Abstract
Fibrin based hydrogels have been employed in vitro as a scaffold to promote tissue formation and investigate underlying molecular mechanisms. These hydrogels support a variety of cellular processes, and are being developed to enhance the presentation of biological cues, or to tailor the biological cues for specific tissues. The presentation of these cues could alternatively be enhanced through gene delivery, which can be employed to induce the expression of tissue inductive factors in the local environment. This report investigates gene delivery within fibrin hydrogels for two in vitro models of tissue growth: i) cell encapsulation within and ii) cell seeding onto the hydrogel. Naked plasmid and lipoplexes can be efficiently entrapped within the hydrogel, and after 1 day in solution more than 70% of the entrapped DNA is retained within the gel, with a sustained release observed for at least 19 days. Encapsulated lipoplexes did not aggregate and retained their original size. Transgene expression in vitro by delivery of lipoplexes was a function of the fibrinogen and DNA concentration. For encapsulated cells, all cells had intracellular plasmid and transgene expression persisted for at least 10 days, with maximal levels achieved at day 1. For cell infiltration, expression levels were less than those observed for encapsulation, and expression increased throughout the culture period. The increasing expression levels suggest that lipoplexes retain their activity after encapsulation; however, interactions between fibrin and the lipoplexes likely limit internalization. The inclusion of non-viral vectors into fibrin-based hydrogels can be employed to induce transgene expression of encapsulated and infiltrating cells, and may be employed with in vitro models of tissue growth to augment the intrinsic bioactivity of fibrin.
Keywords: fibrin, gene delivery, tissue engineering, in vitro transfection
Introduction
Hydrogels serve as a central component for applications in promoting tissue growth, and in basic studies that investigate tissue formation [1, 2]. These scaffolds provide a substrate for cell adhesion and migration and have a high water content, and their physical properties are similar to many tissues [3]. In particular, fibrin is a promising material due to its ability to support cellular processes, as it is naturally derived, and is degraded by cell-secreted proteases, thereby allowing it to be remodeled. Fibrin hydrogels are formed from fibrinogen which exists as a 45-nm dimer comprised of two D domains and a central E domain. Soluble fibrinogen is converted into insoluble fibrin through thrombin-mediated fibrinopeptide release, protofibril formation, and crosslinking in presence of factor XIII to yield a stabilized 3D network of fibrils known as the fibrin clot [4]. Degradation of the fibrin clot occurs on the order of days to weeks, mediated primarily through phagocytosis and plasminogen cleavage [4]. Commercially, fibrin consists of thrombin and fibrinogen cryoprecipitated from blood plasma, which also contains small amounts of fibronectin, transforming growth factor β1 (TGF β 1), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and other biomolecules [5].
Fibrin hydrogels have been widely employed as an in vitro matrix to investigate cellular processes relevant to tissue formation. Cells can be encapsulated within the fibrin hydrogels with maintenance of cell viability, such as in the development of vascular grafts [2, 6]. Alternatively, cells are seeded onto the fibrin hydrogels to investigate cell migration and simulate wound healing [7, 8]. Fibrin supports adhesion and migration of numerous cell types, including endothelial cells, fibroblasts, keratinocytes, myofibroblasts, and mesenchymal stem cells [7–13], through ligation of integrin receptors [14, 15]. Growth factors retained during isolation can stimulate some cellular processes. However, the bioactivity of fibrin is being enhanced or tailored to specific tissues [16–20]. The ability of the matrix to serve as a reservoir for growth factors has been enhanced by covalent tethering to fibrin. Alternatively, heparin has been employed to reversibly bind growth factors.
Localized gene delivery from fibrin represents an alternative approach for enhancing the specific bioactivity of the matrix. Transgene expressing cells serve as bioreactors for localized production of proteins, such as growth factors that can stimulate cell proliferation, migration, differentiation, as well as matrix synthesis [21, 22]. Additionally, gene delivery is versatile, with the potential to target any cellular process, and expression can persist for relative long time periods, which may be advantageous for proteins with short half-lives. Numerous hydrogels have been employed for gene delivery [23], with most biomaterial-based gene delivery systems focusing on non-viral vectors. Hydrogels have been employed to locally deliver non-viral vectors, and to provide a sustained release and maintain elevated concentrations. For regenerative medicine applications, fibrin has been employed as a vehicle on which to transplant ex-vivo engineered cells, or to directly deliver viral and non-viral vectors [24–27]. For non-viral approaches, naked plasmids or plasmid complexed with a transfection reagent have been incorporated and locally released. Fibrin-based gels have been employed with DNA complexes without reduction in activity [28], and modifications to the hydrogel structure may increase the duration of expression [27]. Plasmid release from fibrin in vivo has been able to increase the availability of inductive factors [29]. Fibrin hydrogels are promising for localized gene delivery, though the specific factors limiting gene transfer remain unclear.
In this report, the design of fibrin hydrogels that maximize gene transfer for in vitro models of tissue growth are investigated. We investigated the use of fibrin hydrogels for cell encapsulation, and also for seeding onto the gels to promote infiltration. The specific design conditions that were investigated include the fibrin and thrombin concentrations, and the vector dosage. The extent and duration of expression were monitored using luciferase assays, and the stability of complexes and the distribution of transfected cells were characterized by confocal microscopy. These results identify key design considerations for delivery of non-viral vectors from fibrin hydrogels for use with in vitro models of tissue growth, and may guide the ultimate translation to in vivo applications.
