Abstract
Triggering receptor expressed on myeloid cells (TREM) regulates inflammatory responses to lipopolysaccharide (LPS). In these studies, we analyzed expression of TREM in hepatic macrophages and endothelial cells which play a central role in LPS clearance. LPS administration to C3H/OuJ mice resulted in a rapid induction of TREM-1 and TREM-3, but a decrease in TREM-2 in liver macrophages and endothelial cells. The observation that TREM family members are detectable in endothelial cells is novel and demonstrates that their expression is not limited to myeloid cells. LPS-induced alterations in TREM expression were not evident in cells from C3H/HeJ TLR-4 mutant mice, indicating that the response is dependent on TLR-4. IL-1β and TNFα upregulated TREM-1 and TREM-3 expression and suppressed TREM-2 expression in macrophages and endothelial cells. This activity involved PI3-kinase and p38 MAP kinase signaling. Interestingly, no significant differences were noted in TREM expression between wild-type and TNFR1−/− mice treated with LPS. Treatment of macrophages and endothelial cells with LPS upregulated expression of nitric oxide synthase-2 (NOS-2). This was blocked by TREM-1 Fc/fusion protein, indicating that TREM-1 mediates LPS-induced NOS-2 expression. These results suggest that TREM proteins are important in the inflammatory response of hepatic macrophages and endothelial cells to acute endotoxemia.
Keywords: TNFα, IL-1β, TLR-4, liver, endotoxin
Introduction
Macrophages play an important role in immunological defense by releasing inflammatory mediators and by phagocytosing and destroying pathogens, particulates and macromolecules (Kolios, 2006). To aid in this process, macrophages express a number of cell surface receptors that recognize pathogens or endogenous substances released from damaged tissue during inflammatory responses (Klesney-Tait et al., 2006; Taylor et al., 2005). Many of these receptors belong to a family of pattern recognition receptors, which include toll-like receptors (TLRs), and a protein family referred to as triggering receptor expressed on myeloid cells (TREM) (Taylor et al., 2005). At least three members of the TREM family have been identified including TREM-1, TREM-2 and TREM-3, which play distinct roles in regulating inflammatory responses (Klesney-Tait et al., 2006). Thus, whereas activation of TREM-1 amplifies inflammation, TREM-2 signaling is anti-inflammatory (Gibot et al., 2007; Hamerman et al., 2006; Klesney-Tait et al., 2006; Turnbull et al., 2006). The role of TREM-3 in innate immunity has not been elucidated.
Lipopolysaccharide (LPS) is a constituent of the cell wall of gram-negative bacteria (Raetz and Whitfield, 2002). It is a complex glycolipid composed of a hydrophilic polysaccharide portion and a toxic hydrophobic domain known as lipid A or endotoxin (ETX). LPS contains the pathogen-associated molecular pattern motif and is a potent initiator of inflammation. LPS exerts its biological effects on responsive cell types by complexing with CD14, the adaptor molecule MD2, and TLR-4 (Fujihara et al., 2003; Raetz and Whitfield, 2002). This activates cell signaling cascades leading to increased nuclear binding of transcription factors such as AP-1 and NF-κB, and the release of cytokines like tumor necrosis factor-alpha (TNFα) and interleukin-1 beta (IL-1β), which are thought to contribute to septic shock and tissue injury (Cohen, 2002; Iredale, 2003; Leist et al., 1995). Studies have shown that blockade of TREM-1 protects mice from LPS-induced septic shock and mortality (Bouchon et al., 2001a; Gibot et al., 2006; Gibot et al., 2004). In contrast, macrophages from TREM-2−/− mice are hypersensitive to LPS (Turnbull et al., 2006). These data, together with findings that LPS regulates TREM-1 and TREM-2 expression in phagocytic cells (Bouchon et al., 2001a; Schmid et al., 2002), support the idea that coordinate activation of TREM proteins is important in the outcome of acute inflammatory responses to LPS.
In the liver, both macrophages and endothelial cells of the hepatic sinusoids play a key role in the clearance of LPS from the body (Knolle and Gerken, 2000; Uhrig et al., 2005). In response to LPS, these cells become activated releasing an array of inflammatory mediators which are important in the pathophysiological responses to this bacterially-derived product (Beutler and Kruys, 1995; Fujihara et al., 2003; Iredale, 2003). Previous studies have shown that the response of hepatic macrophages and endothelial cells to LPS is mediated in large part by TLR-4 (Chen, 2006; Iredale, 2003). The role of TREM proteins in this activity is unknown and this was investigated in the present studies.
Materials and Methods
Repurification of LPS
Escherichia coli LPS (serotype 0128:B12, Sigma) was repurified as previously described (Manthey et al., 1994). Briefly, LPS (2.5 mg) was reconstituted in ETX-free water (500 µl) containing 0.2% triethylamine (Netea et al.). Deoxycholate (0.5%, DOC) was added, followed by water-saturated phenol (500 µl). The sample was then vortexed intermittently for 5 min and allowed to separate at room temperature for 5 min. After an additional 5 min on ice, the sample was centrifuged at 4°C for 2 min at 10,000 × g. The aqueous layer was transferred to a new tube and the phenol phase re-extracted with 500 µl of 0.2% TEA/0.5% DOC. The aqueous phases were pooled and subjected to re-extraction with one ml of water-saturated phenol. The resulting aqueous phase was adjusted to 75% ethanol with 30 mM sodium acetate, allowed to precipitate at −20°C for one hr and then centrifuged for 10 min, 4°C, at 10,000 × g. The precipitated pellets were washed with one ml of cold 100% ethanol to remove remaining phenol. Nitrogen gas was used to evaporate the ethanol. LPS powder was resuspended (5 mg/ml) in 0.2% TEA and diluted to 0.25 mg/ml in PBS for animal treatments.
Animals
Male TLR-4-mutant C3H/HeJ mice, control C3H/OuJ mice and wild-type C57BL/6 mice (8–12 weeks) were obtained from Jackson Laboratory (Bar Harbor, ME). C57BL/6 mice with a targeted disruption of the p55 TNFR1 gene were kindly provided by Immunex (Seattle, WA) and bred in our animal facility. All animals were housed under specific-pathogen-free conditions and allowed free access to sterile water and food. The animals received human care in compliance with the institution’s guidelines, as outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences. To induce acute endotoxemia, mice were administered a single intraperitoneal dose of repurified LPS (3 mg/kg). PBS was used as a control.
Hepatic macrophage and endothelial cell isolation
Mice were euthanized with Nembutal (200 mg/kg). Liver sinusoidal cells were isolated as previously described with some modifications (Ahmad et al., 1999). Briefly, the liver was perfused through the portal vein with Ca2+/Mg2+- free Hanks’ balanced salt solution (HBSS, pH 6 7.3) containing 0.5 mM EGTA and 25 mM HEPES, followed by Leibovitz L-15 medium containing 100 U/ml collagenase type IV for 2 min. All buffers were maintained at 37°C during the perfusion. The liver was then extracted, disaggregated, and the resulting cell suspension filtered through 220 µm nylon mesh. Hepatocytes were separated from the nonparenchymal cells by three successive washes (50 × g) for 5 min. Nonparenchymal cells were recovered by centrifugation of the supernatant at 300 × g for 7 min. Macrophages and endothelial cells were then purified according to their size and density on a Beckman J-6 elutriator (Beckman Instruments Inc., Fullerton, CA) equipped with a centrifugal elutriation rotor set at 2500 rpm. The pump speed was set at 12 ml/min to load the cells. Endothelial cells were collected at 17 ml/min and macrophages at 33 ml/min. The purity for macrophages and endothelial cells was greater than 85% as determined by differential staining and by transmission electron microscopy.
cDNA Synthesis and Polymerase Chain Reactions
Cells were stored in RNA LATER solution (Ambion) at −20°C until RNA isolation. DNase I-treated total RNA was extracted using RNeasy Miniprep kit (Qiagen Inc, Valencia, CA) following the manufacturer’s instructions. RNA was quantified using a Nanodrop ND-1000 (Nanodrop Technologies, Wilmington, DE). For cDNA synthesis, RNA (0.2 µg) in 9 µl RNase free water was denatured at 65°C for 4 min, rapidly cooled on ice, and then resuspended in a 20 µl final volume containing 50 mM Tris-HCl pH 8.3, 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 1 mM of each dNTP, 20 mM random hexamers and 200 U Superscript II RNase H− RT (Invitrogen, Carlsbad, CA). After 1 hr incubation at 37°C, RNase H (2 U) was added and the samples incubated for an additional 20 min. The samples were then denatured at 95°C for 4 min and chilled on ice.
