Abstract
Learned associations between effects of abused drugs and the drug administration environment play important roles in drug addiction. Histochemical and electrophysiological studies suggest that these associations are encoded in sparsely distributed nucleus accumbens neurons that are selectively activated by drugs and drug-associated cues. Although correlations between accumbens neuronal activity and responsivity to drugs and drug cues have been observed, no technique exists for selectively manipulating these activated neurons and establishing their causal role in behavioral effects of drugs and drug cues. Here we describe a novel method, termed ‘Daun02-inactivation method’, that selectively inactivates a minority of neurons activated by cocaine in an environment repeatedly paired with cocaine to demonstrate a causal role for these activated neurons in context-specific cocaine-induced psychomotor sensitization in rats. This method provides a new tool to study causal roles of selectively activated neurons in behavioral effects of drugs and drug cues and in other learned behaviors.
Keywords: cocaine sensitization, nucleus accumbens, cell-specific inactivation, striatum, neuronal ensembles
Learned associations between the effects of abused drugs and the drug administration environment play important roles in drug addiction1-9. This form of learning involves the encoding of highly detailed information about drug effects and complex sets of cues in the drug administration environment and thus requires a correspondingly high degree of specificity in the underlying pattern of neural activity. Identification and manipulation of the activated neurons that mediate these learned associations is crucial to understanding the neurobiology of drug addiction.
Neuronal activity within nucleus accumbens mediates many drug-related learned behaviors1, 3, 10, 11, including context-specific psychomotor sensitization7, 8, 12, 13. Psychomotor sensitization refers to the progressive increase in cocaine-induced locomotor activity and stereotypy after repeated drug exposure14, 15 while ‘context-specific’ psychomotor sensitization refers to psychomotor sensitization that is selectively expressed in the drug-associated environmental context but not in a non-drug paired environment7-9, 16. Results from histochemical studies using immediate early gene markers of neuronal activation indicate that following repeated exposure to cocaine in a specific environmental context, only a minority of sparsely distributed accumbens neurons are selectively activated by cocaine in the paired environment, but not in a non-drug paired environment17-20. Thus context-specific neuronal activation correlates with context-specific expression of psychomotor sensitization18.
The implication of the above findings is that only a small number of activated nucleus accumbens neurons are likely to mediate context-specific psychomotor sensitization and other behavioral effects of drugs that are modulated by the drug administration environment. Unfortunately, current experimental methods such as lesions, intracranial injections of pharmacological agents or electrical stimulation target both activated and non-activated neurons and thus cannot selectively manipulate the activated neurons and assess a causal role for these neurons in behavior. This issue is partly addressed by in vivo electrophysiology studies that find temporal correlations between accumbens neuronal activity and exposure to drugs or drug-associated cues21-23. However, results from these electrophysiology studies, like histochemical studies, are correlational and by themselves do not indicate whether the activated neurons play causal roles in behavior.
Here, we describe a novel method, termed ‘Daun02-inactivation method’, which we used to selectively inactivate only those neurons activated by cocaine in an environment repeatedly paired with drug injections. Our results indicate that small subsets (2-3%) of accumbens neurons are selectively activated by cocaine in a specific environment and mediate context-specific cocaine psychomotor sensitization.
Results
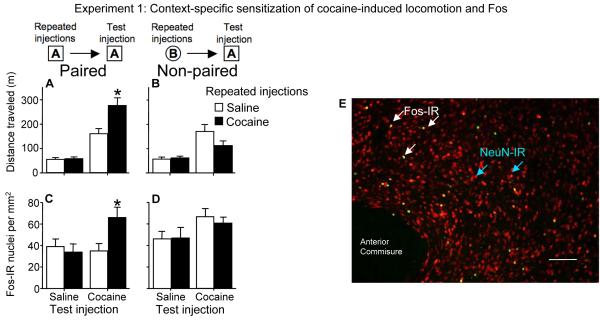
We used context-specific sensitization of cocaine-induced locomotor activity as an animal model of learned associations between the drug and its administration environment7, 9. In this model, cocaine is administered repeatedly to rats in a novel environment outside their home cage. Subsequent cocaine-induced locomotor responses are robustly sensitized in the drug-paired environment, but not in distinct non-paired novel environments, for at least six months following the last cocaine exposure9, 13, 18. In Experiment 1, we injected one set of rats (Paired) with cocaine or saline once daily for 7 days in square locomotor activity chambers with smooth floors (context A) and another set of rats (Non-paired) in round bowls with woodchip bedding (context B). Repeated cocaine injections increased cocaine-induced locomotor activity levels from day 1 to day 7 in the activity chambers (37%) and round bowls (39%) (data not shown). Seven days later, on test day, we injected all rats in the locomotor activity chambers with cocaine or saline. In the Paired groups (Figure 1A), repeated cocaine injections enhanced, and thus sensitized, cocaine-induced locomotor activity, while cocaine-induced locomotion was not enhanced in the Non-paired groups [Figure 1B; three-way ANOVA interaction of prior drug exposure (cocaine, saline), test injection (cocaine, saline), and test environment (paired, non-paired): F(1,56)= 8.9, p=0.004]. Thus, cocaine test injections elicited a sensitized locomotor response only in the environment previously paired with repeated cocaine injections, indicating that a learned association between cocaine effects and its administration environment is critical for the expression of this form of sensitization.