Material and methods
Cells and DNA
Transfection studies were performed with NIH/3T3 cells (ATCC, Manassas, VA) cultured at 37°C and 5% CO2 in cell growth media (Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 1% sodium pyruvate, 1% penicillin-streptomycin, 1.5 g/L NaHCO3, and 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA)). Plasmids encoding for luciferase (pLuc, obtained from National Gene Vector Labs) and a Green Fluorescent Protein (GFP)-Luciferase fusion protein (pEGFP-Luc) were employed, both of which have a CMV promoter. Plasmids were purified from bacteria culture using Qiagen (Valencia, CA) reagents and stored in Tris-EDTA buffer (10 mM Tris, 1 mM EDTA, pH 7.4). Lipoplexes were formed in serum-free DMEM at pH 7.4 by mixing DNA with Transfast™ (Promega, Madison, WI) with a 1:0.5 DNA:Transfast™ ratio, and incubated for 15 min at room temperature.
Fibrin hydrogels
Tisseel™ (Baxter Healthcare, BioScience Division, Westlake Village, CA) fibrin sealant products, kindly provided by Baxter International Inc., were used. Fibrinogen was reconstituted with the supplied aprotinin solution (100 mg/ml) and was diluted in Tris Buffered Saline (TBS) to the working concentrations. Thrombin was reconstituted with 40 μM calcium chloride (500 IU/ml), as supplied and further diluted at 50 UI/ml in 40 μM calcium chloride. Fibrinogen and thrombin solutions were mixed and injected in 24 well plates with a 2 ml Duploject™ kit (Baxter International Inc.) and the polymerization proceeded for 60 min at 37°C in a humid environment. Cell growth media was then added, with replacement every other day. Throughout the text, concentrations of fibrinogen and thrombin are given as the concentration before mixing and polymerization.
Vector Release
DNA release from fibrin gel (25 mg/ml fibrinogen) was characterized using radiolabelled DNA [30]. Naked 32P-DNA or lipoplexes with 32P-DNA were added to a 25 mg/ml fibrinogen solution and hydrogels were formed by mixing with the thrombin solution. Gels were incubated in PBS at 37°C and 32P-DNA released over time was measured by liquid scintillation.
Cell Encapsulation and Transfection
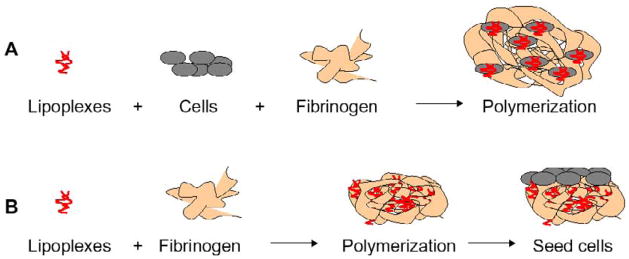
The initial transfection studies focused on the transfection of cells that were encapsulated into the hydrogels, with two techniques for mixing investigated. First, lipoplexes (1 μg of DNA/gel) were incubated with NIH/3T3 cells (60 000 cells/gel) in serum free media for subsequent addition to fibrinogen solutions before polymerization (Fig. 1A). This method maximizes the cellular interactions with the vector and internalization likely occurs primarily during the initial incubation. The effect of the different DNA doses on cell toxicity was evaluated 24 and 48 hours following incorporation in fibrin gels by a LDH test (Roche, Brussels) according to the supplier instructions. A 2D control transfection was performed by plating cells following mixing of the cells and lipoplexes. An additional control was employed to investigate expression resulting from DNA that is internalized while the cells are entrapped within the hydrogel rather than during the initial exposure. In this control, lipoplexes were mixed with fibrinogen solutions followed by addition of cells in serum containing media, and subsequent polymerization (Fig. 1B). Gene expression was quantified by luciferase activity at multiple time points, and was expressed as RLU/104 seeded cells.
Figure 1.
In vitro models for tissue growth. (A) Lipoplexes were incubated with the cells for 30 min prior the addition of fibrinogen and polymerization with thrombin (50 IU/ml). (B) Lipoplexes were mixed with fibrinogen and immediately polymerized with thrombin. Then cells were seeded on top of the fibrin gel.
Visualization of DNA-cell-fibrin interactions
Confocal imaging was performed to visualize plasmid fate after incorporation in fibrin gels (Leica TCS SP2 Confocal Scanning Laser Microscope, and Zeiss LSM multiphoton). Three-dimensional plasmid distribution in fibrin was visualized by labeling pLuc with label IT tracker CX- Rhodamine (MirusBio, Madison, WI). Lipoplexes (1 μg DNA/gel) were formed and then mixed with fibrinogen solution (25 mg/ml) and polymerized in chambered coverglass (Lab-Tek II, ThermoFisher Scientific, Waltham, MA). Images were captured beginning at the bottom of the hydrogel and progressed to a depth of 120 μm into the gel. A total of 40 images were captured, each with an effective thickness of 3 μm. Samples were analyzed 1 hour and 48 hours after polymerization. Rhodamine was detected between 590 and 600 nm after excitation at 543 nm (1 mV GreNe laser source).
