Summary
Although soil contains only traces of soluble carbohydrates, plant roots take up glucose and sucrose efficiently when supplied in artificial media. Soluble carbohydrates and other small metabolites found in soil are in part products from exudation from plant roots. The molecular nature of the transporters for uptake and exudation is unknown. Here, fluorescence resonance energy transfer (FRET) glucose and sucrose sensors were used to characterize accumulation and elimination of glucose and sucrose in Arabidopsis roots tips. Using an improved image acquisition set-up, FRET responses to perfusion with carbohydrates were detectable in roots within less than 10 sec and over a wide concentration range. Accumulation was fully reversible within 10-180 sec after glucose or sucrose had been withdrawn; elimination may be caused by metabolism and/or efflux. The rate of elimination was unaffected by pre-incubation with high concentrations of glucose, suggesting that elimination is not due to accumulation in a short-term buffer such as the vacuole. Glucose and sucrose accumulation was insensitive to protonophores, was comparable in media differing in potassium levels, and was similar at pH 5.8, 6.8 and 7.8, suggesting that both influx and efflux may be mediated by proton-independent transport systems. High-resolution expression mapping in root tips showed that only a few proton-dependent transport of the STP (Sugar Transport Protein) and SUT/SUC (Sucrose Transporter/Carrier) families are expressed in the external cell layers of root tips. The root expression maps may help to pinpoint candidate genes for uptake and release of carbohydrates from roots.
Keywords: glucose transport, sucrose transport, uniport, quantitative imaging, FRET
Introduction
Plant roots can absorb many metabolites from the rhizosphere. Plants also secrete a wide spectrum of compounds into the rhizosphere (Walker et al., 2003); for example, 5-21% of photosynthetically fixed carbon may be exported as root exudates (Marschner, 1996). Exudation products include carbohydrates such as pentoses, hexoses, and di- and trisaccharides (Jalali and Suryanarayana, 1971). Reporter-based biosensors have been used to determine the concentrations of sugars and amino acids in soil; the highest abundance of sucrose was found near the tip of Avena roots (Farrar et al., 2003; Jaeger et al., 1999). Exudation appears to be controlled and may be altered, for example during pathogen attack (Walker et al., 2003). Although many plant glucose and sucrose/H+ co-transporters have been identified, the molecular mechanisms responsible for uptake and release and their control are not understood (Lalonde et al., 2004). Identification of efflux transporters would help to advance our understanding of plant/soil interactions, but may also be relevant in the context of phloem loading (efflux from mesophyll or phloem parenchyma cells) and for sucrose efflux from the seed coat for supply of developing embryos (Zhou et al., 2007).
While the efflux of metabolites is difficult to measure, uptake of metabolites into cells or tissues is typically measured using radiotracers. Drawbacks of this approach include the fact that short-term studies with 14C- or 3H-labeled carbohydrates cannot differentiate the products, which are subject to metabolic conversion and compartmentation (see for example Javelle et al., 2005), issues that become even more relevant when analyzing intact tissues as a consequence of exchange between cells. For efflux, vesicles, cells or tissues are pre-loaded with isotopes, but measurements are limited by the amounts of tracer available for efflux.
Genetically encoded molecular nanosensors for metabolites enable analysis of uptake, exchange and release of metabolites in individual cells from intact organs. Moreover, the sensors can be expressed specifically either in the cytosol or targeted to cellular compartments (Chaudhuri et al., 2007). FRET sensors make use of ligand-dependent changes in protein conformation, which are used as a proxy for measuring analyte concentrations. FRET sensors for carbohydrates utilize members of the bacterial periplasmic binding protein superfamily (PBPs) as recognition elements (Tam and Saier, 1993). The conformational change induced by the target molecule, here glucose or sucrose, is converted into a macroscopic observable (emission of fluorescent light) via reporter elements (Lalonde et al., 2005). Reporter elements are sterically separated donor-acceptor FRET pairs of fluorescent proteins; typically GFP spectral variants such as eCFP and eYFP or the more robust Venus variant (Fehr et al., 2002; Miyawaki et al., 1997).
Sugar FRET sensors have been developed for monosaccharides such as arabinose, ribose, glucose and galactose, and disaccharides such as sucrose and maltose (Fehr et al., 2002, 2003; Kaper et al., 2008; Lager et al., 2003, 2006). Glucose nanosensors have been used to measure sugar uptake and homeostasis in living animal cells, and subcellular analyte levels have been determined using nuclear- or ER-targeted versions (Fehr et al., 2004, 2005; Okumoto et al., 2005). Optimized versions of the glucose nanosensors have also been used to analyze the contribution of individual members of the human GLUT glucose transporter family to glucose uptake in mammalian cells using siRNA inhibition (Takanaga et al., 2008).