Figure 1.
Environment modulates cocaine-induced locomotor activity and accumbens neuronal activity in sensitized rats. (A) Prior repeated cocaine injections enhanced cocaine-induced locomotor activity in Paired rats that received cocaine injections (15 mg/kg, i.p.) in the same locomotor activity chambers (context A), but not in (B) Non-paired rats that received the same repeated cocaine injections in a different environment (context B). In parallel, prior repeated cocaine injections enhanced cocaine-induced Fos expression in (C) Paired rats, but not in (D) Non-paired rats. Values are expressed as the mean±SEM using n=7-8 rats per group. * indicates a significant difference relative to cocaine challenge following repeated saline administration. (E) Fos and NeuN immunohistochemistry in nucleus accumbens of sensitized rats after the cocaine test injection in the Paired environment. Green-labeled nuclei indicate Fos expression; red-labeled nuclei indicate expression of the general neuronal nuclei marker NeuN; green/yellow-labeled nuclei are double-labeled for both Fos and NeuN expression. White bar indicates length of 100 micrometers.
We previously identified a neural correlate of context-specific sensitization to cocaine in the nucleus accumbens13. The ability of cocaine to induce the neural activity marker Fos24-27 in nucleus accumbens is strongly enhanced for at least six months following sensitization in a novel environment13, 17, 20. Here, we found that specific environments modulate this enhanced Fos response. Repeated cocaine injections enhanced cocaine-induced Fos expression in the Paired groups (Figure 1C), but not in the Non-paired groups [Figure 1D; three-way ANOVA interaction of prior drug exposure (cocaine, saline), test injection (cocaine, saline), and test environment (paired, non-paired): F(1,55)= 7.7, p=0.008]. As with the locomotor response, cocaine test injections produced an enhanced Fos response only in the environment previously paired with repeated cocaine injections. Considered alongside our previous findings that the cocaine administration environment modulates not only neuronal activation but also the specific pattern of neurons that are activated during expression of context-specific sensitization18, the present finding indicates that activity of a small proportion of nucleus accumbens neurons correlates with and could mediate learned associations underlying this behavior.
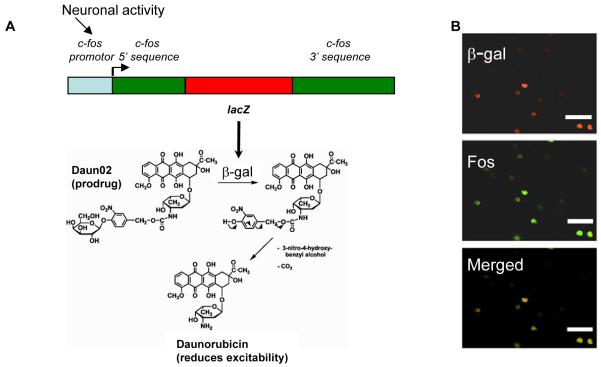
However, it remains unknown whether these activated neurons play a causal role in mediating the context-specific sensitized response. Until now, no tools existed to selectively manipulate the small number of sparsely distributed neurons activated during a drug-related learned behavior. This difficulty is exemplified by using double-labeling for Fos and the neuronal marker NeuN: only 2-3% of all accumbens neurons were activated by cocaine during the sensitized locomotor response (Figure 1E, Supplementary figure i). How do we manipulate these neurons without affecting the surrounding neurons? We developed a novel technique for inactivating only those accumbens neurons activated during context-specific sensitized locomotion. We used c-fos-lacZ transgenic rats developed by Drs. J. Morgan and T. Curran (St. Jude Children’s Hospital, Memphis, TN) that contain a transgene with a c-fos promoter regulating expression of the bacterial lacZ gene, which encodes the protein β-galactosidase28 (Figure 2A) that can be detected with X-gal staining (Figure 4C, Supplementary figure ii). When injected into cocaine sensitized c-fos-lacZ rats in the drug-paired environment, cocaine induced β-galactosidase exclusively in Fos-expressing accumbens neurons; conversely all Fos was expressed only in β-galactosidase nuclei (Figure 2B). This allowed us to use a prodrug called Daun02, which is converted by β-galactosidase to daunorubicin29 (Figure 2A), which has been shown to reduce calcium ion (Ca2+)-dependent action potentials in neuroblastoma cells30. Thus, we could selectively inactivate β-galactosidase expressing neurons activated in the presence of cocaine and paired stimuli in the administration environment, and monitor the degree of inactivation by measuring β-galactosidase expression.
Figure 2.