To detect cellular internalization of plasmid, pLuc was labeled with Label IT Tracker Cy3 (MirusBio). Lipoplexes (1μg DNA/gel) were mixed with cells and then fibrinogen, and hydrogels were polymerized in chambered coverglass. Alternatively, for the control, lipoplexes (6μg DNA/gel) were incubated with fibrinogen and then with NIH/3T3 cells for subsequent polymerization. To visualize cell-DNA co-localization, cells were labeled with Syto61 red fluorescent nucleic acid stain (Invitrogen) just before imaging. After 24 hours of culture, Syto61 (red) was detected between 644 and 733 nm after excitation at 633 nm (10 mV He/Ne laser source) and Cy3 (blue) between 570 and 590 nm after excitation at 543 nm (1 mV Gre Ne laser source). To discriminate between cell surface bound and internalized lipoplexes, a xy confocal analysis along a xz axis was performed on samples composed of cells preliminary incubated with YOYO-1 DNA labeled lipoplexes (green) internalized in fibrin gels. Cells were stained prior to incubation with a cell tracker (CellTracker™ Red CMTPX, Invitrogen). A stack of pictures was acquired and using the orthogonal view tool (Zeiss lsm software), localization of lipoplexes compared to cells was performed. Red was detected between 577 and 602 nm after excitation at 561 nm (DPSS 561-10 laser source) and YOYO-1 between 491 and 509 after excitation at 488 nm (Argon/2 laser source).
To visualize the distribution of transfected cells within the hydrogel pEGFP-Luc-lipoplexes (1μg DNA/gel) were used. Transfected cells were identified by expression of GFP using fluorescence microscopy, with all encapsulated cells identified by staining with the fluorescent dye Syto61. GFP produced by transfected cells was detected between 500 and 550 nm after excitation at 480 nm (30 mV Ar/Kr laser source). This limited range of wavelengths was used to filter out cell autofluorescence.
Cell Infiltration and Transfection
Transfection of infiltrating cells was investigated by encapsulating lipoplexes within the hydrogel and then seeding cells onto the hydrogel. Lipoplexes with plasmid encoding for pLuc (1 and 6 μg of DNA/gel) were mixed with fibrinogen (25 mg/ml fibrinogen) and directly polymerized. The resulting gels were seeded with NIH/3T3 cells (50 000 cells/gel) (Fig. 1B). Luciferase expression was measured at Day 3, Day 6 and Day 11.
Statistics
Results from the studies were analyzed using ANOVA for multiple comparisons followed by post-hoc testing to identify specific conditions. Post-hoc testing was performed using the non-parametric Mann-Whitney and Kruskall-Wallis tests (significance P<0.05). In the studies, the sample size was n=3 unless otherwise stated with replicates performed.
Results and discussion
Vector Release
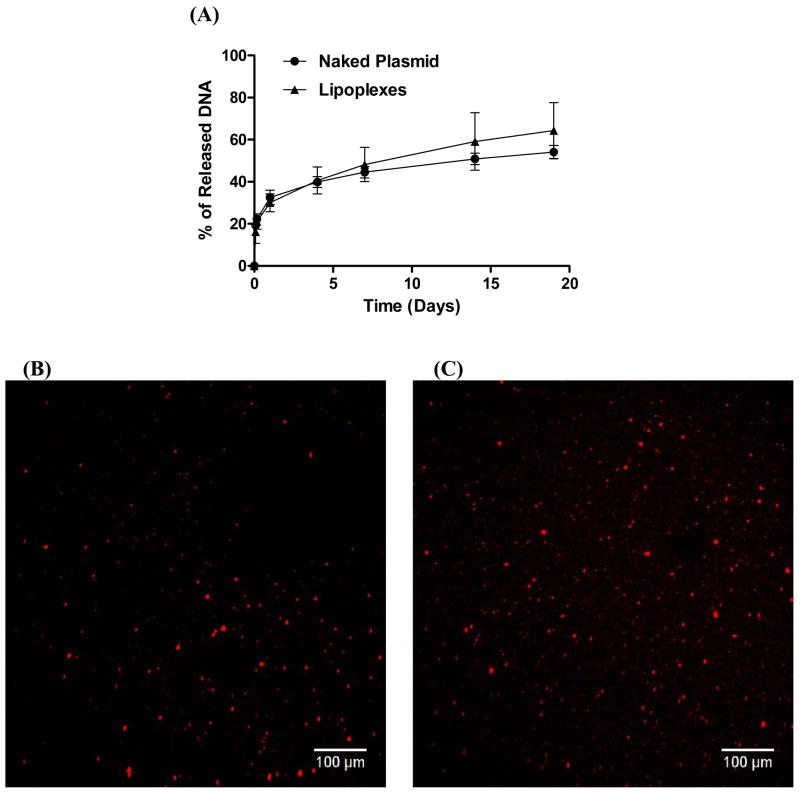
We initially investigated the release of lipoplexes from fibrin gels, with naked plasmid serving as a control. No significant difference was observed between the release profile of naked plasmid and lipoplexes (Fig. 2). An initial burst of 21% and 22% was observed after 4 hours for plasmid and lipoplexes, respectively. Thus, after 1 day, more than 70% of the DNA was retained in the fibrin gel, which is desirable for transfecting cells entrapped within the hydrogel or infiltrating into the hydrogel. A sustained release was observed for the 19 remaining days during which 54% and 64% of the incorporated plasmid and lipoplexes, respectively, were released. The similar release profiles for plasmid and lipoplexes despite differences in size suggests that release was not controlled by the hydrogel mesh size. In our studies, both vectors have negative zeta potentials [31] and may interact with the material to influence release. Naked plasmid and PEI-complexed DNA did not have varied released from fibrin gels [27], similar to our observations.
Figure 2.
Characterization of lipoplex release and morphology. (A) Release kinetics using 32P-DNA incorporated in fibrin gels (25 mg/ml fibrinogen, 50 IU/ml thrombin). (B, C) Lipoplex distribution in fibrin. Lipoplexes (1 μg) were formed and mixed with rhodamine-labeled pLuc plasmid, which was then mixed with fibrinogen (25 mg/ml). Lipoplexes were visualized by confocal microscopy (B) 1 hour and (C) 48 hours following polymerization.
