spontaneous loss.
FIG. 6.
Spontaneous pregnancy losses are associated with the level of peritoneal fluid (PF) concentration of TIMP1 (mean ± SD). Peritoneal fluid TIMP1 concentrations were significantly higher in Endo rats with SAbs than in Sham rats without SAbs. Different letters indicate significant differences between groups (P = 0.011, ANOVA).
Experiment 5: Multigenerational Effects of Endometriosis on Oocyte Quality and Preimplantation Embryo Development
Oocytes and preimplantation embryos were collected from the offspring (F1 generation) of Endo and Sham mothers. The F1 offspring had no surgical intervention and were allowed to develop to sexual maturity in our vivarium. Oocytes from Endo F1 rats (i.e., the daughters of Endo mothers) had elongated cytoplasm and scattered chromosomes compared with oocytes from Sham F1 rats (i.e., the daughters of Sham mothers) (Fig. 4C). Endo F1 zygotes displayed fragmented cytoplasm, lack of pronuclear apposition, and hypercondensed female pronuclei, which were not found in Sham F1 daughters (Fig. 4F). On Day 5, Sham F1 blastocysts were formed with normal interphase microtubule networks in their blastomeres, but Endo F1 Day 5 embryos failed to develop past the eight-cell stage and displayed missing or abnormal organization of microtubules. The F1 daughters of Endo rats at Day 5 looked the same as Endo F0 Day 5 embryos (data not shown).
When Endo rat F1 daughters (n = 3) were allowed to gestate to Day 15, two of three failed to become pregnant, and one became pregnant with 11 implantation sites and four spontaneous losses. When Sham rat F1 daughters (n = 3) were allowed to gestate to Day 15, one failed to become pregnant, but two became pregnant with 17 normal implantation sites and no spontaneous losses.
DISCUSSION
In this study, surgically induced endometriosis in the rat led to ovarian dysfunction, anomalous oocyte quality and embryo development, and early pregnancy loss. We have shown that Endo rats had significantly fewer follicles and CL and an increased number of LUFS compared with Shams. It was anticipated that there would be differences in follicle and CL numbers when comparing nonpregnant with pregnant rats, but it seems that endometriosis impairs the ovary regardless of pregnancy status. This study validates the work of Moon et al. [29], who found more LUFS and fewer follicles in Endo rats compared with controls. However, they did not identify the mechanism that causes these anomalies or examine oocyte/embryo quality.
TIMP1 levels were elevated in peritoneal fluid of Endo rats compared with Shams. Curiously, these data are not consistent with findings that TIMP1 concentrations in the peritoneal fluid of women with endometriosis were decreased compared with those of control subjects [55]. Reasons for this disparity might be related to species or to the fact that the women in the prior study had advanced stages of endometriosis compared with the rats in this study, which had newly established lesions. As the stage of endometriosis changes, so does the activity of the endometriotic lesions [48, 56]. Moreover, the women in the previous study [55] were patients seeking treatment for pain associated with endometriosis and not for infertility. Because the previous study measured TIMP1 by radioimmunoassay and the present study used an established rat TIMP1 ELISA, methodologic variations may also contribute to the differences.
The data herein support our hypothesis that TIMP1 secreted by endometriotic lesions into the peritoneal fluid localizes in the theca of preovulatory follicles and alters MMP activity that is critical for ovulation, thereby contributing to the mechanism associated with ovarian dysfunction and entrapment of oocytes in LUFS. In women, LUFS has been associated with unexplained infertility and endometriosis [57]. We propose that this mechanism is related to TIMP1 localization in antral follicles based on the excess TIMP1 localized to the thecal cells of Endo rats but not of Sham rats. However, it is also possible that the altered cytokine environment or another endometriosis-associated factor may stimulate the production of excess TIMP1 in the ovary. Experiments to evaluate the role of TIMP1 in this mechanism are ongoing in our laboratory.