Schematic mechanism for Daun02 inactivation in c-fos-lacZ rats. (A) The c-fos-lacZ transgene contains a c-fos promoter that drives expression of lacZ, which encodes the protein β-galactosidase (β-gal) (adapted from Schilling et al.39). β-galactosidase can catalyze the prodrug Daun02 into the daunorubicin (adapted from Farquhar et al.29), which reduces cellular excitability30. (B) Cocaine-induced neuronal activity induces β-galactosidase expression (red-labeled nuclei) and Fos-expressing (green-labeled nuclei) neurons in nucleus accumbens of sensitized c-fos-lacZ rats. Nuclei double-labeled for both β-galactosidase and Fos appear yellow to orange in the Merged image panel and indicate colocalization of β-galactosidase and Fos proteins. We performed quantitative analysis of cocaine-induced Fos and β-galactosidase co-localization in accumbens from three sensitized c-fos-lacZ rats. β-galactosidase was expressed in 100% of the Fos expressing neurons from all three rats and vice versa. Thus Daun02 can inactivate activated neurons that express both β-galactosidase and Fos. White bar indicates length of 50 micrometers.
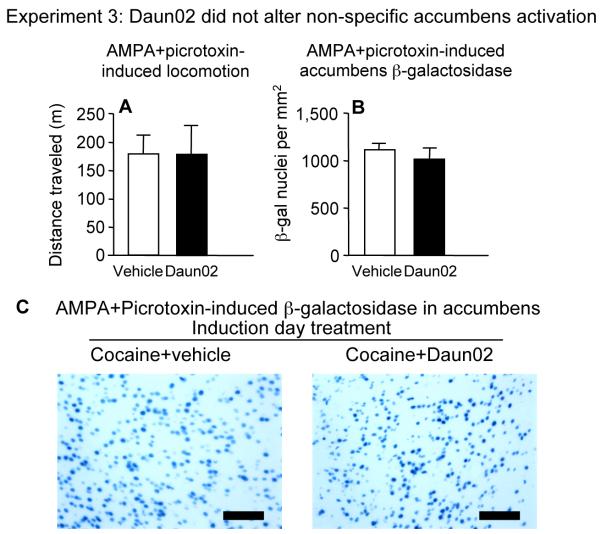
Figure 4.
Experiment 3: Daun02 on induction day did not alter locomotor activity (A) and β-galactosidase expression (B) in nucleus accumbens of c-fos-lacZ rats following accumbens AMPA+picrotoxin infusions on test day. (C) Representative images of X-gal staining for visualization of β-galactosidase. Black bar indicates length of 100 micrometers. Values are expressed as mean±SEM distance traveled during 1 h and mean±SEM density of β-galactosidase-labeled nuclei in nucleus accumbens following intra-accumbens AMPA+picrotoxin (n=7-8)
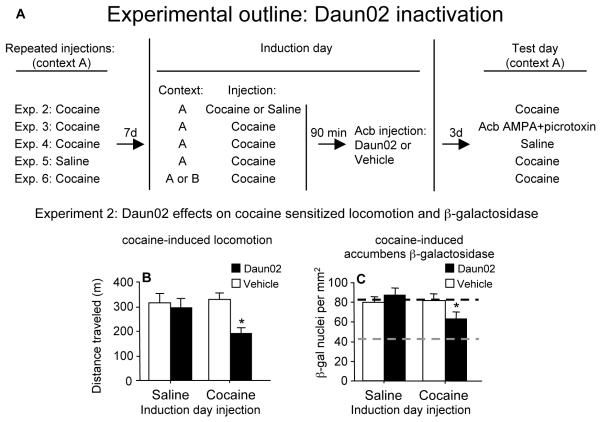
The timeline for all Daun02 inactivation experiments is shown in Figure 3A. In Experiment 2, we sensitized cfos-lacZ rats to cocaine using the same procedure described above for wild-type rats. Repeated cocaine injections increased cocaine-induced locomotor activity levels approximately 38% from day 1 to day 7 (F(1,58)=21.3, p<0.0001), which is comparable to wild-type rats in Experiment 1. Seven days later, on induction day, rats were injected with either cocaine to induce β-galactosidase or saline as a control that does not significantly induce β-galactosidase. Ninety minutes later, when β-galactosidase was near maximal levels, Daun02 or vehicle was bilaterally infused into the accumbens and the rats were returned to their home cages for three more days to produce cell-specific inactivation. On test day, all rats were injected with cocaine to assess cocaine-induced locomotor activity and accumbens β-galactosidase expression. Thus, all values shown in Figures 3B and 3C indicate levels following cocaine injections on test day, while ‘Saline’ and ‘Cocaine’ on the x-axes refer to injections on induction day, three days prior to test day.
Figure 3.