The distribution and size of lipoplexes within the gel were subsequently characterized. Lipoplexes were observed as distinct particles and were distributed evenly following polymerization. There was no observable increase in the size of the lipoplex particles after 48 hours, suggesting that the hydrogel stabilized the particles against aggregation (Fig. 2B, C). Lipoplexes have been observed to interact with biomaterials and proteins [32–36] and thus entrapped lipoplexes were expected to associate with fibrin, which may limit aggregation as well as slow release [36].
Cell Encapsulation
Fibrinogen Concentration
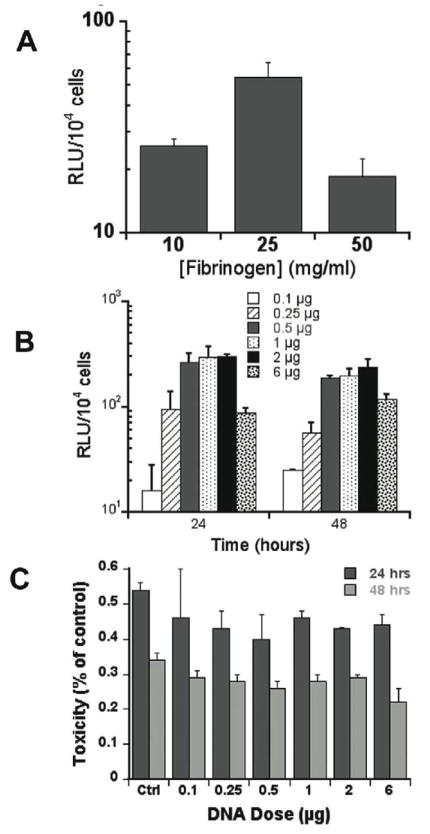
Transfection studies initially investigated the role the fibrinogen concentration in promoting transfection of encapsulated cells. The thrombin concentration has been reported to affect cell proliferation, migration and differentiation [4, 12, 37] less than the fibrinogen concentration. Thus, the thrombin concentration was fixed and the influence of fibrinogen concentration was investigated. Hydrogels were formed by mixing complexes with cells, followed by addition of fibrinogen at 10 to 50 mg/ml and polymerization with 50 IU/ml μg/ml thrombin (Fig. 1A), which maximizes the exposure of cells to lipoplexes. Transfection was observed in all fibrin hydrogel conditions. A fibrinogen concentration of 25 mg/mL provided a 2 and 3-fold higher luciferase expression compared to 10 mg/mL and 50 mg/mL, respectively (Fig. 3A). The increased expression at 25 mg/mL may reflect the balance between gel stability and cell proliferation. In general, fibrin gels with high fibrinogen concentrations (70–100 mg/ml) have decreased cell proliferation and migration relative to hydrogels with low fibrinogen concentrations (10 mg/mL) [4, 12]. However, fibrin hydrogels formed with 10 mg/mL fibrinogen had significant degradation after 7 days and limited the duration of culture. Since the greatest transfection was observed at 25 mg/mL fibrinogen, and gels retained their integrity for two weeks in vitro at this concentration, all subsequent studies were performed under this condition.
Figure 3.
Fibrinogen concentration and transgene expression. (A) NHI3T3 cells (60,000 cells) and 1 μg of lipoplexes were entrapped within hydrogels formed at a range of fibrinogen concentrations. (B) DNA concentration and transgene expression. Transfection efficiency was evaluated by measuring the gene expression of luciferase 24 and 48 hours following polymerization, expressed in RLU/104 seeded cells. (C) Cytotoxicity test (LDH) 24 and 48h after culture in fibrin hydrogels.
DNA concentration
Transgene expression was subsequently quantified as a function of the vector concentration. Luciferase expression increased as the DNA loading increased from 0.1 to 0.5 μg, plateaued between 0.5 to 2 μg, and then decreased as the DNA content reached 6 μg. For all DNA dosages, the expression levels were stable between 24 and 48 hours after transfection (Fig. 3B). This dose dependence of transfection is expected based on previous reports, though the specific values differ due to the presence of the hydrogel [29]. The decreased expression at 6 μg may result from cytotoxicity at the elevated DNA dosages. Lactate dehydrogenase was employed as a measure of cytotoxicity, and was measured immediately after cell exposure to complexes, and at 24 and 48 hours after culture in fibrin hydrogels. Immediately after cell exposure, the levels of LDH were elevated for a 6 μg dose relative to a dose of 1 or 2 μg (data not shown, p<0.05). After 24 or 48 hours of culture, the LDH response decreased between 24 and 48 hours, without substantial differences for the varying DNA doses (Fig. 3C).