Real time quantitative PCR was performed using the ABI Prism 7000 Sequence Detection System. Each reaction contained 0.01 µg cDNA template, 1X primer set consisting of two unlabeled PCR primers and a FAM dye-labeled TaqMan probe, and TaqMan universal PCR master mix in a 20 µl final volume. All reagents including TREM-1, TREM-2, TREM-3 and 18S rRNA primer sets were obtained from Applied Biosystems (Foster City, CA). The thermal cycling parameters were set for the following conditions: one 2-min cycle at 50°C, one 10-min cycle at 95°C (for AmpliTaq gold enzyme activation), and 40 cycles of 95°C for 15 s and 60°C for 1 min. Normalization for the relative quantity of mRNA was accomplished by comparison to 18S rRNA. Increases in mRNA level are presented as fold induction relative to untreated controls, which were arbitrarily assigned a value of one.
For semi-quantitative RT-PCR, each reaction contained 0.01 µg cDNA template, 0.8 µM primer pair, 1X PCR buffer w/ 15 mM MgCl2, 0.1 mM dNTP and 0.5 U Taq polymerase in 20 µl reaction. Amplification was initiated by 15 sec denaturation at 95°C, followed by 21 cycles of 15 sec at 95°C, 25 sec at 56°C and 90 sec at 76°C. TNF-α, IL-1β and 18S primer sets were purchased from Ambions. PCR products were run on agarose gels and visualized by ethidium bromide staining.
Western Blotting
Cells were lysed in buffer containing 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS, 1% protease inhibitor and 1% phosphatase inhibitors in PBS. Lysates were clarified by centrifugation at 16,000 g for 15 min at 4°C. Protein concentrations were measured using a BCA protein assay kit (Pierce Chemical Co., Rockford, IL). Samples were fractioned on a 10% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ). Nonspecific binding was blocked using 5% milk in Tris-buffered saline (TBS) (w/ 0.1% Tween 20). Membranes were incubated overnight (4°C) with primary antibody in 1% milk-TBS; washed for 1 hr using TBS (w/ 0.1% Tween 20), and then incubated for 1 hr with secondary antibody (1:5000) in 2.5% milk-TBS (w/ 0.1% Tween 20). Antibody binding was visualized by autoradiography using enhanced chemiluminescence ECL detection reagents (Amersham Life Science, Arlington Heights, IL). Polyclonal rabbit anti-mouse p44/42 and p38 MAP kinases were obtained from Upstate Cell Signaling (Charlottesville, VA) and horse-radish peroxidase (HRP)-conjugated anti-rabbit antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The dilutions of primary antibodies were as follows: p38 and p44/42 MAP kinases (1:1300); and phospho-p38 and phospho-p44/42 MAP kinases (1:1000). Ten micrograms of protein were loaded onto each lane of the gel.
Statistics
All experiments were repeated two to four times using four mice per treatment group. Data were analyzed by one-way analysis of variance using SigmaStat 3.5. A P value of ≤ 0.05 was considered statistically significant.
Results
Effects of acute endotoxemia on expression of TREM; role of TLR-4
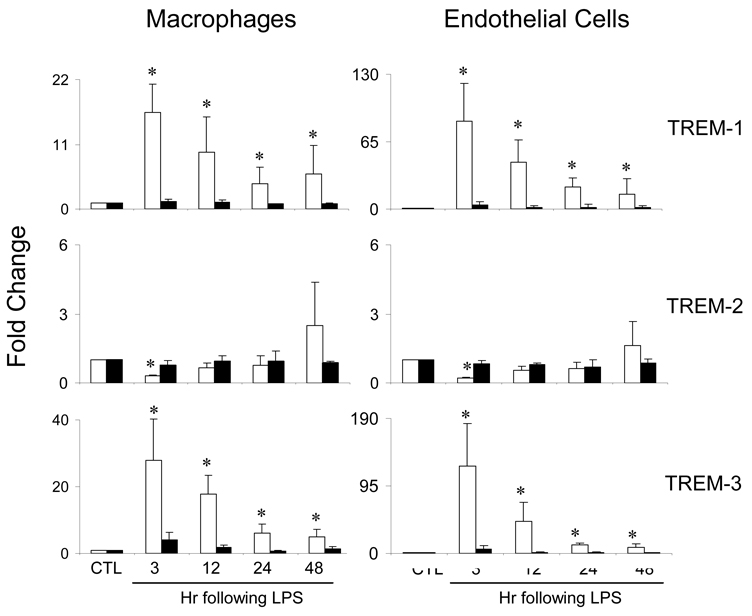
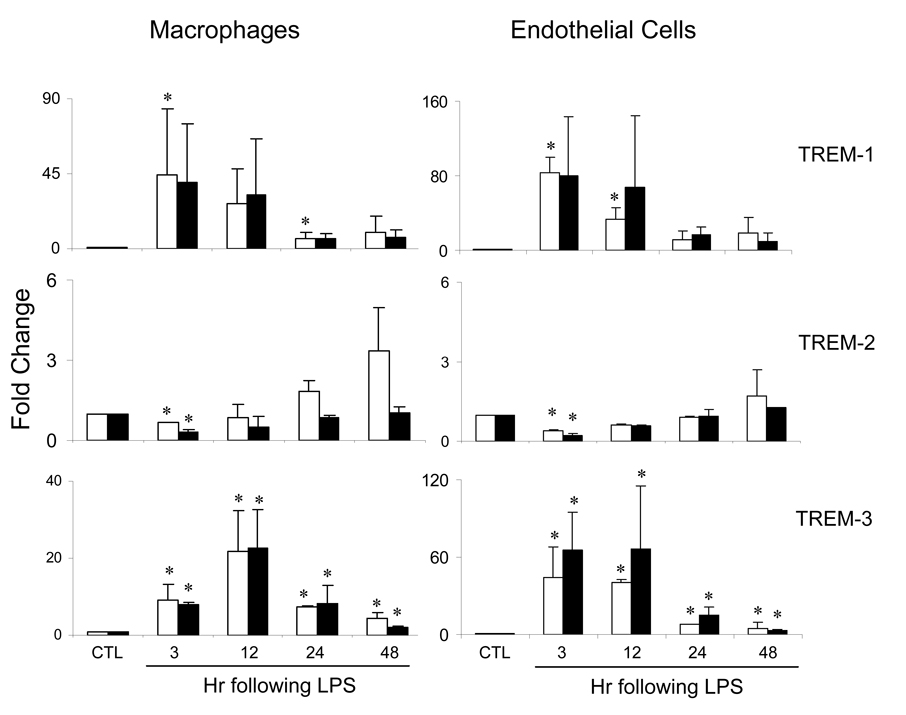
In initial studies, we compared expression of TREM mRNA in hepatic sinusoidal cells from control C3H/OuJ and TLR-4 mutant C3H/HeJ mice and the effects of acute endotoxemia on this activity. Macrophages and endothelial cells from livers of both C3H/OuJ and C3H/HeJ mice were found to constitutively express TREM-1, TREM-2 and TREM-3 mRNA (Table 1). In cells from C3H/OuJ mice, induction of acute endotoxemia was associated with a marked increase in TREM-1 and TREM-3 expression, which was evident within 3 hr, and a decrease in TREM-2 expression (Figure 1). In both cell types, expression of all three genes subsequently decreased. In contrast to C3H/OuJ mice, acute endotoxemia had no major effects on expression of TREM mRNA in cells from TLR-4 mutant C3H/HeJ mice (Figure 1).
Table 1.