Timeline for Daun02 inactivation experiments (A). Rats were repeatedly injected once daily for 7 days with cocaine (15 mg/kg, i.p) or saline. On induction day, after 7 days of withdrawal, the rats were injected with either cocaine (20 mg/kg) or saline. Ninety minutes later, rats received an intracranial infusion of 2 micrograms of Daun02 or vehicle bilaterally into the nucleus accumbens (Acb). On test day, 3 days after intracranial infusions, the rats were injected with cocaine (15 mg/kg) or saline. Experiment 2: Daun02 attenuated cocaine-induced locomotion and β-galactosidase expression in nucleus accumbens of c-fos-lacZ rats. Rats were repeatedly injected with cocaine from day 1 to day 7, and seven days later on induction day, rats were injected with cocaine or saline followed by accumbens Daun02 or vehicle infusions. On test day, three days later, all rats were injected with cocaine. Daun02 attenuated cocaine-induced locomotor activity (B) and accumbens β-galactosidase levels (C) on test day when rats were previously injected with cocaine, but not saline, on induction day. Black dashed line indicates maximum levels of cocaine-induced β-galactosidase (as determined from vehicle induction group) and grey dashed line indicates baseline levels of β-galactosidase (as determined from saline test injection group in Experiment 4; Supplementary Figure v). Values are expressed as mean±SEM distance traveled during 1 h following cocaine test injections (n=14-21) and mean±SEM density of β-galactosidase-labeled nuclei in nucleus accumbens (n=14-16). * indicates a significant difference relative to that in rats infused with vehicle following cocaine injections on induction day.
Nucleus accumbens injections of Daun02 attenuated cocaine-induced locomotor activity (Figure 3B) in rats that previously received cocaine, but not saline, on induction day (interaction of Daun02 infusions (Daun02, vehicle) with Induction day injection (cocaine, saline): F(1,67)=5.03, p=0.028). Indeed, Daun02 reduced cocaine-induced locomotor activity to levels similar to the acute cocaine response observed on day 1 of the sensitization treatment. Daun02 also attenuated cocaine-induced β-galactosidase expression (Figure 3C) in rats that previously received cocaine, but not saline, on induction day (interaction of Daun02 infusions with Induction day injection: F(1,53)=4.52, p=0.038). For perspective, when the number of β-galactosidase nuclei induced following cocaine injections (Figure 3C) are compared to the number induced following saline control injections (Experiment 4; Supplementary figure v), Daun02 produced a 50% decrease in the number of β-galactosidase nuclei induced specifically by cocaine. As an anatomical control, infusions of Daun02 into caudate-putamen one millimeter dorsal to our test site did not alter sensitized locomotor responses to cocaine test injections (p=0.36). In addition, when wild-type rats were treated with the identical procedure used in Experiment 2, Daun02 did not have a significant effect on cocaine-induced locomotor activity which further indicates a lack of non-specific effects on neurons that do not produce β-galactosidase (Supplementary figure iii). Altogether, these findings demonstrate that Daun02 inactivation requires cocaine-induced β-galactosidase found in strongly activated accumbens neurons. If Daun02 had produced non-specific inactivation of accumbens neurons, then Daun02 infusions would also have attenuated cocaine-induced locomotor activity and β-galactosidase expression in rats injected with saline on induction day.
We next examined whether Daun02-mediated attenuation of cocaine-induced locomotion and β-galactosidase was associated with non-specific effects in the accumbens. To examine the possibility that Daun02-mediated effects were due to generalized neurotoxicity in the accumbens, we examined cresyl violet-stained sections of nucleus accumbens from rats in Experiment 2. Sections did not indicate gross alterations or decrease in size of nucleus accumbens architecture and no indication of gliosis at higher-level magnification (Supplementary figure iv). To examine the possibility that Daun02 effects were due to non-specific impairments of accumbens function, we assessed Daun02 effects on non-specific activation of accumbens neurons in Experiment 3. Rats were treated identically to those in Experiment 2, but a mixture of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) and picrotoxin (a GABAa receptor antagonist) was infused into the accumbens on test day to produce robust (over 12 fold more β-galactosidase expression than cocaine) non-specific activation of accumbens neurons. Daun02 did not affect AMPA+picrotoxin-induced locomotion (Figure 4A, p=0.94) or the number of β-galactosidase-labed nuclei (Figure 4B, p=0.39, images in 4C). This lack of effect indicates that when cocaine was injected on induction day in Experiment 2, subsequent Daun02-dependent decreases in cocaine-induced locomotion and β-galactosidase were not due to non-specific inactivation of accumbens neurons. Taken together, Daun02 inactivation after expression of context-specific sensitization does not appear to produce significant non-specific effects on nucleus accumbens structure and function.
In Experiment 4, we assessed the effects of Daun02 inactivation on spontaneous or novelty-induced locomotor activity. Daun02 infusions on induction day did not alter locomotor activity (p=0.65) or β-galactosidase expression (p=0.72) after saline test injections (Supplementary figure v). This finding indicates that Daun02 inactivation of accumbens neurons is specific for only cocaine-induced locomotor activity and β-galactosidase expression.
In Experiment 5, we examined whether Daun02 inactivation was effective after an acute cocaine injection. We repeatedly injected saline, instead of cocaine, to c-fos-lacZ rats and on induction day, rats received cocaine followed by accumbens infusions of Daun02 or vehicle. On test day, Daun02 did not alter subsequent cocaine-induced locomotion (p=0.61; Supplementary figure vi), indicating that Daun02 inactivation requires prior sensitization to cocaine.