Extent and Duration of Transgene Expression
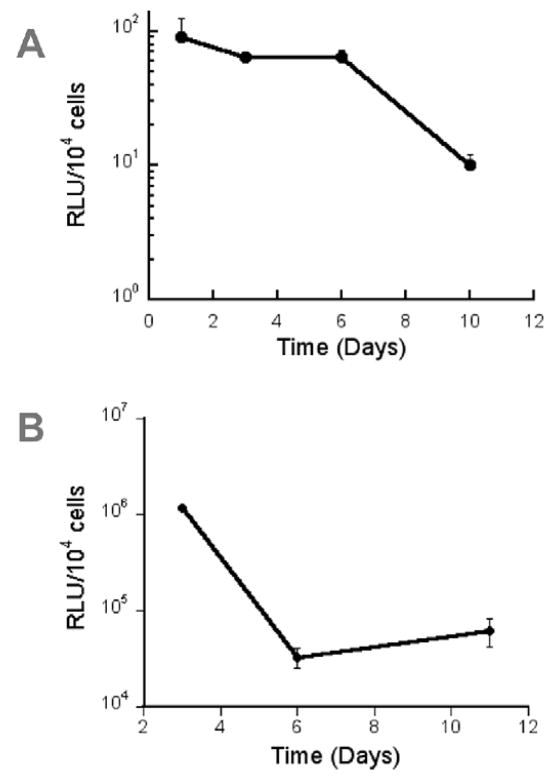
The kinetics of transgene expression were subsequently investigated for cells encapsulated within hydrogels. Detectable amounts of luciferase were produced for 10 days (Fig. 4A). Expression levels were stable during the first 6 days and decreased 6.6-fold at day 10 day. This duration of expression was subsequently compared to 2D transfection with the same quantities of DNA. For 2D transfection, expression was greatest at 3 days, decreased 35-fold at day 6, and remained consistent through day 11. Interestingly, 3D culture of cells has been proposed as a means to extend transgene expression [38]. Expression decreased substantially between days 3 and 6 for 2D transfection, and between days 6 and 11 for 3D transfection. However, the level of transgene expression is significantly higher in 2D. This reduced expression in 3D likely results from either interactions between fibrin and the lipoplexes that limits cell internalization, or decreased trafficking of vectors through the cell following internalization.
Figure 4.
Duration of transgene expression. NHI3T3 cells (60 000 cells) and 1 μg of lipoplexes were (A) mixed with fibrinogen (25 mg/ml) for subsequent polymerization or (B) plated onto 2D polystyrene.
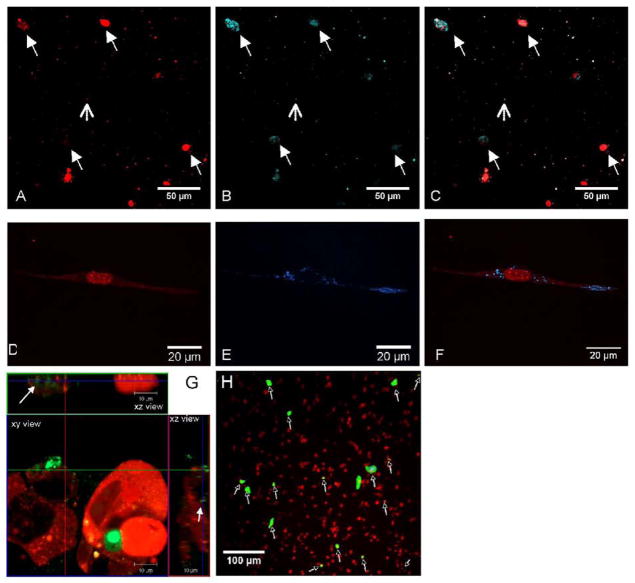
The cellular association and internalization of DNA was subsequently investigated (Fig. 5) to determine the relative distribution of DNA between the cells and hydrogel. The nucleic acid dye Syto61 was used to visualize the distribution of cells within the fibrin gel. Cells (red) fluorescence) and lipoplexes containing Cy3 labeled plasmid (blue fluorescence) were uniformly distributed in the gel (Fig. 5 A–F). Co-localization of Syto-61 and Cy3 labeled plasmid indicated that all cells had associated plasmid, consistent with reports for transfection by bolus addition of complexes. Confocal slices through the cells indicated that some of the cellular associated DNA was present intracellularly (Fig. 5G).
Figure 5.
Co-localization of cells and lipoplexes in fibrin. Lipoplexes, (1 μg of DNA/gel) and NHI3T3 cells (60,000 cells) were incorporated in fibrin gels (25 mg/ml fibrinogen, 50 IU/ml Thrombin). Images were captured by confocal microscopy for (A,D) cells stained with Syto61 (red) and (B,E) DNA labeled with Cy3 prior to complex formation (blue). (C,F) Co-localization of DNA with cells was visualized by superposition of the two pictures. (G) Confocal slice demonstrating intracellular DNA. (H) Transgene expression by cells within the gel was visualized by GFP production. Lipoplexes containing pGFP-Luc plasmid (1 μg of DNA/gel) and NHI3T3 cells (60,000 cells) were incorporated in fibrin gels.
To visualize the extent of cell transfection and the distribution of transfected cells, transfection studies were performed using lipoplexes containing pEGFP-Luc. Co-localization of GFP (green fluorescence) and cells stained with Syto61 (red fluorescence) identified transfected cells (Fig. 5H). These cells were homogeneously distributed; however, the number of transfected cells was relatively low (less than 10%) after 48 hours, which is significantly less than obtained with bolus transfection of NIH/3T3 cells (≈30%) [32]. The decreased number of transfected cells within the hydrogel relative to the standard 2D transfection is consistent with the decreased level of transgene expression. Given that bolus delivery and hydrogel encapsulation have similar DNA distribution, yet the percentage of transfected cells is less in the hydrogel, the 3D environment may reduce the efficiency of intracellular trafficking, or the hydrogel may limit the quantity of DNA internalized.