Endo rats produced fewer and poor-quality oocytes compared with Sham rats (experiment 3). It is possible that these Endo oocytes are abnormal because of maturation in the anomalous ovary. Adding to the problem is the fact that after ovulation the oocyte complex is exposed directly to the peritoneal fluid, which contains anomalous TIMP1 concentrations. Because TIMP1 can enter into the nucleus and affect the normal cell cycle [58], the overabundance of TIMP1 from the Endo phenotype could cause abnormal nuclear development. Endo rats had fewer Day 1 zygotes, and their zygotes had delayed or anomalous development compared with those of Sham rats. Again, these abnormalities and delays could originate from a suboptimal ovarian environment, but the problem may also be exacerbated by direct contact with the excess TIMP1 in the peritoneal fluid. Embryos were delayed or arrested at the eight-cell or 16-cell stage when collected from Endo rats at the time when blastocysts should be present. This provides further evidence that the Endo oocytes and embryos have delayed development. The preimplantation embryos are still in direct contact with and influence of the peritoneal fluid at this time. Excessive TIMP1 may continue to intensify this problem. Experiments elucidating the effect of excessive TIMP1 directly on oocytes and embryos are being conducted in our laboratory.
Our most intriguing results show that oocytes and embryos from the F1 generation that had no surgical intervention (endometriosis-free daughters of Endo mothers) had the same anomalies of oocytes and preimplantation embryo development and quality as their Endo mothers, whereas the F1 generation without surgery from Sham mothers did not manifest these irregularities. In addition, it seems that these F1 generation rats tended to have reduced fecundity and spontaneous abortions like their Endo mothers. To our knowledge, these results show for the first time that developmental exposure to maternal endometriosis may have a negative effect on oocyte quality and embryo development in subsequent generations. However, these data need to be confirmed in a larger study.
First-degree relatives of women with endometriosis are more likely to have endometriosis themselves. Some studies [59, 60] indicated that endometriosis is a heritable disease of polygenetic or even near-mendelian inheritance. Other investigators argue that endometriosis is epigenetically heritable, including hypermethylation patterns in women with endometriosis as the target for disease [61–63]. Because our multigenerational data suggest the potential for epigenetic mechanisms in reduced fecundity in endometriosis, ongoing studies in our laboratory are investigating this phenomenon.
Only Endo rats had SAbs. We and others have described endometrial anomalies in women with endometriosis [43, 54, 64–67], which may contribute to reduced fecundity. Hence, an endometrial factor cannot be ruled out as a cause of reduced fecundity in endometriosis. The fact that there was no difference noted in viable fetal sac sizes between Endo and Sham rats supports the hypothesis that the decrease in fecundity may be a result of compromised oocyte and preimplantation embryo quality. Yet, the possibility also exists that endometrial secretory proteins cause a dysfunction in the implantation process. Anomalous endometrial MMP and TIMP concentrations have been noted in endometriosis patients and animal models [47–50], which may interfere with the establishment, maintenance, and outcome of pregnancy. In fact, our data showing that the peritoneal fluid TIMP1 concentrations are correlated with the occurrence of spontaneous losses suggest that anomalous levels of TIMP1 in Endo rats may be involved in this mechanism (Fig. 6). Just as successful pregnancy is beneficial but not curative in women with endometriosis [68, 69], successful pregnancy in rats also decreases the size of the lesion [27], which we have found is associated with a decrease in endometriotic TIMP1 production in the peritoneal fluid.
These results offer further evidence that surgical induction of endometriosis in rats leads to ovulatory dysfunction and compromised oocyte and preimplantation embryo quality, causing a decrease in fecundity and spontaneous abortions. However, the specific mechanism of TIMP and MMP involvement in endometriosis-associated infertility remains undefined. These results suggest that TIMP1 may be involved in the pathogenesis of endometriosis-associated infertility by altering the MMP/TIMP balance that is required for normal reproductive function.
Acknowledgments
We would like to thank Dr. Cindy Besch-Wiliford, Dr. Heide Schatten, Dr. Wade Davis, and the laboratory staff in the Department of Obstetrics, Gynecology and Women's Health Division of Perinatal Research, particularly Dr. Jennifer Luth, Henda Nabli, Julie Birt, and Randy Zimmer.