As shown in Experiment 1, the learned association mediating context-specific locomotor sensitization is expressed only in the cocaine-paired environment. By extension, the specific neurons mediating this learned association should also be activated only in the cocaine-paired environment. Therefore, cocaine injections in an alternate non-paired environment on induction day should not activate these context-specific activated neurons and consequently Daun02 should fail to attenuate subsequent sensitized responding in the paired environment on test day. We tested this prediction in Experiment 6 where c-fos-lacZ rats received repeated cocaine injections in locomotor activity chambers (context A) according to our usual procedure, but on induction day, we injected half of these rats with cocaine in the paired environment (context A) and the other half with cocaine in an alternate non-paired environment (context B) followed by accumbens infusions of Daun02 or vehicle. On test day, we injected all rats with cocaine in context A and assessed locomotor activity. Daun02 attenuated cocaine-induced locomotor activity [Figure 5; interaction of Daun02 infusions (Daun02, vehicle) with Induction day context (A, B): F(1,55)=5.94, p=0.018] in rats that received cocaine on induction day in the paired environment (context A), but not in rats that received cocaine in the alternate non-paired environment (context B). The lack of Daun02 effects when infused in the alternate environment indicates that the neurons selectively activated by cocaine in the cocaine-paired environment remained intact and preserved the learned association.
Figure 5.
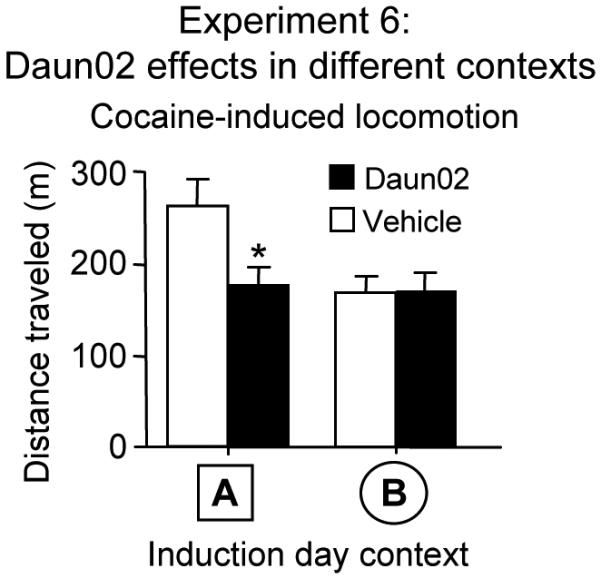
Experiment 6: While Daun02 attenuated locomotor activity when cocaine was previously injected in the Paired environment (context A), Daun02 did not alter cocaine-induced locomotor activity on test day in c-fos-lacZ rats that were previously injected on induction day with cocaine in an alternate non-paired environment (context B) prior to Daun02 infusions. Cocaine-induced locomotion on test day was significantly higher than Day 1 for both context A and B groups infused with vehicle. Values are expressed as mean±SEM distance traveled during 1 h following test injections (n=10-15 for context A; n=17 for context B). * indicates a significant difference relative to that in rats infused with vehicle following cocaine injections on induction day.
Discussion
Our Daun02 inactivation procedure allowed us to demonstrate a causal role between a small subset of nucleus accumbens neurons selectively activated by cocaine in the drug-paired environment and context-specific locomotor sensitization to the drug. Daun02 disrupted behavior only when these neurons were activated by cocaine in the paired environment and did not produce non-specific impairments in accumbens function or structure. We found that Daun02 was effective only when neurons were activated by cocaine, but not saline, on induction day. Daun02 was effective in attenuating cocaine-induced, but not saline-induced or AMPA+picrotoxin-induced locomotion and accumbens activity on test day. Daun02 was effective only in reducing sensitized cocaine-induced locomotor activity but not locomotion following the first acute injection of cocaine. Most importantly, Daun02 required context-specific cocaine-induced activation of a small subset of accumbens neurons in the drug-paired environment while Daun02 had no effect when cocaine was injected in an alternate non-paired environment on induction day. Overall, the dependence of Daun02 on stimuli-induced endogenous neuronal activity in behaving animals permits examination of context-specific activation of a small proportion of selectively activated neurons in drug-induced behavior.
The learned association between cocaine and the drug administration environment following context-specific locomotor sensitization to cocaine appears to be mediated by enhanced activation of a small number of sparsely distributed neurons in the nucleus accumbens. Results from our previous study indicate that the exact pattern of these activated neurons is determined at least in part by cocaine-induced interoceptive cues and associated environmental cues present during drug injections18. Following a cocaine sensitization treatment in this previous study, test injections of cocaine in the formerly cocaine-paired environment activated 87% of the same neurons that were repeatedly activated in nucleus accumbens during prior repeated cocaine administration. This overlap in activation pattern was significantly less when test injections of cocaine were administered in an alternate unpaired environment. The results from this previous study are congruent with our present results that indicated Daun02 disrupted sensitized locomotion only when cocaine was injected in the cocaine-paired environment on induction day, but not when injected in an alternate unpaired environment. Altogether these studies lead us to speculate that during repeated cocaine injections in a novel environment, the learned association between environment and cocaine effects becomes encoded in specific neuronal ensembles31, 32.