Constitutive TREM mRNA expression in macrophages and endothelial cells. Cells were isolated from livers of C3H/OuJ or C3H/HeJ mice.TREM mRNA was quantified by real time PCR.TREM expression levels were normalized to 18S gene expression. Data are mean ± SD (n=8–12) from 2–3 separate experiments.
| OuJ | HeJ | |
|---|---|---|
| Macrophages | ||
| TREM-1 | 1.2±0.1 | 8.6±4.4 |
| TREM-2 | 105.6±2.3 | 126.7±3.1 |
| TREM-3 | 11.9±0.4 | 23.8±1.9 |
| Endothelial Cells | ||
| TREM-1 | 3.6±0.1 | 20.3±6.3 |
| TREM-2 | 21.1±5.1 | 31.7±1.4 |
| TREM-3 | 10.7±0.3 | 29.7±2.5 |
Fig. 1.
Effects of acute endotoxemia on TREM mRNA expression. Macrophages and endothelial cells were isolated from livers of control C3H/OuJ (white bars) or TLR-4 mutant C3H/HeJ (black bars) mice 3–48 hr after administration of LPS or control (CTL). TREM mRNA was quantified by real time PCR. Data are presented as fold change relative to CTL. Each bar is the mean ± SD (n=12) from 3–4 separate experiments. *Significantly different (p<0.05) from CTL.
Effects of acute endotoxemia on IL-1β and TNFα expression in liver macrophages and endothelial cells
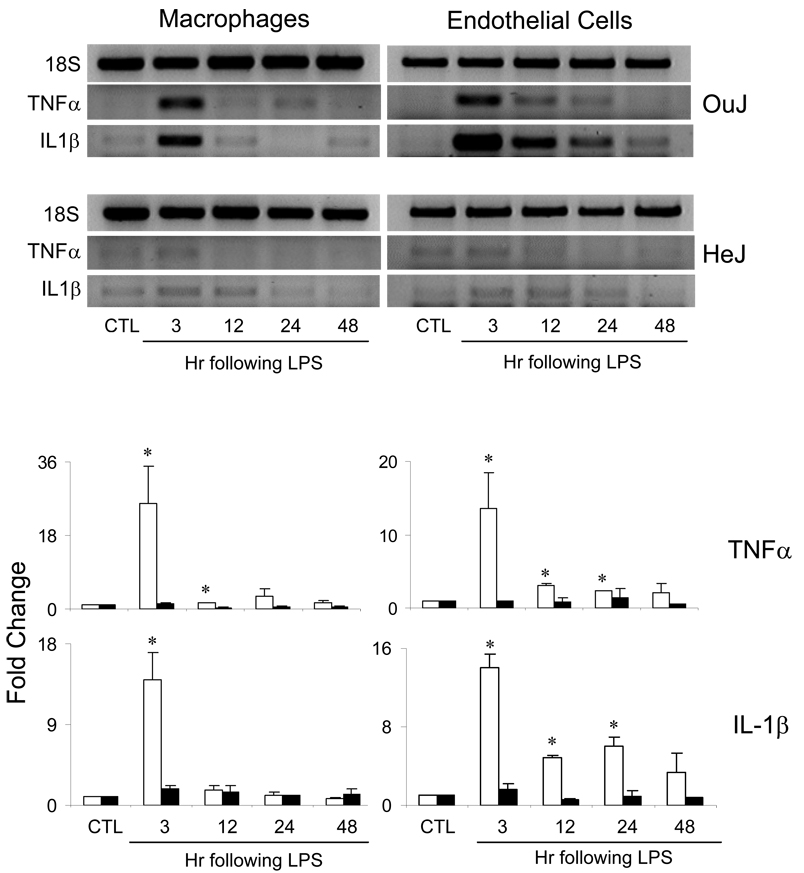
A number of inflammatory mediators important in hepatic responsiveness are generated during acute endotoxemia, including IL-1β and TNFα (Cohen, 2002; Iredale, 2003). We next determined if liver macrophages and endothelial cells were activated after LPS administration to generate these proinflammatory cytokines. Low levels of IL-1β and TNFα mRNA were detectable in cells from untreated mice. Induction of acute endotoxemia caused a marked increase in macrophage and endothelial cell expression of IL-1β and TNFα mRNA, which was evident within 3 hr (Figure 2). Whereas in macrophages, IL-1 β and TNFα expression rapidly returned to control levels, in endothelial cells, expression of these mediators persisted for at least 24 hr although at reduced levels. In C3H/HeJ TLR-4 mutant mice, acute endotoxemia had no major effects on expression of IL-1β or TNFα in either cell type (Figure 2).
Fig. 2.
Effects of acute endotoxemia on IL-1β and TNFα mRNA expression. Macrophages and endothelial cells were isolated from livers of C3H/OuJ (white bars) or C3H/HeJ (black bars) mice 3–48 hr after administration of LPS or control (CTL). Upper panel: Samples were run on agarose gels and visualized by ethidium bromide staining. Lower panel: Samples were quantified by real time PCR. Data are presented as fold change relative to CTL. Each bar is the mean ± SD (n=8–12) from 2–3 separate experiments. *Significantly different (p<0.05) from CTL.
Effects of IL-1β and TNFα on expression of TREM
We next determined if IL-1β or TNFα modulated TREM mRNA expression in hepatic sinusoidal cells. In macrophages and endothelial cells from C3H/OuJ mice treated with control, both TNFα and IL-1β were found to upregulate expression of TREM-1 and TREM-3 (Table 2). In contrast, TREM-2 expression decreased in response to TNFα and IL-1β. In cells from C3H/HeJ TLR-4 mutant mice, IL-1β and TNFα also significantly reduced TREM-2 expression and increased TREM-1 and TREM-3 expression (Table 2). The effects of the cytokines on TREM expression persisted for at least 10 hr. Induction of acute endotoxemia had no significant effect on the response of macrophages and endothelial cells to TNFα or IL-1β in either mouse strain. Interestingly, macrophages from C3H/HeJ mice were, in general, more responsive to IL-1β or TNFα than cells from C3H/OuJ mice.
Table 2.
Effects of IL-1β and TNFα on TREM expression. Macrophages and endothelial cells, isolated from livers of C3H/OuJ or C3H/HeJ mice 24 hr after administration of LPS or control (CTL), were incubated with IL-1β (10 ng/ml),TNFα (10 ng/ml) or medium for 6 and/or 10 hr. TREM mRNA was quantified by real time PCR. Data are presented as fold change relative to cells treated with medium alone which was normalized to one. Each value is the mean ± SD (n=8–20) from 3–5 separate experiments.
| OuJ | HeJ | |||||
|---|---|---|---|---|---|---|
| CTL | LPS | CTL | LPS | |||
| 6 hr | 10 hr | 10 hr | 6 hr | 10 hr | 10 hr | |
| Macrophages | ||||||
| TREM-1 | ||||||
| TNFα | 3.4±0.2* | 2.1±0.1* | 2.3±0.1* | 9.9±1.0* | 4.3±0.5* | 6.5±1.0* |
| IL-1β | 1.8±0.2 | 1.8±0.6 | 1.6±0.3 | 5.7±0.4* | 3.9±0.2* | 6.5±2.0* |
| TREM-2 | ||||||
| TNFα | 0.6±0.1 | 0.4±0.1* | 0.5±0.1* | 0.2±0.1* | 0.2±0.1* | 0.2±0.1* |
| IL-1β | 0.7±0.1* | 0.6±0.1* | 0.8±0.1 | 0.3±0.1* | 0.5±0.1* | 0.5±0.1* |
| TREM-3 | ||||||
| TNFα | 3.2±0.1* | 1.8±0.2* | 1.8±0.1* | 5.3±1.0* | 5.0±0.8* | 5.0±0.9* |
| IL-1β | 2.7±0.1* | 1.6±0.4 | 1.2±0.2 | 1.9±0.2 | 1.6±0.1* | 1.5±0.2* |
| Endothelial Cells | ||||||
| TREM-1 | ||||||
| TNFα | 1.5±0.1* | 2.5±0.6* | 1.9±0.2 | 4.0±0.3* | 2.4±0.3* | 2.4±0.3* |
| IL-1β | 2.0±0.4 | 2.0±0.5 | 2.2±0.4* | 4.5±0.1* | 1.7±0.2 | 1.7±0.3 |
| TREM-2 | ||||||
| TNFα | 0.3±0.1* | 0.5±0.1* | 0.4±0.1* | 0.3±0.1* | 0.4±0.1* | 0.6±0.1* |
| IL-1β | 0.3±0.1* | 0.6±0.1* | 0.5±0.1* | 0.5±0.1* | 0.5±0.1* | 0.6±0.1* |
| TREM-3 | ||||||
| TNFα | 3.3±0.4* | 1.6±0.3 | 1.6±0.3 | 1.8±0.1* | 1.8±0.1* | 1.7±0.2* |
| IL-1β | 1.7±0.1* | 1.6±0.2 | 1.5±0.5 | 1.5±0.1 | 1.4±0.1* | 1.0±0.1 |
Significantly different (p<0.05) from cells treated with medium.