Footnotes
1Supported in part by the University of Missouri Research Board and NIH HD57445-01 (to K.L.S.-T.), seed funding from the Food for the 21st Century Program of the University of Missouri (to P.S.), and NIH T32 RR-07004 (to P.S.). Presented in part at the 53rd Annual Meeting of the Society of Gynecological Investigation, March 15, 2007, Reno, Nevada; and the 41st Annual Meeting of the Society for the Study of Reproduction, May 29, 2008, Kailua-Kona, Hawaii.
REFERENCES
- Garrido N, Pellicer A, Remohi J, Simon C.Uterine and ovarian function in endometriosis. Semin Reprod Med 2003; 21: 183–192. [DOI] [PubMed] [Google Scholar]
- Halis G, Arici A.Endometriosis and inflammation in infertility. Ann N Y Acad Sci 2004; 1034: 300–315. [DOI] [PubMed] [Google Scholar]
- Gomez-Torres MJ, Acien P, Campos A, Velasco I.Embryotoxicity of peritoneal fluid in women with endometriosis: its relation with cytokines and lymphocyte populations. Hum Reprod 2002; 17: 777–781. [DOI] [PubMed] [Google Scholar]
- Strathy JH, Molgaard CA, Coulam CB, Melton LJ., IIIEndometriosis and infertility: a laparoscopic study of endometriosis among fertile and infertile women. Fertil Steril 1982; 38: 667–672. [DOI] [PubMed] [Google Scholar]
- Haney AF.Endometriosis-associated infertility. Baillieres Clin Obstet Gynaecol 1993; 7: 791–812. [DOI] [PubMed] [Google Scholar]
- Muse KN, Wilson EA.How does mild endometriosis cause infertility? Fertil Steril 1982; 38: 145–152. [DOI] [PubMed] [Google Scholar]
- Brosens I.Endometriosis and the outcome of in vitro fertilization. Fertil Steril 2004; 81: 1198–1200. [DOI] [PubMed] [Google Scholar]
- Hahn DW, Carraher RP, Foldesy RG, McGuire JL.Experimental evidence for failure to implant as a mechanism of infertility associated with endometriosis. Am J Obstet Gynecol 1986; 155: 1109–1113. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Fedorkow DM, Collins JA.A quantitative overview of controlled trials in endometriosis-associated infertility. Fertil Steril 1993; 59: 963–970. [PubMed] [Google Scholar]
- Navarro J, Garrido N, Remohi J, Pellicer A.How does endometriosis affect infertility? Obstet Gynecol Clin North Am 2003; 30: 181–192. [DOI] [PubMed] [Google Scholar]
- De Hondt A, Peeraer K, Meuleman C, Meeuwis L, De Loecker P, D'Hooghe TM.Endometriosis and subfertility treatment: a review. Minerva Ginecol 2005; 57: 257–267. [PubMed] [Google Scholar]
- Garrido N, Navarro J, Garcia-Velasco J, Remoh J, Pellice A, Simon C.The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update 2002; 8: 95–103. [DOI] [PubMed] [Google Scholar]
- Ozkan S, Murk W, Arici A.Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci 2008; 1127: 92–100. [DOI] [PubMed] [Google Scholar]
- Allaire C.Endometriosis and infertility: a review. J Reprod Med 2006; 51: 164–168. [PubMed] [Google Scholar]
- Cahill DJ, Hull MG.Pituitary-ovarian dysfunction and endometriosis. Hum Reprod Update 2000; 6: 56–66. [DOI] [PubMed] [Google Scholar]
- Doody MC, Gibbons WE, Buttram VC., JrLinear regression analysis of ultrasound follicular growth series: evidence for an abnormality of follicular growth in endometriosis patients. Fertil Steril 1988; 49: 47–51. [DOI] [PubMed] [Google Scholar]
- Tummon IS, Maclin VM, Radwanska E, Binor Z, Dmowski WP.Occult ovulatory dysfunction in women with minimal endometriosis or unexplained infertility. Fertil Steril 1988; 50: 716–720. [PubMed] [Google Scholar]