Our Daun02 inactivation technique has the broader potential to reveal the causal roles that selectively activated subsets of neurons play in a variety of learned behaviors, such as cue-induced drug seeking33-35, fear conditioning36, or spatial learning37. By revealing behaviorally relevant alterations produced in a small number of selected neurons, the present technique in rats and a recently reported technique in mice38, combined with electrophysiological and molecular characterization of these neurons during a learning task, provide new tools to study the neuronal mechanisms of learned behaviors.
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Drug Abuse, Intramural Research Program. We thank Emily Wentzell for her excellent editorial assistance, Kriss Knestaut for breeding the cfos-lacZ rats and Tristan Adams-Deutsch, Charles Pickens, Kristina Wihbey, for technical assistance. We are especially thankful to Yavin Shaham for his comments over the years and for his help in writing this manuscript and to Roy Wise for providing the inspiration this study.
Appendix
Methods
Subjects
Male c-fos-lacZ transgenic rats28 and Sprague-Dawley rats (Charles River, Raleigh, NC, USA) were housed individually in standard plastic cages in a temperature-and humidity-controlled room. They were maintained on a 12 : 12 h reverse light : dark cycle (lights on at 21.00 h), and allowed free access to food and water. They were acclimatized to these housing conditions for a minimum of 7 days prior to drug treatments. Experimental procedures were approved by the NIDA Animal Care and Use Committee.
Experiment 1: Cocaine-induced locomotor sensitization in wild-type rats
Male Sprague-Dawley rats were divided into 8 groups of 7-8 rats each. We administered 7 once daily injections of cocaine (15 mg/kg, i.p.) or saline to 4 groups of ‘Paired’ rats in locomotor activity chambers (context A: square chamber, dark, silent, smooth floor) and to 4 groups of ‘Non-paired’ rats in a distinct novel environment (context B: round chamber, brighter lighting, music by Marilyn Manson played continuously, woodchip bedding on floor). Seven days later, we placed rats in the locomotor activity chambers (context A) for 30 min and then injected all rats with cocaine (20 mg/kg, i.p.) or saline in the locomotor activity chambers and assessed locomotor activity for 60 minutes. Two hours after the test injections, all rats were deeply anesthetized with isoflurane and perfused transcardially with 4% paraformaldehyde.
Fos immunohistochemistry
Brain sections were processed for Fos immunohistochemistry and quantified as described previously13, 17. We used Fos antibody (1:10,000 dilution of Ab-5; Calbiochem, San Diego, CA) and developed the sections using an Elite ABC kit from VectorLabs (Burlingame, CA) and diaminobenzidine (DAB).
Fos and NeuN double-labeling
Eight more rats were treated identically as the Paired groups above and all rats were perfused with paraformaldehyde two hours after test injections of cocaine (20 mg/kg i.p.) or saline (n=4). The general procedure for fluorescence immunohistochemistry and quantification was similar to that described previously18. Sections were incubated with Fos antibody (1:10,000 dilution of Ab-5, Calbiochem) and NeuN antibody (1:2000 dilution of MAB377, Chemicon, Temecula, CA) and labeled with secondary antibodies conjugated to Alexa 488 and 568 fluorophores, respectively.
β-galactosidase and Fos immunohistochemistry with c-fos-lacZ rats
We used transgenic c-fos-lacZ rats that had been bred for 35-40 generations on a Sprague-Dawley background28. We first examined colocalization of Fos and β-galactosidase expression in these rats. All c-fos-lacZ rats received repeated cocaine injections as described above for Paired groups. Rats were perfused with paraformaldehyde two hours after the test injections of cocaine (20 mg/kg i.p.). Sections were incubated with Fos antibody (1:10,000 dilution of Ab-5, Calbiochem) and β-galactosidase goat polyclonal antibody (1:1,000 dilution of 4600-1409, Biogenesis, Oxford, U.K.) and labeled with secondary antibodies conjugated to Alexa 488 and 568 fluorophores, respectively.
Experiments 2-6: Implanting cannula into c-fos-lacZ rats
We implanted guide cannula into the accumbens for Daun02 inactivation experiments using previously described surgical procedures13. Transgenic rats (250-350 g) were anesthetized with equithesin (including 60 mg/kg sodium pentobarbital + 225 mg/kg chloral hydrate; i.p.). For nucleus accumbens infusions, permanent guide cannulae (23 G; Plastics One, Roanoke, VA) were implanted bilaterally at a 10° angle with the tip 1 mm dorsal to the nucleus accumbens; coordinates were anteroposterior +1.6 mm, mediolateral ±3.0 mm, and dorsoventral -6.0 mm relative to Bregma40. Guide cannula coordinates for control infusions into the caudate-putamen were anteroposterior +1.6 mm, mediolateral ±3.0 mm, and dorsoventral -5.0 mm relative to Bregma. Rats were allowed to recover for at least 5 days before beginning experiments.