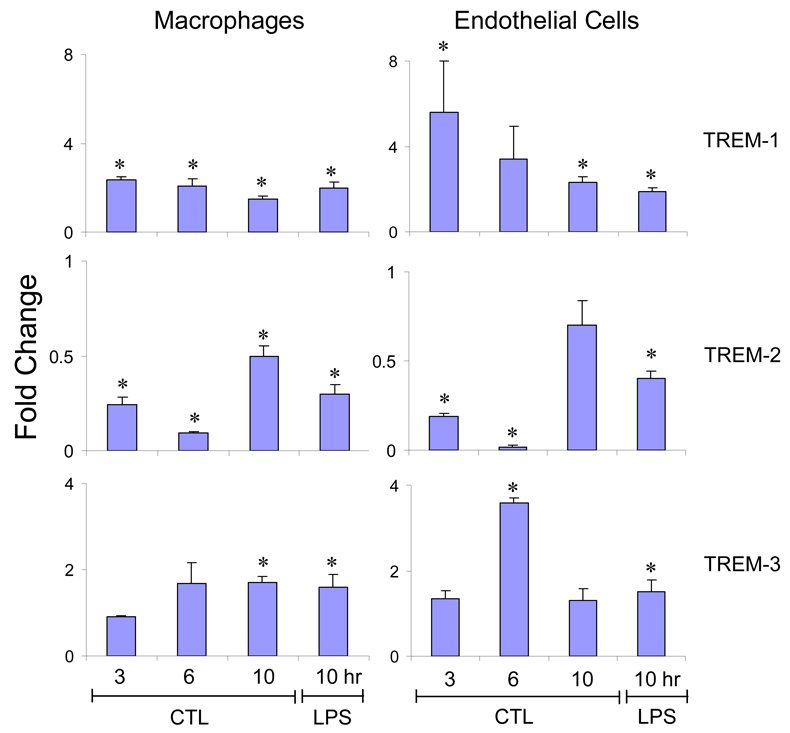
For comparison, we also analyzed the effects of LPS treatment of the cells on TREM expression in C3H/OuJ mice. Incubation of both macrophages and endothelial cells with LPS (100 ng/ml) caused a significant increase in TREM-1 and TREM-3 expression (Figure 3). While an increase in TREM-1 expression was evident within 3 hr, increases in TREM-3 were delayed for 6–10 hr. In contrast, a decrease in TREM-2 expression was noted in both cell types, which was maximal at 6 hr. Endothelial cells were more sensitive to the effects of LPS than macrophages. Similar results were observed with 10 and 500 ng/ml of LPS (data not shown). Induction of acute endotoxemia did not alter the responsiveness of the cells to LPS in culture.
Fig. 3.
Effects of LPS on TREM expression. Macrophages and endothelial cells, isolated from livers of C3H/OuJ mice 24 hr after administration of LPS or CTL were incubated with LPS (100 ng/ml) or medium for 3, 6 and/or 10 hr. TREM mRNA was quantified by real-time PCR. Data are presented as fold change relative to cells treated with medium which was normalized to one. Each bar is the mean ± SD (n=8–12) from 2–3 separate experiments. *Significantly different (p<0.05) from cells treated with medium.
Role of the MAP kinase and PI3-kinase in cytokine-induced alterations in TREM expression
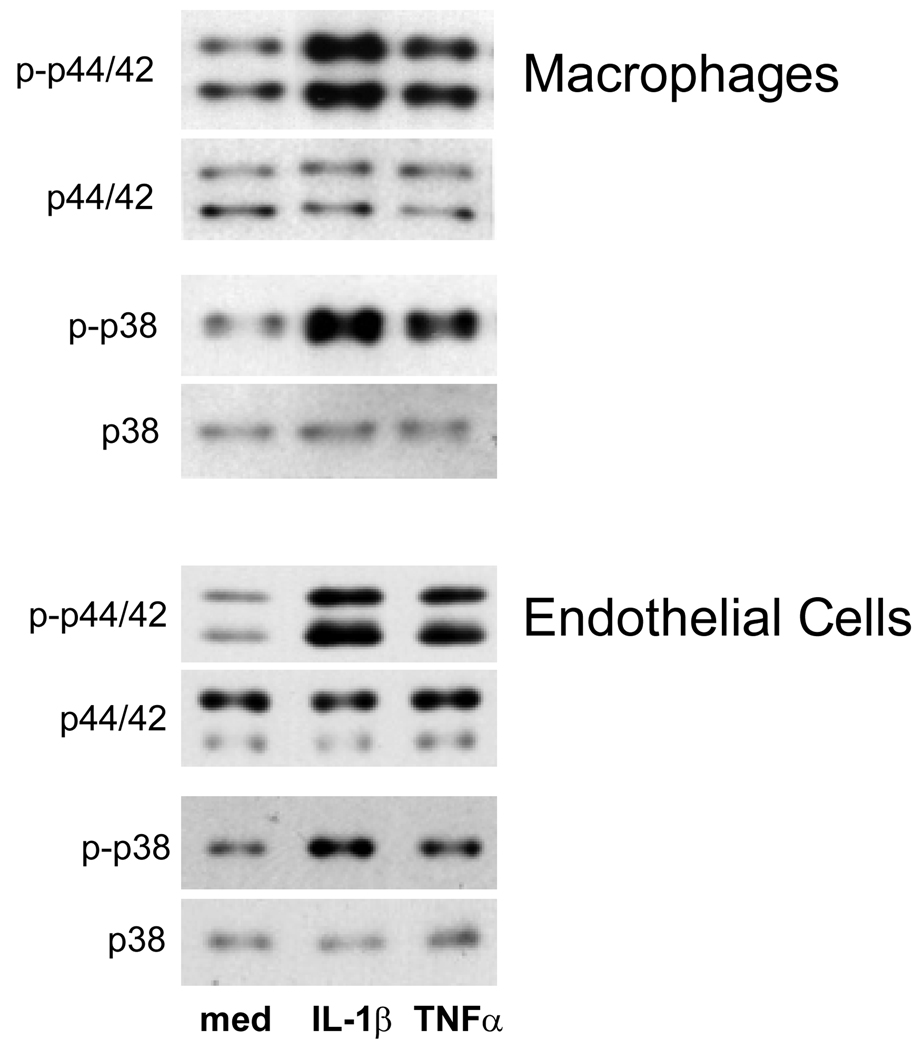
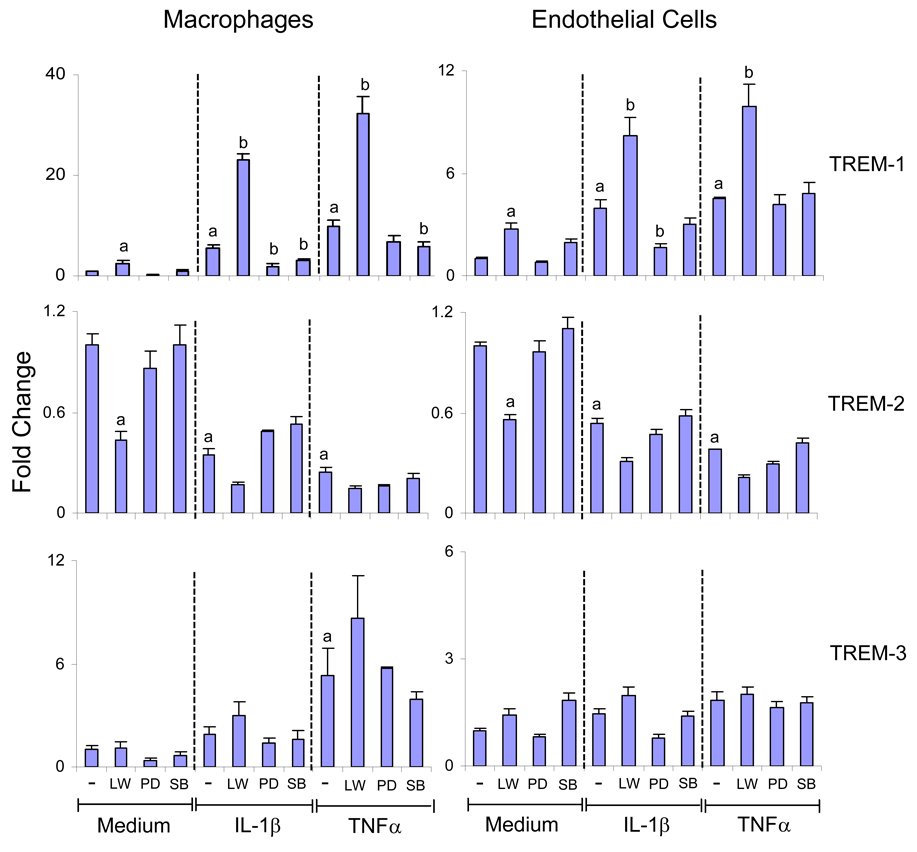
IL-1β and TNFα exert their biological effects, in part, by activation of p44/42 and p38 MAP kinase (Chen et al., 2001; Tian et al., 2000). To assess the role of these signaling molecules in cytokine-induced TREM expression, we used specific kinase inhibitors. In these studies, macrophages from TLR-4 mutant C3H/HeJ mice were analyzed since these cells were more responsive to IL-1β and TNFα than cells from control C3H/OuJ mice (Table 2). For comparison, endothelial cells from C3H/HeJ mice were also studied. On western blots, macrophages and endothelial cells were found to constitutively express both total and phosphorylated p44/42 and p38 MAP kinases (Figure 4). Incubation of the cells with IL-1β or TNFα resulted in increased expression of activated or phosphorylated p44/42 and p38 MAP kinases in both cell types. In contrast, total MAP kinase proteins remained unchanged. PD98059, an inhibitor of p44/42 MAP kinase, significantly reduced IL-1β but not TNFα-induced TREM-1 expression in macrophages and endothelial cells (Figure 5). Whereas SB202190, an inhibitor of p38 MAP kinase, suppressed IL-1β or TNFα- induced increases in TREM-1 in macrophages, no effects were observed in endothelial cells. Neither PD98059 nor SB202190 had any major effects on cytokine-mediated decreases in TREM-2 in either macrophages or endothelial cells. IL-1β and TNFα are also known to activate PI3-kinase (Kristof et al., 2003), and the role of this enzyme in regulating TREM expression was analyzed next. LY294002 and wortmannin, selective inhibitors of PI3-kinase (Walker et al., 2000), were found to augment constitutive and IL-1β or TNFα-induced TREM-1 expression in macrophages and endothelial cells (Figure 5). These inhibitors were also found to inhibit constitutive expression of TREM-2 in both cell types without significantly altering the effects of IL-1β or TNFα on TREM-2. In contrast, PI3-kinase and MAP kinase inhibitors had no effects on cytokine-induced alterations in TREM-3 expression in the cells.