- Hull MG, Williams JA, Ray B, McLaughlin EA, Akande VA, Ford WC.The contribution of subtle oocyte or sperm dysfunction affecting fertilization in endometriosis-associated or unexplained infertility: a controlled comparison with tubal infertility and use of donor spermatozoa. Hum Reprod 1998; 13: 1825–1830. [DOI] [PubMed] [Google Scholar]
- Groll M.Endometriosis and spontaneous abortion. Fertil Steril 1984; 41: 933–935. [DOI] [PubMed] [Google Scholar]
- Tanbo T, Omland A, Dale PO, Abyholm T.In vitro fertilization/embryo transfer in unexplained infertility and minimal peritoneal endometriosis. Acta Obstet Gynecol Scand 1995; 74: 539–543. [DOI] [PubMed] [Google Scholar]
- Bergendal A, Naffah S, Nagy C, Bergqvist A, Sjoblom P, Hillensjo T.Outcome of IVF in patients with endometriosis in comparison with tubal-factor infertility. J Assist Reprod Genet 1998; 15: 530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittaway DE, Vernon C, Fayez JA.Spontaneous abortions in women with endometriosis. Fertil Steril 1988; 50: 711–715. [DOI] [PubMed] [Google Scholar]
- Geber S, Paraschos T, Atkinson G, Margara R, Winston RM.Results of IVF in patients with endometriosis: the severity of the disease does not affect outcome, or the incidence of miscarriage. Hum Reprod 1995; 10: 1507–1511. [DOI] [PubMed] [Google Scholar]
- Harlow CR, Cahill DJ, Maile LA, Talbot WM, Mears J, Wardle PG, Hull MG.Reduced preovulatory granulosa cell steroidogenesis in women with endometriosis. J Clin Endocrinol Metab 1996; 81: 426–429. [DOI] [PubMed] [Google Scholar]
- Tummers P, De Sutter P, Dhont M.Risk of spontaneous abortion in singleton and twin pregnancies after IVF/ICSI. Hum Reprod 2003; 18: 1720–1723. [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL.Using rats as a research model for the study of endometriosis. Ann N Y Acad Sci 2002; 955: 318–327,.340–342, 396–406. [DOI] [PubMed] [Google Scholar]
- Vernon MW, Wilson EA.Studies on the surgical induction of endometriosis in the rat. Fertil Steril 1985; 44: 684–694. [PubMed] [Google Scholar]
- Berkley KJ, Cason A, Jacobs H, Bradshaw H, Wood E.Vaginal hyperalgesia in a rat model of endometriosis. Neurosci Lett 2001; 306: 185–188. [DOI] [PubMed] [Google Scholar]
- Moon CE, Bertero MC, Curry TE, London SN, Muse KN, Sharpe KL, Vernon MW.The presence of luteinized unruptured follicle syndrome and altered folliculogenesis in rats with surgically induced endometriosis. Am J Obstet Gynecol 1993; 169: 676–682. [DOI] [PubMed] [Google Scholar]
- Pal AK, Biswas S, Goswami SK, Kabir SN.Effect of pelvic endometrial implants on overall reproductive functions of female rats. Biol Reprod 1999; 60: 954–958. [DOI] [PubMed] [Google Scholar]
- Matrisian LM.Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet 1990; 6: 121–125. [DOI] [PubMed] [Google Scholar]
- Hulboy DL, Rudolph LA, Matrisian LM.Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod 1997; 3: 27–45. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Osteen KG.Cyclic changes in the matrix metalloproteinase system in the ovary and uterus. Biol Reprod 2001; 64: 1285–1296. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Adler RR, Rappolee DA, Pedersen RA, Werb Z.Genes for extracellular-matrix-degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes Dev 1989; 3: 848–859. [DOI] [PubMed] [Google Scholar]
- Inderdeo DS, Edwards DR, Han VK, Khokha R.Temporal and spatial expression of tissue inhibitors of metalloproteinases during the natural ovulatory cycle of the mouse. Biol Reprod 1996; 55: 498–508. [DOI] [PubMed] [Google Scholar]
- Bagavandoss P.Differential distribution of gelatinases and tissue inhibitor of metalloproteinase-1 in the rat ovary. J Endocrinol 1998; 158: 221–228. [DOI] [PubMed] [Google Scholar]