Vector-Material Interactions
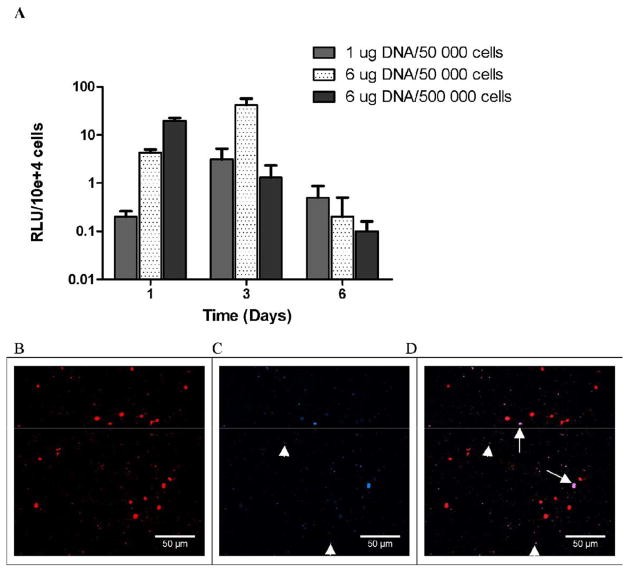
The extent of gene expression that results from the initial incubation with cells relative to expression that results from vector internalization after culture within fibrin was investigated by altering the mixing order for the vector, cells and material. This control conditions consists of pre-encapsulation of the vector within the fibrinogen solutions prior to the addition of cells and subsequent polymerization, Transfection was observed by cells within the hydrogel for this vector pre-encapsulation; however, the levels of expression were significantly lower relative to direct vector incubation with cells prior to encapsulation. Relative to the cell-vector pre-mixing approach, expression levels decreased by 424-fold, 19-fold, and 114-fold at Day 1, 3, and 6 respectively for the pre-encapsulated vector (Fig. 6A). In addition, while expression did not significantly vary during the initial 6 days of culture for cell-vector mixing, pre-encapsulation of the vector had expression increase from days 1 to 3, which decreased by day 6. The maximum expression was observed at Day 3 for the hydrogels formed with 50,000 cells and 6 μg DNA (41.7 RLU/104 seeded cells). We also investigated significant increases in cell density as a means to increase cell-vector association. Increasing the cell density by an order of magnitude produced the highest expression at day 1, which decreased through days 3 and 6. Confocal imaging of hydrogels formed with the pre-encapsulated vector after 24h indicated that Cy3-labeled plasmid was homogeneously distributed throughout the gel (Fig. 6B), yet the plasmid was visualized in less than 20% of the detected cells (solid arrows) (Fig. 6C & D). This small number of cells with intracellular plasmid is consistent with the low levels of transfection.
Figure 6.
(A) Lipoplexes-fibrinogen interaction influences transgene expression. Lipoplexes (1 and 6 μg of DNA) were incubated 30 minutes with fibrinogen (25 mg/ml) prior to cell addition (50,000 or 500,000 NIH3T3 cells) and polymerization. Transfection efficiency was evaluated by measuring the gene expression of luciferase over time, expressed in RLU/104 seeded cells. (B) Co-localization of cells and lipoplexes within fibrin gels. Lipoplexes (6 μg) were incubated 30 minutes with fibrinogen (25 mg/ml) prior to cell addition (50 000 NIH3T3 cells). (B) Cells were stained with Syto61 (red) and (C) DNA was labeled with Cy3 (blue) prior to complex formation. (D) Internalization of DNA by cells was visualized by superposition of the two pictures.
Taken together, these cell and imaging studies indicate that transfection results primarily from the initial cell/DNA interactions, rather than uptake by cells following encapsulation within the hydrogel. This initial exposure produces transgene expression that persists at high levels for 6 days and subsequently decreases. The relatively low expression that arises by vector internalization following cell encapsulation may be increased through the design of either the biomaterial or the vector. Modifying the vector with protective copolymers has been employed to decrease vector-material interactions, and may similarly increase delivery in this format [40].
Cell Infiltration and Gene Delivery
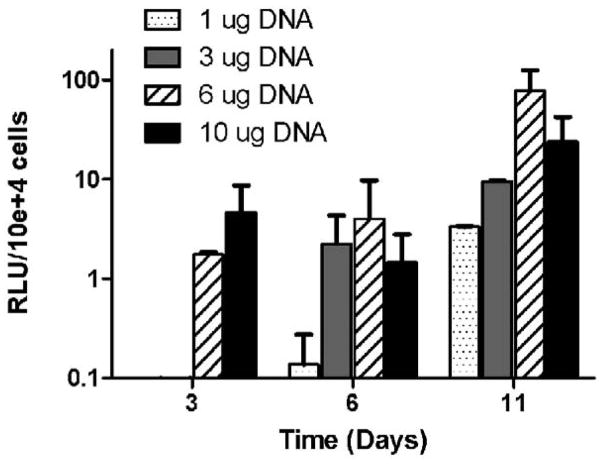
The transfection of cells infiltrating into the hydrogels was subsequently investigated by seeding cells onto lipoplex-loaded fibrin hydrogels (Fig. 1B). At 3 and 6 days following cell seeding, luciferase expression was low for all conditions yet increased significantly by Day 11 (Fig. 7). Between Day 6 and Day 11, transgene expression increased 24-, 4-, 20-, and 16-times for 1, 3, 6 and 10 μg of DNA, respectively. Consistent with hydrogel encapsulation, transgene expression was dose-dependent. The highest expression was observed for 6 μg of DNA/gel, higher amounts being probably cytotoxic. This dosage giving maximal transgene expression is significant increased relative to that used for vector-incubated encapsulated cells, likely due to the association of the lipoplexes with the fibrin during the encapsulation.
Figure 7.
Cell infiltration and gene delivery. Fibrin gels were formed (25 mg/ml fibrinogen, 50 IU/ml thrombin) incorporating lipoplexes (1, 3, 6, and 10 μg of DNA). 1 hour following polymerization 50,000 NIH3T3 cells were seeded on top of gels.