Fig. 4.
Effects of IL-1β and TNFα on MAP kinase expression. Macrophages and endothelial cells isolated from livers of C3H/HeJ mice were incubated with IL-1 β (10 ng/ml), TNFα (10 30 ng/ml) or medium for 20 min. Protein lysates were analyzed by western blotting for expression of total and phosphorylated p38 and p44/42 MAP kinases. One representative of two separate experiments is shown.
Fig. 5.
Regulation of IL-1β and TNFα induced TREM expression by PI3K and MAP kinases. Macrophages and endothelial cells were isolated from C3H/HeJ mice. After overnight incubation, the cells were incubated with LY294002 (5 µM) and wortmannin (1 µM) (LW), PD98059 (PD, 10 µM), SB202190 (SB, 2.5 µM) or medium for 20 min and then with IL-1β (10 ng/ml), TNFα (10 ng/ml) or medium. RNA was isolated 6 hr later and TREM mRNA quantified by real time PCR. Data are presented as fold change relative to cells treated with medium alone. Each bar is the mean ± SD (n=8) from 2 separate experiments. aSignificantly different (p<0.05) from cells treated with medium; bSignificantly different (p<0.05) from cells treated with IL-1β or TNFα alone.
Role of TNFR1 in TREM expression
In further studies, we analyzed the role of TNFα signaling via TNFR1 in regulating TREM expression using TNFR1−/− mice. As observed in C3H/OuJ mice, macrophages and endothelial cells from wild-type mice were found to constitutively express TREM-1, TREM-2 and TREM-3 mRNA. In cells from both wild type and TNFR1−/− mice induction of acute endotoxemia was associated with a marked increase in TREM-1 and TREM-3 expression, which was evident within 3 hr and persisted for 12 hr (Figure 6). In contrast, TREM-2 expression decreased following LPS administration in both cell types. No significant differences in TREM expression were observed between wild type and TNFR1−/− mice (Figure 6).
Fig. 6.
Effects of loss of TNFR1 on TREM expression. Macrophages and endothelial cells were isolated from livers of wild-type (white bars) or TNFR1−/− (black bars) mice 3–48 hr after administration of LPS or control (CTL). TREM mRNA was quantified by real time PCR. Data are presented as fold change relative to CTL. Each bar is the mean ± SD (n=12) from 3 separate experiments. *Significantly different (p<0.05) from CTL.
Role of TREM-1 in LPS-induced activation of macrophages and endothelial cells
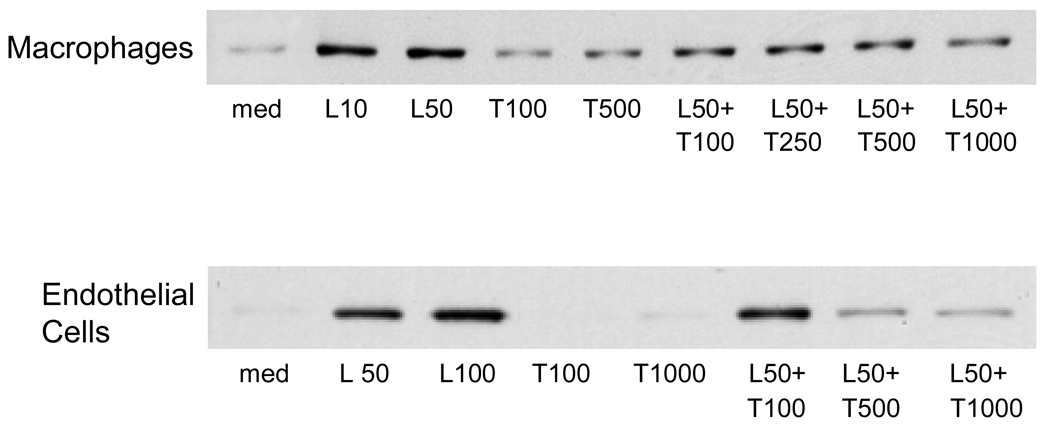
To assess the function of TREM-1 in LPS-induced responses in hepatic macrophages and endothelial cells, we used a TREM-1/Fc fusion protein, which blocks activation of TREM-1 (Bouchon et al., 2001a). LPS was found to upregulate NOS-2 protein in both macrophages and endothelial cells from C3H/OuJ mice (Figure 7). Preincubation of the cells with TREM-1/Fc fusion protein blocked the effects of LPS on NOS-2 expression. Treatment of the cells with TREM-1/Fc fusion protein alone had no effect on expression of NOS-2.