- Wang H, Wen Y, Mooney S, Li H, Behr B, Polan ML.Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase expression in human preimplantation embryos. Fertil Steril 2003; 80(suppl 2):736–742. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Mann JS, Huang MH, Keeble SC.Gelatinase and proteoglycanase activity during the periovulatory period in the rat. Biol Reprod 1992; 46: 256–264. [DOI] [PubMed] [Google Scholar]
- Reich R, Tsafriri A, Mechanic GL.The involvement of collagenolysis in ovulation in the rat. Endocrinology 1985; 116: 522–527. [DOI] [PubMed] [Google Scholar]
- Bany BM, Schultz GA.Tissue inhibitor of matrix metalloproteinase-3 expression in the mouse uterus during implantation and artificially induced decidualization. Mol Reprod Dev 2001; 59: 159–167. [DOI] [PubMed] [Google Scholar]
- Nothnick WB, Soloway P, Curry TE., JrAssessment of the role of tissue inhibitor of metalloproteinase-1 (TIMP-1) during the periovulatory period in female mice lacking a functional TIMP-1 gene. Biol Reprod 1997; 56: 1181–1188. [DOI] [PubMed] [Google Scholar]
- Nothnick WB.Disruption of the tissue inhibitor of metalloproteinase-1 gene results in altered reproductive cyclicity and uterine morphology in reproductive-age female mice. Biol Reprod 2000; 63: 905–912. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Woessner JF, Jr, Koos RD, Sear CH, LeMaire WJ.Inhibitors of mammalian tissue collagenase and metalloproteinases suppress ovulation in the perfused rat ovary. Endocrinology 1988; 122: 1715–1721. [DOI] [PubMed] [Google Scholar]
- Osteen KG, Yeaman GR, Bruner-Tran KL.Matrix metalloproteinases and endometriosis. Semin Reprod Med 2003; 21: 155–164. [DOI] [PubMed] [Google Scholar]
- Seli E, Berkkanoglu M, Arici A.Pathogenesis of endometriosis. Obstet Gynecol Clin North Am 2003; 30: 41–61. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Vernon MW.Polypeptides synthesized and released by rat ectopic uterine implants differ from those of the uterus in culture. Biol Reprod 1993; 48: 1334–1340. [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL, Penney LL, Zimmer RL, Wright JA, Zhang Y, Surewicz K.Partial purification and amino acid sequence analysis of endometriosis protein-II (ENDO-II) reveals homology with tissue inhibitor of metalloproteinases-1 (TIMP-1). J Clin Endocrinol Metab 1995; 80: 3784–3787. [DOI] [PubMed] [Google Scholar]
- Cox KE, Piva M, Sharpe-Timms KL.Differential regulation of matrix metalloproteinase-3 gene expression in endometriotic lesions compared with endometrium. Biol Reprod 2001; 65: 1297–1303. [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL, Zimmer RL, Jolliff WJ, Wright JA, Nothnick WB, Curry TE.Gonadotropin-releasing hormone agonist (GnRH-a) therapy alters activity of plasminogen activators, matrix metalloproteinases, and their inhibitors in rat models for adhesion formation and endometriosis: potential GnRH-a-regulated mechanisms reducing adhesion formation. Fertil Steril 1998; 69: 916–923. [DOI] [PubMed] [Google Scholar]