Experimental procedure for Daun02 inactivation
The detailed timeline for Daun02 experiments is shown in Figure 3A. In Experiment 2, c-fos-lacZ rats received 7 once daily injections of cocaine (15 mg/kg i.p.), using the same procedure described above for Paired rats. On ‘induction day’, seven days later, we injected all rats with cocaine (20 mg/kg, i.p.) or saline in the locomotor activity chambers. Ninety minutes later, 2 μg of Daun0229 or vehicle (50% ACSF, 50% DMSO) in 0.5 μL volume was bilaterally infused over 2 minutes into the nucleus accumbens or the dorsal control site, caudate-putamen. Injectors extended 1 mm beyond the end of the guide cannula. Accumbens cannula placement was verified in cresyl violet stained sections (Supplementary figure vii). Rats were returned to their home cages for three days after infusions. The three-day time point was chosen based on unpublished pilot studies on the time course of the effectiveness of Daun02. On test day, rats were placed in locomotor activity chambers for 30 minutes and then injected with cocaine (15 mg/kg i.p.) and locomotor activity was monitored for 60 minutes. Rats were perfused with paraformaldehyde two hours after test injections. Brain sections were processed for X-gal histochemistry. For Experiments 3-6 and wild-type controls, the same experimental timeline (e.g. once daily repeated injections, induction day, and test day) and cocaine doses were used, but with the following modifications. For Experiment 3, the similar procedure was used except on test day rats were infused with alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) (1.0mM, 00-5, Ascent Scientific, Bristol, U.K.)+picrotoxin (2.7mM, P176, Sigma, St. Louis, USA) bilaterally in the accumbens. For wild-type controls, two groups of wild-type rats were repeatedly injected with cocaine once daily for 7 days, and on induction day all rats were injected with cocaine followed by Daun02 or vehicle infusions. On test day, all rats were then injected with cocaine. In Experiment 4, rats were repeatedly injected with cocaine once daily for 7 days, and on induction day all rats were injected with cocaine followed by Daun02 or vehicle infusions. On test day, all rats were then injected with saline. Experiment 5, rats were repeatedly injected with saline once daily for 7 days, and on induction day all rats were injected with cocaine followed by Daun02 or vehicle infusions. In Experiment 6, rats were repeatedly injected with cocaine once daily for 7 days in locomotor activity boxes (context A). On induction day, half of these rats were injected with cocaine in the round chambers (context B; described above for Experiment 1) while the other half was injected with cocaine in the activity boxes (context A), followed by Daun02 or vehicle infusions. On test day, all rats were injected with cocaine in the locomotor activity boxes (context A).
X-Gal histochemistry
Coronal sections (30 μm) were cut in a cryostat at -20°C and collected in 10 mM PBS. Free floating sections were washed 3 times for 10 minutes each in PBS followed by incubation in reaction buffer (0.1 M X-gal, 100 mM sodium phosphate, 100 mM sodium chloride, 5 mM EGTA, 2 mM MgCl2, 0.2% Triton X-100, 0.05 M K3FeCN6, 0.05 M K4FeCN6) for 3 hours at 37°C with gentle shaking. Sections were washed with 3 times for 10 minutes each in PBS and mounted onto chrom-alum coated slides. Slides were dried, dehydrated through a graded series of alcohol (70, 95, 95, 100, 100% ethanol) and cleared with Citrasolv (Fisher Scientific, Hampton, NH) before coverslipping with Permount (Fisher Scientific).
Quantification of histochemical results
Bright-field images of nucleus accumbens were captured under a 5X objective lens and digitized using a CCD camera (Coolsnap Photometrics, Roper Scientific Inc., Trenton, NJ) attached to a Zeiss Axioskop 2 light microscope. β-galactosidase-expressing nuclei characterized by blue nuclear staining were counted from these images using IPLab software for Macintosh (Scanalytics, Inc., Fairfax, VA) (Supplementary figure ii). We chose a threshold level that detected moderate to darkly stained nuclei but not lightly stained nuclei. We counted nuclei from three rectangular sampling areas of 0.15 mm2 each around the accumbens injection site from 2-3 coronal sections per rat. Image capture and quantification were conducted by an observer blind to the experimental conditions.
Data analysis
Locomotor activity and histochemical data were analyzed using factorial two- or three-way analyses of variance (ANOVA). Fisher’s PLSD post hoc tests were used to make specific group comparisons when appropriate. Effects were considered significant when P<0.05. In Experiments 2-5, 12 out of 199 rats that ran less than 70 m for two or more days during repeated cocaine injections were eliminated from the study due to abnormally low cocaine responses during sensitization. Of the remaining 187 rats, 8 rats with test day locomotion values two standard deviations beyond the mean were excluded. Rats were also excluded from analysis when injection sites were outside the intended boundaries; for example when they were too rostral (approximately 2.5 mm anterior to Bregma) or too caudal (approximately 1.2 mm anterior to Bregma). The rostral accumbens was chosen because nearly all cocaine-induced Fos is expressed within the rostral but not caudal portions of the nucleus accumbens17. Finally we excluded rats with obvious signs of necrosis and rats that we later determined to be c-fos-lacZ negative.