Fig. 7.
Regulation of NOS-2 expression by TREM-1. Macrophages and endothelial cells, isolated from livers of control C3H/OuJ mice, were preincubated with TREM-1/Fc (T, ng/ml) fusion protein for 1 hr and then with LPS (L, ng/ml) or medium for 24 hr. Protein lysates were then analyzed by western blotting for expression of NOS-2. One representative of three separate experiments is shown.
Discussion
TREM proteins regulate innate immunity and inflammatory responses to microbial products, including ETX (Klesney-Tait et al., 2006). Whereas inflammatory responses are in general increased following TREM-1 activation, they are attenuated by TREM-2 (Bleharski et al., 2003; Bouchon et al., 2001a; Gibot et al., 2006; Gibot et al., 2007; Hamerman et al., 2006; Klesney-Tait et al., 2006; Takahashi et al., 2005; Turnbull et al., 2006). Hepatic macrophages and endothelial cells play an important role in ETX-induced inflammatory responses in the liver (Fujihara et al., 2003; Knolle and Gerken, 2000). The present studies were designed to assess expression of TREM family members in these cells during acute endotoxemia and the role of the TLR-4 in this response.
Acute endotoxemia resulted in a rapid increase in expression of TREM-1 mRNA in macrophages and endothelial cells from C3H/OuJ mice, which was maximal within 3 hr. A similar rapid induction of TREM-1 has been observed in cultured mononuclear phagocytes stimulated with LPS, and in mouse peritoneal macrophages after sepsis induced by cecal ligation and puncture (Gibot et al., 2004; Gibot et al., 2005; Knapp et al., 2004; Murakami et al., 2006). Activation of TREM-1 in mononuclear phagocytes has been reported to lead to the generation of chemotactic proteins including monocyte chemotactic protein-1 and -3, macrophage inflammatory protein-1α and interleukin-8 (IL-8), as well as IL-1 and TNFα, and in neutrophils to induce phagocytosis, and production of reactive oxygen species, myeloperoxidase and IL-8 (Bleharski et al., 2003; Bouchon et al., 2000; Fortin et al., 2006; Murakami et al., 2006; Tessarz et al., 2007). Upregulation of TREM-1 in liver sinusoidal cells may be important in the recruitment of macrophages and neutrophils into liver during acute endotoxemia and their subsequent activation. This is supported by findings that costimulation of phagocytic cells with LPS and a TREM-1 agonist synergistically enhances inflammatory responses (Bleharski et al., 2003; Bouchon et al., 2001a; Fortin et al., 2006), while blockade of TREM-1 protects mice from LPS-induced septic shock and mortality (Bouchon et al., 2001a; Gibot et al., 2006; Klesney-Tait et al., 2006). In contrast to our findings, Wong-Baeza et al. (Wong-Baeza et al., 2006), reported that LPS-induced TREM-1 expression is regulated post-transcriptionally in human monocytes. Thus, while LPS upregulated TREM-1 protein, it had no effects on TREM-1 mRNA levels. Differences between these results and ours may reflect the unique response of mouse and human mononuclear phagocytes to LPS. We also found that TREM-3 mRNA was upregulated in liver macrophages and endothelial cells in response to acute endotoxemia. The role of TREM-3 in hepatic inflammatory responses is unknown. The fact that TREM-3 is induced by LPS in RAW264.7 macrophages and MT2 T cells, and is located in the TREM gene cluster on mouse chromosome 17 indicates that it functions as an activating receptor and may also participate in innate immunity (Chung et al., 2002; Klesney-Tait et al., 2006).
Hepatic macrophages and endothelial cells were found to constitutively express inhibitory TREM-2 mRNA. This may be important in limiting the response of these cells to endogenous LPS. In contrast to TREM-1 and TREM-3, TREM-2 expression rapidly (within 3 hr) declined in macrophages and endothelial cells after LPS administration to C3H/OuJ mice. Similar decreases in TREM-2 have been described in isolated monocytes and microglial cells treated with LPS and/or IFNγ (Bouchon et al., 2001b; Schmid et al., 2002). Using TREM-2−/− mice, Turnbull et al. (Turnbull et al., 2006) recently demonstrated that TREM-2 functions to inhibit macrophage activation. Downregulation of TREM-2 in hepatic macrophages and endothelial cells may contribute to increased proinflammatory mediator production in the liver during acute endotoxemia. It has been reported that knockdown of TREM-2 increases TNFα and NOS-2 expression and abrogates microglial cell phagocytosis (Takahashi et al., 2005). It is possible that TREM-2 also regulates production of inflammatory mediators and phagocytosis in resident liver macrophages and endothelial cells, and this remains to be determined.
Recent studies suggest that TREM proteins synergize with TLR-4 and other pathogen recognition receptors (Klesney-Tait et al., 2006; Netea et al., 2006). In further studies, we investigated the role of TLR-4 in regulating TREM expression in liver cells during acute endotoxemia. In contrast to cells from C3H/OuJ mice, LPS administration had negligible effects on expression of TREM mRNA in macrophages and endothelial cells from C3H/HeJ TLR-4 mutant mice. These findings are novel and indicate that LPS-induced alterations in TREM-1 are dependent on TLR-4 (Knapp et al., 2004; Wong-Baeza et al., 2006). Interestingly, constitutive expression of TREM-1, TREM-2 and TREM-3 was noted in macrophages and endothelial cells from C3H/HeJ mice, suggesting that alternative signaling pathways control TREM expression during homeostasis. Constitutive TREM expression in these mice may reflect compensatory bacterial recognition responses in the absence of TLR-4 signaling.
LPS administration to animals has previously been reported to upregulate IL-1β and TNFα expression in rat liver macrophages (Fukui, 2005; Luster et al., 1999). Similarly, the present studies show that expression of IL-1β and TNFα increases rapidly (within 3 hr) in hepatic macrophages, as well as endothelial cells, after LPS treatment of C3H/OuJ mice. This induction was not observed in C3H/HeJ mice, providing further evidence that hepatic nonparenchymal responses to LPS are dependent on TLR-4 (Chen, 2006; Ozato et al., 2002; Scott et al., 2005). In cultured macrophages and endothelial cells from both C3H/OuJ and C3H/HeJ mice, IL-1β and TNFα̣ induced TREM-1 and TREM-3 expression, but suppressed TREM-2 expression. Whereas similar inhibitory effects of TNFα on TREM-2 have been described in monocyte-derived dendritic cells (Bouchon et al., 2001a), published reports on the effects of TNFα on TREM-1 expression are conflicting. Thus, while some studies have shown that TNFα upregulates TREM-1 expression in mononuclear phagocytes, others report no effect (Bleharski et al., 2003; Knapp et al., 2004; Murakami et al., 2006; Schenk et al., 2005). Differences in these results and our studies may be due to distinct origin of the macrophages examined and/or the culture conditions. Interestingly, IL-1β and TNFα were found to be more effective in inducing macrophage TREM-1 and TREM-3 expression in C3H/HeJ mice when compared to C3H/OuJ mice. Increases in the sensitivity of the mutant mice to cytokines may be due to a loss of LPS-induced inhibitory proteins, such as suppressor of cytokine signaling (SOCS) or Janus Kinase (JAK)-signal transducer and activator of transcription (STAT) activation (Baetz et al., 2004; Shuai and Liu, 2003). The cytoplasmic domains of TLR and IL-1R have been shown to exhibit homology (Aderem and Ulevitch, 2000; Qureshi et al., 1999). Several intracellular pathways induced by LPS, IL-1 and TNFα overlap, including serine-threonine innate immunity kinase (IRAK), TNFR-associated factor 6 (TRAF6), MAP kinases and NF-κB (Huang et al., 2003; Luster et al., 1999; Martin and Wesche, 2002; Medzhitov, 2001; Qureshi et al., 1999). Compensatory increases in these pathways may also account for increased sensitivity of C3H/HeJ mice to IL-1β and TNFα (Adi et al., 1992; Johnson et al., 1997).