This observation of increased expression with time in culture may reflect an effect of cell infiltration and also vector-hydrogel interactions. With vector retained in the hydrogel after the first day, cell migration through the hydrogel would increase exposure to the vector and could lead to internalization of the lipoplexes. In addition, the expression profile may result from the slow release of vector from the hydrogel. This delay in availability due to sequestering by the fibrin would also delay cellular internalization, consistent with increase in expression over time. Taken together, these results would suggest that the rate of cell migration and the vector-material interactions will be important considerations for maximizing gene transfer within a hydrogel.
Conclusions
In this report, we demonstrate the potential of fibrin hydrogels as gene delivery system and characterized transgene expression for use with in vitro models of tissue growth. Fibrin hydrogel allowed a sustained release of DNA, naked or complexed, during at least 19 days. Transfection was dependent on fibrinogen and DNA concentrations, with the maximal expression dependent upon a balance between the hydrogel stability, maintaining cell viability, and promoting cell-vector interactions. The duration of expression depended upon the specific in vitro model (encapsulation or infiltration). For encapsulated cells, transgene expression was maintained for at least 10 days, with greatest levels at day 1. For cell infiltration, the expression increased throughout the 11 days of the study. Results of the release study and the encapsulation control transfection suggest an interaction between lipoplexes and fibrinogen, which decreased the transfection efficiency. These studies have identified the hydrogel properties that influence gene delivery, and the use of alternative cells that are either more (e.g., HEK293T) or less (e.g., neurons) transfectable will also influence the expression profile. Fibrin-based gene delivery systems are promising tools in the scope of, but not limited to, tissue engineering and regenerative medicine applications.
Acknowledgments
We thank Baxter International Inc., and more particularly Drs Tawil and Hagerman (Baxter BioSurgery, Global R&D, Biotheraputics & Regenerative Medicine). This work was supported by Baxter International Inc., the Belgium American Education Foundation (B.A.E.F), the Fonds National de la Recherche Scientifique (Belgium), the US Army TATRC W81XWH-05-1-0381 and the NIH RO1 GM066830.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288(3):H1451–1460. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 2.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98(1):25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 3.Peretti GM, Xu JW, Bonassar LJ, Kirchhoff CH, Yaremchuk MJ, Randolph MA. Review of injectable cartilage engineering using fibrin gel in mice and swine models. Tissue Eng. 2006;12(5):1151–1168. doi: 10.1089/ten.2006.12.1151. [DOI] [PubMed] [Google Scholar]
- 4.Ho W, Tawil B, Dunn JC, Wu BM. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12(6):1587–1595. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 5.Newton C, Goodwin C, Helgerson S, Spaethe R. The Behavior of Human Mesenchymal Stem Cells in 3D Fibrin Clots: Dependence on Fibrinogen Concentration and Clot Structure. In 8th World Biomaterials Congress; Sydney, Australia. 2004. [Google Scholar]
- 6.Liu JY, Swartz DD, Peng HF, Gugino SF, Russell JA, Andreadis ST. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res. 2007;75(3):618–628. doi: 10.1016/j.cardiores.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Horch RE, Bannasch H, Kopp J, Andree C, Stark GB. Single-cell suspensions of cultured human keratinocytes in fibrin-glue reconstitute the epidermis. Cell Transplant. 1998;7(3):309–317. doi: 10.1177/096368979800700309. [DOI] [PubMed] [Google Scholar]
- 8.Meana A, Iglesias J, Del Rio M, Larcher F, Madrigal B, Fresno MF, et al. Large surface of cultured human epithelium obtained on a dermal matrix based on live fibroblast-containing fibrin gels. Burns. 1998;24(7):621–630. doi: 10.1016/s0305-4179(98)00107-7. [DOI] [PubMed] [Google Scholar]
- 9.Ye Q, Zund G, Benedikt P, Jockenhoevel S, Hoerstrup SP, Sakyama S, et al. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2000;17(5):587–591. doi: 10.1016/s1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 10.Sporn LA, Bunce LA, Francis CW. Cell proliferation on fibrin: modulation by fibrinopeptide cleavage. Blood. 1995;86(5):1802–1810. [PubMed] [Google Scholar]
- 11.Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez-del-Pulgar S, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res. 2003;66(2):102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 12.Cox S, Cole M, Tawil B. Behavior of human dermal fibroblasts in three-dimensional fibrin clots: dependence on fibrinogen and thrombin concentration. Tissue Eng. 2004;10(5–6):942–954. doi: 10.1089/1076327041348392. [DOI] [PubMed] [Google Scholar]
- 13.Bensaid W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24(14):2497–2502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 14.Clark RA. Fibrin is a many splendored thing. J Invest Dermatol. 2003;121(5):xxi–xxii. doi: 10.1046/j.1523-1747.2003.12575.x. [DOI] [PubMed] [Google Scholar]
- 15.Chernousov MA, Carey DJ. alphaVbeta8 integrin is a Schwann cell receptor for fibrin. Exp Cell Res. 2003;291(2):514–524. doi: 10.1016/s0014-4827(03)00409-9. [DOI] [PubMed] [Google Scholar]
- 16.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF--fibrin matrices for endothelialization. J Control Release. 2001;72(1–3):101–113. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 17.Wong C, Inman E, Spaethe R, Helgerson S. Fibrin-based biomaterials to deliver human growth factors. Thromb Haemost. 2003;89(3):573–582. [PubMed] [Google Scholar]