References
- 1.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 2.Siegel S. Morphine analgesic tolerance: its situation specificity supports a Pavlovian conditioning model. Science. 1976;193:323–325. doi: 10.1126/science.935870. [DOI] [PubMed] [Google Scholar]
- 3.Wise RA. Dopamine, learning and motivation. Nat. Rev. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 4.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat. Rev. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 5.Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol. Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 7.Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behav. Pharm. 2004;15:327–339. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56(Suppl 1):160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav. Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- 10.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 11.Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol. Biochem. Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- 12.Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J. Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hope BT, Simmons DE, Mitchell TB, Kreuter JD, Mattson BJ. Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur. J. Neurosci. 2006;24:867–875. doi: 10.1111/j.1460-9568.2006.04969.x. [DOI] [PubMed] [Google Scholar]
- 14.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 15.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 16.Vezina P, Giovino AA, Wise RA, Stewart J. Environment-specific cross-sensitization between the locomotor activating effects of morphine and amphetamine. Pharmacol. Biochem. Behav. 1989;32:581–584. doi: 10.1016/0091-3057(89)90201-3. [DOI] [PubMed] [Google Scholar]
- 17.Crombag HS, Jedynak JP, Redmond K, Robinson TE, Hope BT. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav. Brain Res. 2002;136:455–462. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 18.Mattson BJ, et al. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur. J. Neurosci. 2008;27:202–212. doi: 10.1111/j.1460-9568.2007.05984.x. [DOI] [PubMed] [Google Scholar]
- 19.Rademacher DJ, Napier TC, Meredith GE. Context modulates the expression of conditioned motor sensitization, cellular activation and synaptophysin immunoreactivity. Eur. J. Neurosci. 2007;26:2661–2668. doi: 10.1111/j.1460-9568.2007.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todtenkopf MS, Mihalakopoulos A, Stellar JR. Withdrawal duration differentially affects c-fos expression in the medial prefrontal cortex and discrete subregions of the nucleus accumbens in cocaine-sensitized rats. Neuroscience. 2002;114:1061–1069. doi: 10.1016/s0306-4522(02)00272-5. [DOI] [PubMed] [Google Scholar]
- 21.Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J. Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carelli RM. Activation of accumbens cell firing by stimuli associated with cocaine delivery during self-administration. Synapse. 2000;35:238–242. doi: 10.1002/(SICI)1098-2396(20000301)35:3<238::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 23.Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr. Opin. Neurobiol. 2004;14:763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Sgambato V, Abo V, Rogard M, Besson MJ, Deniau JM. Effect of electrical stimulation of the cerebral cortex on the expression of the Fos protein in the basal ganglia. Neuroscience. 1997;81:93–112. doi: 10.1016/s0306-4522(97)00179-6. [DOI] [PubMed] [Google Scholar]
- 25.Berretta S, Robertson HA, Graybiel AM. Neurochemically specialized projection neurons of the striatum respond differentially to psychomotor stimulants. Prog. Brain Res. 1993;99:201–205. doi: 10.1016/s0079-6123(08)61347-3. [DOI] [PubMed] [Google Scholar]
- 26.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Ann. Rev. Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 27.Canales JJ, Graybiel AM. Patterns of gene expression and behavior induced by chronic dopamine treatments. Ann. Neurol. 2000;47:S53–59. [PubMed] [Google Scholar]
- 28.Kasof GM, et al. Kainic acid-induced neuronal death is associated with DNA damage and a unique immediate-early gene response in c-fos-lacZ transgenic rats. J. Neurosci. 1995;15:4238–4249. doi: 10.1523/JNEUROSCI.15-06-04238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farquhar D, et al. Suicide gene therapy using E. coli beta-galactosidase. Cancer Chemother. Pharmacol. 2002;50:65–70. doi: 10.1007/s00280-002-0438-2. [DOI] [PubMed] [Google Scholar]
- 30.Santone KS, Oakes SG, Taylor SR, Powis G. Anthracycline-induced inhibition of a calcium action potential in differentiated murine neuroblastoma cells. Cancer Res. 1986;46:2659–2664. [PubMed] [Google Scholar]
- 31.Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 32.Graybiel AM. Habits, rituals, and the evaluative brain. Ann. Rev. Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 33.Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J. Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav. Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- 35.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 36.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Moser EI, Paulsen O. New excitement in cognitive space: between place cells and spatial memory. Curr. Opin. Neurobiol. 2001;11:745–751. doi: 10.1016/s0959-4388(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 38.Han JH, et al. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- 39.Schilling K, Luk D, Morgan JI, Curran T. Regulation of a fos-lacZ fusion gene: a paradigm for quantitative analysis of stimulus-transcription coupling. Proc. Natl. Acad. Sci. U.S.A. 1991;88:5665–5669. doi: 10.1073/pnas.88.13.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.