PI3-kinase and MAP kinase signaling have been implicated in the regulation of inflammatory responses initiated by LPS (Morrison and Davis, 2003; Ono and Han, 2000; Weinstein et al., 2000). Inhibition of PI3-kinase has previously been shown to block LPS-induced TREM-1 expression in monocytes (Knapp et al., 2004). In contrast, the present studies show that blockade of PI3-kinase with LY294002 and wortmannin augmented both constitutive and IL-1β or TNFα induced increases in TREM-1 mRNA in hepatic macrophages and endothelial cells indicating that PI3-kinase is a negative regulator of TREM-1 expression. Differences between these findings may be due to the specific inflammatory stimuli used and/or distinct responses of human monocytes and mouse liver nonparenchymal cells. In contrast to TREM-1, PI3-kinase inhibition reduced constitutive TREM-2 expression, but had no significant effects on cytokine mediated decreases in TREM-2. These findings suggest that only constitutive expression of TREM-2 requires PI3-kinase activity. In contrast to PI3-kinase inhibitors, MAP kinase inhibitors suppressed cytokine-induced alterations in TREM-1. However, their effects depended on the cytokine and the cell type. Thus, in macrophages while both p44/42 and p38 MAP kinases are important in IL1-β-induced TREM-1 expression, only p38 MAP kinase is involved in TNFα-induced TREM-1 expression. In contrast, in endothelial cells, only p44/42 MAP kinase mediates the effects of IL-1β on TREM-1 expression. These findings are in contrast to a recent report by Knapp et al. (Knapp et al., 2004) who showed no significant effects of inhibiting MAP kinases on LPS-induced TREM-1 expression in monocytes. This may reflect differences in signaling pathways mediated by LPS and various cytokines in the mouse and human cells. Of note, MAP kinases do not appear to mediate the effects of cytokines on TREM-2 or TREM-3 expression in macrophages or endothelial cells. Similarly, PI3-kinase activity does not appear to play a role in expression of TREM-3 in either cell type. Further studies are ongoing to elucidate the pathways controlling expression of these TREM family members in liver macrophages and endothelial cells.
TNFα-induced inflammatory responses are mediated in large part, by signaling via TNFR1 (Luster et al., 1999; Peschon et al., 1998). Recent studies have shown TNFR1−/− mice are protected from LPS-induced liver injury (Shimizu et al., 2005). We found that there were no significant differences between wild type and TNFR1−/− mice with respect to TREM expression during acute endotoxemia. This suggests that TNFR1does not play a major role in vivo in regulating TREM mRNA expression in either macrophages or endothelial cells. It is possible that induction of TREM by TNFα is due to activation of TNFR2, and this remains to be determined.
Activation of TREM-1 is associated with release of proinflammatory mediators and reactive oxygen and nitrogen intermediates by phagocytic leukocytes (Bouchon et al., 2000; Gibot et al., 2007; Radsak et al., 2004). These activities have been linked to hepatotoxicity (Jaeschke and Hasegawa, 2006; Laskin and Laskin, 2001; Laskin and Pendino, 1995). We found that blockade of TREM-1 activation prevented LPS-induced NOS-2 expression in both macrophages and endothelial cells. This suggests that TREM-1 contributes to cellular inflammatory responses to LPS in part, by upregulating NOS-2. Our findings are in accord with previous studies demonstrating that blockade of TREM-1 attenuates LPS-induced nitric oxide accumulation in the plasma of rats and provide additional support for the idea that TREM-1 activation contributes to oxidative stress during LPS-induced inflammatory responses (Gibot et al., 2006; Radsak et al., 2004).
In summary, the present studies demonstrate that liver sinusoidal macrophages and endothelial cells express TREM-1, TREM-2 and TREM-3 mRNA, and that this activity is altered during acute endotoxemia. Our findings that TREM genes are expressed in endothelial cells are novel and suggest that TREM expression is not limited to myeloid cells. We have previously shown that hepatic endothelial cells express low levels of the macrophage antigens, CD68 and F4/80 (Chen, 2006). The findings that TREM is expressed by hepatic endothelial cells provides further evidence that these cells share functional and antigenic properties with resident liver macrophages (Knolle et al., 1999; McCloskey et al., 1992). TREM proteins contribute to both pro- and anti-inflammatory responses to microbial products (Hamerman et al., 2006; Klesney Tait et al., 2006; Turnbull et al., 2006). Coordinate regulation of TREM activation may be important in determining the outcome of acute endotoxemia. Thus, elucidating mechanisms regulating TREM expression may allow development of drug targets for limiting LPS-induced inflammation and injury.
Acknowledgements
This work was supported by NIH Grants GM034310, ES004738, AR055073, ES005022 and CA100994.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Adi S, Pollock AS, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Role for monokines in the metabolic effects of endotoxin. Interferon-gamma restores responsiveness of C3H/HeJ mice in vivo. J. Clin. Invest. 1992;89:1603–1609. doi: 10.1172/JCI115755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Gardner CR, Yurkow EJ, Laskin DL. Inhibition of macrophages with gadolinium chloride alters intercellular adhesion molecule-1 expression in the liver during acute endotoxemia in rats. Hepatology. 1999;29:728–736. doi: 10.1002/hep.510290324. [DOI] [PubMed] [Google Scholar]
- Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J. Biol. Chem. 2004;279:54708–54715. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- Beutler B, Kruys V. Lipopolysaccharide signal transduction, regulation of tumor necrosis factor biosynthesis, and signaling by tumor necrosis factor itself. J. Cardiovasc. Pharmacol. 1995;25 Suppl 2:S1–S8. doi: 10.1097/00005344-199500252-00002. [DOI] [PubMed] [Google Scholar]
- Bleharski JR, Kiessler V, Buonsanti C, Sieling PA, Stenger S, Colonna M, Modlin RL. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 2003;170:3812–3818. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001a;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- Bouchon A, Hernandez-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J. Exp. Med. 2001b;194:1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LC, Laskin JD, Laskin DL. Regulation of inflammatory mediator production in the liver during acute endotoxemia. The Toxicologist. 2006;90(1):349. [Google Scholar]
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem. Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- Chung DH, Seaman WE, Daws MR. Characterization of TREM-3, an activating receptor on mouse macrophages: definition of a family of single Ig domain receptors on mouse chromosome 17. Eur. J. Immunol. 2002;32:59–66. doi: 10.1002/1521-4141(200201)32:1<59::AID-IMMU59>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- Fortin CF, Lesur O, Fulop T., Jr Effects of TREM-1 activation in human neutrophils: activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int. Immunol. 2006;19:41–50. doi: 10.1093/intimm/dxl119. [DOI] [PubMed] [Google Scholar]
- Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H, Ikeda H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol. Ther. 2003;100:171–194. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Fukui H. Relation of endotoxin, endotoxin binding proteins and macrophages to severe alcoholic liver injury and multiple organ failure. Alcohol Clin. Exp. Res. 2005;29:172S–179S. doi: 10.1097/01.alc.0000189278.30237.e9. [DOI] [PubMed] [Google Scholar]
- Gibot S, Buonsanti C, Massin F, Romano M, Kolopp-Sarda MN, Benigni F, Faure GC, Bene MC, Panina-Bordignon P, Passini N, Levy B. Modulation of the triggering receptor expressed on the myeloid cell type 1 pathway in murine septic shock. Infect. Immun. 2006;74:2823–2830. doi: 10.1128/IAI.74.5.2823-2830.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibot S, Kolopp-Sarda MN, Bene MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J. Exp. Med. 2004;200:1419–1426. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibot S, Massin F, Le Renard P, Bene MC, Faure GC, Bollaert PE, Levy B. Surface and soluble triggering receptor expressed on myeloid cells-1: expression patterns in murine sepsis. Crit. Care. Med. 2005;33:1787–1793. doi: 10.1097/01.ccm.0000172614.36571.75. [DOI] [PubMed] [Google Scholar]