- 18.Pandit AS, Wilson DJ, Feldman DS. Fibrin scaffold as an effective vehicle for the delivery of acidic fibroblast growth factor (FGF-1) J Biomater Appl. 2000;14(3):229–242. doi: 10.1177/088532820001400303. [DOI] [PubMed] [Google Scholar]
- 19.Lee AC, Yu VM, Lowe JB, 3rd, Brenner MJ, Hunter DA, Mackinnon SE, et al. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184(1):295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 20.Kipshidze N, Chekanov V, Chawla P, Shankar LR, Gosset JB, Kumar K, et al. Angiogenesis in a patient with ischemic limb induced by intramuscular injection of vascular endothelial growth factor and fibrin platform. Tex Heart Inst J. 2000;27(2):196–200. [PMC free article] [PubMed] [Google Scholar]
- 21.Collier S, Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthritis Cartilage. 1995;3(2):127–138. doi: 10.1016/s1063-4584(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 22.Scherping SC, Jr, Schmidt CC, Georgescu HI, Kwoh CK, Evans CH, Woo SL. Effect of growth factors on the proliferation of ligament fibroblasts from skeletally mature rabbits. Connect Tissue Res. 1997;36(1):1–8. doi: 10.3109/03008209709160209. [DOI] [PubMed] [Google Scholar]
- 23.De Laporte L, Cruz Rea J, Shea LD. Design of modular non-viral gene therapy vectors. Biomaterials. 2006;27(7):947–954. doi: 10.1016/j.biomaterials.2005.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escamez MJ, Carretero M, Garcia M, Martinez-Santamaria L, Mirones I, Duarte B, et al. Assessment of optimal virus-mediated growth factor gene delivery for human cutaneous wound healing enhancement. J Invest Dermatol. 2008;128(6):1565–1575. doi: 10.1038/sj.jid.5701217. [DOI] [PubMed] [Google Scholar]
- 25.Breen AM, Dockery P, O’Brien T, Pandit AS. The use of therapeutic gene eNOS delivered via a fibrin scaffold enhances wound healing in a compromised wound model. Biomaterials. 2008;29(21):3143–3151. doi: 10.1016/j.biomaterials.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Breen A, Dockery P, O’Brien T, Pandit A. Fibrin scaffold promotes adenoviral gene transfer and controlled vector delivery. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32039. [DOI] [PubMed] [Google Scholar]
- 27.Saul JM, Linnes MP, Ratner BD, Giachelli CM, Pun SH. Delivery of non-viral gene carriers from sphere-templated fibrin scaffolds for sustained transgene expression. Biomaterials. 2007;28(31):4705–4716. doi: 10.1016/j.biomaterials.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 28.Trentin D, Hall H, Wechsler S, Hubbell JA. Peptide-matrix-mediated gene transfer of an oxygen-insensitive hypoxia-inducible factor-1alpha variant for local induction of angiogenesis. Proc Natl Acad Sci U S A. 2006;103(8):2506–2511. doi: 10.1073/pnas.0505964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andree C, Voigt M, Wenger A, Erichsen T, Bittner K, Schaefer D, et al. Plasmid gene delivery to human keratinocytes through a fibrin-mediated transfection system. Tissue Eng. 2001;7(6):757–766. doi: 10.1089/107632701753337708. [DOI] [PubMed] [Google Scholar]
- 30.A. Amersham International plc. Labelling of DNA with 32P by nick translation. Technical Bulletin. 1980;80(3) [Google Scholar]
- 31.Tsutsumi T, Hirayama F, Uekama K, Arima H. Evaluation of polyamidoamine dendrimer/[alpha]-cyclodextrin conjugate (generation 3, G3) as a novel carrier for small interfering RNA (siRNA) Journal of Controlled Release. 2007;119(3):349–359. doi: 10.1016/j.jconrel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Bengali Z, Pannier AK, Segura T, Anderson BC, Jang JH, Mustoe TA, et al. Gene delivery through cell culture substrate adsorbed DNA complexes. Biotechnol Bioeng. 2005;90(3):290–302. doi: 10.1002/bit.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houchin-Ray T, Swift LA, Jang JH, Shea LD. Patterned PLG substrates for localized DNA delivery and directed neurite extension. Biomaterials. 2007;28(16):2603–2611. doi: 10.1016/j.biomaterials.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houchin-Ray T, Whittlesey KJ, Shea LD. Spatially patterned gene delivery for localized neuron survival and neurite extension. Mol Ther. 2007;15(4):705–712. doi: 10.1038/mt.sj.6300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pannier AK, Anderson BC, Shea LD. Substrate-mediated delivery from self-assembled monolayers: effect of surface ionization, hydrophilicity, and patterning. Acta Biomater. 2005;1(5):511–522. doi: 10.1016/j.actbio.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pannier AK, Wieland JA, Shea LD. Surface polyethylene glycol enhances substrate-mediated gene delivery by nonspecifically immobilized complexes. Acta Biomater. 2008;4(1):26–39. doi: 10.1016/j.actbio.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catelas I, Sese N, Wu BM, Dunn JC, Helgerson S, Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12(8):2385–2396. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 38.Xie Y, Yang ST, Kniss DA. Three-dimensional cell-scaffold constructs promote efficient gene transfection: implications for cell-based gene therapy. Tissue Eng. 2001;7(5):585–598. doi: 10.1089/107632701753213200. [DOI] [PubMed] [Google Scholar]
- 39.Varga K, Jurkuvenaite A, Wakefield J, Hong JS, Guimbellot JS, Venglarik CJ, et al. Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J Biol Chem. 2004;279(21):22578–22584. doi: 10.1074/jbc.M401522200. [DOI] [PubMed] [Google Scholar]
- 40.Scherer F, Schillinger U, Putz U, Stemberger A, Plank C. Nonviral vector loaded collagen sponges for sustained gene delivery in vitro and in vivo. J Gene Med. 2002;4(6):634–643. doi: 10.1002/jgm.298. [DOI] [PubMed] [Google Scholar]