- Zhou HE, Nothnick WB.The relevancy of the matrix metalloproteinase system to the pathophysiology of endometriosis. Front Biosci 2005; 10: 569–575. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Zimmer RL, Griffin WT, Penney LL.Polypeptides synthesized and released by human endometriosis differ from those of the uterine endometrium in cell and tissue explant culture. Fertil Steril 1993; 60: 839–851. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Bertero MC, Lyon BP, Muse KN, Vernon MW.Follicular atresia and infertility in rats treated with a gonadotropin-releasing hormone antagonist. Endocrinology 1990; 127: 25–31. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Bertero MC, Muse KN, Vernon MW.Spontaneous and steroid-induced recurrence of endometriosis after suppression by a gonadotropin-releasing hormone antagonist in the rat. Am J Obstet Gynecol 1991; 164: 187–194. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Stereological Methods, Vol. 1: Practical Methods for Biological Morphometry. London:: Academic Press;; 1979. [Google Scholar]
- Sharpe-Timms KL, Keisler LW, McIntush EW, Keisler DH.Tissue inhibitor of metalloproteinase-1 concentrations are attenuated in peritoneal fluid and sera of women with endometriosis and restored in sera by gonadotropin-releasing hormone agonist therapy. Fertil Steril 1998; 69: 1128–1134. [DOI] [PubMed] [Google Scholar]
- Vernon MW, Beard JS, Graves K, Wilson EA.Classification of endometriotic implants by morphologic appearance and capacity to synthesize prostaglandin F. Fertil Steril 1986; 46: 801–806. [PubMed] [Google Scholar]
- Marik J, Hulka J.Luteinized unruptured follicle syndrome: a subtle cause of infertility. Fertil Steril 1978; 29: 270–274. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Li H, Yamashita K, Guo XK, Hoshino T, Yoshida S, Shinya T, Hayakawa T.Cell cycle-associated accumulation of tissue inhibitor of metalloproteinases-1 (TIMP-1) in the nuclei of human gingival fibroblasts. J Cell Sci 1998; 111(pt 9):1147–1153. [DOI] [PubMed] [Google Scholar]
- Bischoff F, Simpson JL.Genetics of endometriosis: heritability and candidate genes. Best Pract Res Clin Obstet Gynaecol 2004; 18: 219–232. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Treloar SA, Lin J, Weeks DE, Nyholt DR, Mangion J, MacKay IJ, Cardon LR, Martin NG, Kennedy SH, Montgomery GW.Significant evidence of one or more susceptibility loci for endometriosis with near-Mendelian inheritance on chromosome 7p13–15. Hum Reprod 2007; 22: 717–728. [DOI] [PubMed] [Google Scholar]
- Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW.Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol 2005; 193: 371–380. [DOI] [PubMed] [Google Scholar]
- Wu Y, Strawn E, Basir Z, Halverson G, Guo SW.Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 2006; 1: 106–111. [DOI] [PubMed] [Google Scholar]
- Wu Y, Strawn E, Basir Z, Halverson G, Guo SW.Aberrant expression of deoxyribonucleic acid methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertil Steril 2007; 87: 24–32. [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL.Endometrial anomalies in women with endometriosis. Ann N Y Acad Sci 2001; 943: 131–147. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Bagot C, Kardana A, Olive D, Arici A.HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod 1999; 14: 1328–1331. [DOI] [PubMed] [Google Scholar]
- Braun DP, Dmowski WP.Endometriosis: abnormal endometrium and dysfunctional immune response. Curr Opin Obstet Gynecol 1998; 10: 365–369. [DOI] [PubMed] [Google Scholar]
- Chung HW, Wen Y, Chun SH, Nezhat C, Woo BH, Lake Polan M.Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: a rationale for endometriotic invasiveness. Fertil Steril 2001; 75: 152–159. [DOI] [PubMed] [Google Scholar]
- McArthur JW, Ulfelder H.The effect of pregnancy upon endometriosis. Obstet Gynecol Surv 1965; 20: 709–733. [DOI] [PubMed] [Google Scholar]
- Hanton EM, Malkasian GD, Dockerty MB, Pratt JH.Endometriosis: symptomatic during pregnancy. Am J Obstet Gynecol 1966; 95: 1165–1166. [DOI] [PubMed] [Google Scholar]