- Gibot S, Massin F, Marcou M, Taylor V, Stidwill R, Wilson P, Singer M, Bellingan G. TREM-1 promotes survival during septic shock in mice. Eur. J. Immunol. 2007;37:456–466. doi: 10.1002/eji.200636387. [DOI] [PubMed] [Google Scholar]
- Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J. Immunol. 2006;177:2051–2055. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- Huang LY, Aliberti J, Leifer CA, Segal DM, Sher A, Golenbock DT, Golding B. Heat-killed Brucella abortus induces TNF and IL-12p40 by distinct MyD88-dependent pathways: TNF, unlike IL-12p40 secretion, is Toll-like receptor 2 dependent. J Immunol. 2003;171:1441–1446. doi: 10.4049/jimmunol.171.3.1441. [DOI] [PubMed] [Google Scholar]
- Iredale JP. Regulating hepatic inflammation: pathogen-associated molecular patterns take their toll. Hepatology. 2003;37:979–982. doi: 10.1053/jhep.2003.50224. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Gheusi G, Segreti S, Dantzer R, Kelley KW. C3H/HeJ mice are refractory to lipopolysaccharide in the brain. Brain Res. 1997;752:219–226. doi: 10.1016/s0006-8993(96)01454-0. [DOI] [PubMed] [Google Scholar]
- Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat. Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- Knapp S, Gibot S, de Vos A, Versteeg HH, Colonna M, van der Poll T. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J. Immunol. 2004;173:7131–7134. doi: 10.4049/jimmunol.173.12.7131. [DOI] [PubMed] [Google Scholar]
- Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol. Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- Knolle PA, Germann T, Treichel U, Uhrig A, Schmitt E, Hegenbarth S, Lohse AW, Gerken G. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. J. Immunol. 1999;162:1401–1407. [PubMed] [Google Scholar]
- Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J. Gastroenterol. 2006;12:7413–7420. doi: 10.3748/wjg.v12.i46.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristof AS, Marks-Konczalik J, Billings E, Moss J. Stimulation of signal transducer and activator of transcription-1 (STAT1)-dependent gene transcription by lipopolysaccharide and interferon-gamma is regulated by mammalian target of rapamycin. J. Biol. Chem. 2003;278:33637–33644. doi: 10.1074/jbc.M301053200. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Laskin JD. Role of macrophages and inflammatory mediators in chemically induced toxicity. Toxicology. 2001;160:111–118. doi: 10.1016/s0300-483x(00)00437-6. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Pendino KJ. Macrophages and inflammatory mediators in tissue injury. Annu. Rev. Pharmacol. Toxicol. 1995;35:655–677. doi: 10.1146/annurev.pa.35.040195.003255. [DOI] [PubMed] [Google Scholar]
- Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55 kDa TNF receptor is necessary and sufficient for TNF-induced liver failure, hepatocyte apoptosis, and nitrite release. J. Immunol. 1995;154:1307–1316. [PubMed] [Google Scholar]
- Luster MI, Simeonova PP, Gallucci R, Matheson J. Tumor necrosis factor alpha and toxicology. Crit. Rev. Toxicol. 1999;29:491–511. doi: 10.1080/10408449991349258. [DOI] [PubMed] [Google Scholar]
- Manthey CL, Perera PY, Henricson BE, Hamilton TA, Qureshi N, Vogel SN. Endotoxin-induced early gene expression in C3H/HeJ (Lpsd) macrophages. J. Immunol. 1994;153:2653–2663. [PubMed] [Google Scholar]
- Martin MU, Wesche H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim. Biophys. Acta. 2002;1592:265–280. doi: 10.1016/s0167-4889(02)00320-8. [DOI] [PubMed] [Google Scholar]
- McCloskey TW, Todaro JA, Laskin DL. Lipopolysaccharide treatment of rats alters antigen expression and oxidative metabolism in hepatic macrophages and endothelial cells. Hepatology. 1992;16:191–203. doi: 10.1002/hep.1840160130. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nature Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Akahoshi T, Hayashi I, Endo H, Kawai S, Inoue M, Kondo H, Kitasato H. Induction of triggering receptor expressed on myeloid cells 1 in murine resident peritoneal macrophages by monosodium urate monohydrate crystals. Arthritis Rheum. 2006;54:455–462. doi: 10.1002/art.21633. [DOI] [PubMed] [Google Scholar]
- Netea MG, Azam T, Ferwerda G, Girardin SE, Kim SH, Dinarello CA. Triggering receptor expressed on myeloid cells-1 (TREM-1) amplifies the signals induced by the NACHT-LRR (NLR) pattern recognition receptors. J. Leukoc. Biol. 2006;80:1454–1461. doi: 10.1189/jlb.1205758. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Ozato K, Tsujimura H, Tamura T. Toll-like receptor signaling and regulation of cytokine gene expression in the immune system. Biotechniques. 2002 Suppl:66–68. 70–72 passim. [PubMed] [Google Scholar]
- Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J. Exp. Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radsak MP, Salih HR, Rammensee HG, Schild H. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J. Immunol. 2004;172:4956–4963. doi: 10.4049/jimmunol.172.8.4956. [DOI] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk M, Bouchon A, Birrer S, Colonna M, Mueller C. Macrophages expressing triggering receptor expressed on myeloid cells-1 are underrepresented in the human intestine. J. Immunol. 2005;174:517–524. doi: 10.4049/jimmunol.174.1.517. [DOI] [PubMed] [Google Scholar]
- Schmid CD, Sautkulis LN, Danielson PE, Cooper J, Hasel KW, Hilbush BS, Sutcliffe JG, Carson MJ. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J. Neurochem. 2002;83:1309–1320. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MJ, Liu S, Su GL, Vodovotz Y, Billiar TR. Hepatocytes enhance effects of lipopolysaccharide on liver nonparenchymal cells through close cell interactions. Shock. 2005;23:453–458. doi: 10.1097/01.shk.0000160939.08385.f1. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Yamada Y, Okuno M, Ohnishi H, Osawa Y, Seishima M, Moriwaki H. Liver injury induced by lipopolysaccharide is mediated by TNFR-1 but not by TNFR-2 or Fas in mice. Hepatol. Res. 2005;31:136–142. doi: 10.1016/j.hepres.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nature Rev. Immunol. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- Tessarz AS, Weiler S, Zanzinger K, Angelisova P, Horejsi V, Cerwenka A. Non-T Cell Activation Linker (NTAL) Negatively Regulates TREM-1/DAP12-Induced Inflammatory Cytokine Production in Myeloid Cells. J. Immunol. 2007;178:1991–1999. doi: 10.4049/jimmunol.178.4.1991. [DOI] [PubMed] [Google Scholar]
- Tian W, Zhang Z, Cohen DM. MAPK signaling and the kidney. Am. J. Physiol. Renal Physiol. 2000;279:F593–F604. doi: 10.1152/ajprenal.2000.279.4.F593. [DOI] [PubMed] [Google Scholar]
- Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, Colonna M. Cutting edge: TREM-2 attenuates macrophage activation. J. Immunol. 2006;177:3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- Uhrig A, Banafsche R, Kremer M, Hegenbarth S, Hamann A, Neurath M, Gerken G, Limmer A, Knolle PA. Development and functional consequences of LPS tolerance in sinusoidal endothelial cells of the liver. J. Leukoc. Biol. 2005;77:626–633. doi: 10.1189/jlb.0604332. [DOI] [PubMed] [Google Scholar]
- Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- Weinstein SL, Finn AJ, Dave SH, Meng F, Lowell CA, Sanghera JS, DeFranco AL. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-beta. J. Leukoc. Biol. 2000;67:405–414. doi: 10.1002/jlb.67.3.405. [DOI] [PubMed] [Google Scholar]
- Wong-Baeza I, Gonzalez-Roldan N, Ferat-Osorio E, Esquivel-Callejas N, Aduna-Vicente R, Arriaga-Pizano L, Astudillo-de la Vega H, Villasis-Keever MA, Torres-Gonzalez R, Estrada-Garcia I, Lopez-Macias C, Isibasi A. Triggering receptor expressed on myeloid cells (TREM-1) is regulated post-transcriptionally and its ligand is present in the sera of some septic patients. Clin. Exp. Immunol. 2006;145:448–455. doi: 10.1111/j.1365-2249.2006.03158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]