In plants, a series of FRET glucose sensors have been used to measure steady-state glucose levels in the cytosol of epidermal leaf cells as well as intact roots of soil-grown plants (Deuschle et al., 2006). The results showed that, under the conditions tested, root glucose levels in the absence of external supply are significantly lower compared to leaf epidermis. The glucose gradient across the plasma membrane in both cell types was much steeper than expected, and no evidence of tight homeostatic control was seen. The sucrose nanosensor has not previously been used in vivo. Here, an improved detection system was used for a detailed analysis of glucose and sucrose flux in Arabidopsis root tips of intact seedlings grown on solidified full-nutrient (FN) medium. The results provide evidence of protonophore-insensitive and pH-independent transport systems in root tips. Consistent with this finding, known proton-coupled sugar transporters are expressed at a low level in root tips. A comprehensive high-resolution root expression map (Brady et al., 2007) was used to identify potential candidates responsible for this function.
Results
Cytosolic steady-state glucose levels depend on external supply
Roots of Arabidopsis plants expressing an affinity series of FRET glucose sensors grown in soil have been used to identify concentration-dependent FRET changes (Deuschle et al., 2006). The transgenic lines provided an opportunity to measure steady-state levels in vivo. However, the use of soil-grown plants has limitations, e.g. due to injury caused by removal from soil and subsequent washing. Therefore, transgenic plants grown on agar plates were used for further analyses. Plants were germinated on FN medium (Loqué et al., 2005) solidified with 0.7% agar. In total, 64.5% of 535 individual plants expressing glucose or sucrose sensors showed reliable responses to perfusion of either of the carbohydrates.
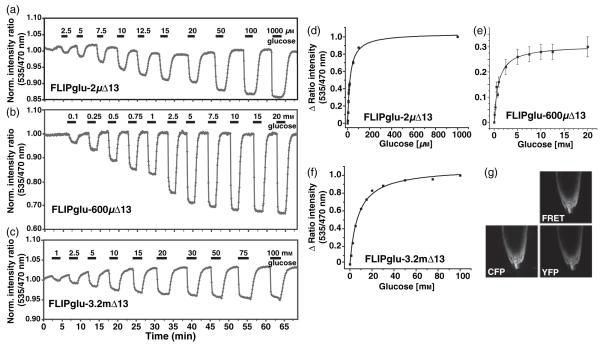
Plants expressing one of three glucose nanosensors with in vitro affinities for glucose ranging from 2 μm to 3.2 mm were used to determine the relative accumulation of glucose in the cytosol at various external supply levels. Measurements were carried out using primary root tips from plants expressing FLIPglu-2μΔ13, FLIPglu-600μΔ13 or FLIPglu-3.2mΔ13 in FN medium at pH 5.8 using a pulse protocol in which defined sugar concentrations were added by perfusion. Glucose was removed after the FRET response had reached a plateau (Figure 1). Glucose accumulation was readily reversible when glucose was removed from the perfusion medium. The maximum ratio change (Δ ratio) at various glucose concentrations was plotted and fitted to a single-site binding isotherm to determine the in vivo K0.5, i.e. the external glucose concentration at which the cytosolic levels correspond to the Kd of the respective sensor. The apparent K0.5 for FLIPglu-2μΔ13 was 10 μm; thus, after reaching steady state during perfusion with 10 μm external glucose, the sensor was at its in vitro Kd, suggesting that the cytosolic glucose concentration was about 2 μm. For FLIPglu-600μΔ13, the extracellular concentration required for half saturation of the sensor was 1 mm (Figure 1d-f). As the maximal in vitro Δ ratio for FLIPglu-3.2mΔ13 was lower than for the other variants, it is not possible to conclude whether the observed saturation is caused by saturation of the uptake systems or of the sensor. Overall, the extracellular levels required for reaching cytosolic glucose levels that correspond to the Kd of the respective sensor were always higher by a factor of 1.7-5 compared to their in vitro Kd. This suggests that metabolism and compartmentation contribute significantly to the reduced steady-state levels in the cytosol, and that the transporters cannot accumulate glucose to levels above the extracellular concentration supplied. The sensors are expressed under the control of the CaMV 35S promoter, which is active in all cell types of the root tip (Figures 1g and 5d; Movie S1). The epifluorescence system probably detects fluorescence from various cell layers in the z-axis. The analysis was carried out by analyzing various regions of interest in the root tip; no apparent differences in response were detected between different regions of the root tip (Figure S1).
Figure 1.
Glucose-induced FRET changes in the cytosol of intact roots.
(a-c) The FRET sensors FLIPglu-2μΔ13 (a), FLIPglu-600μΔ13 (b), and FLIPglu-3.2mΔ13 (c) respond to glucose perfusion in stably transformed rdr6-11 Arabidopsis roots after 5, 3 or 2 days of transfer to sugar-free medium, respectively. Quantitative data were derived by pixel-by-pixel integration of the ratiometric images. The y axis gives the ratio of eYFP intensity (ET535/30m) to eCFP intensity (ET470/24m). The bars above each graph represent the duration of perfusion with the indicated sugar concentrations.
(d-f) Saturation curves derived from the data shown in (a)-(c) for FLIPglu-2μΔ13 (d), FLIPglu-600μΔ13 (e) and FLIPglu-3.2mΔ13 (f).
(g) Confocal images showing the expression pattern of FLIPglu-600μΔ13 in the CFP, YFP and FRET channels in root tips.
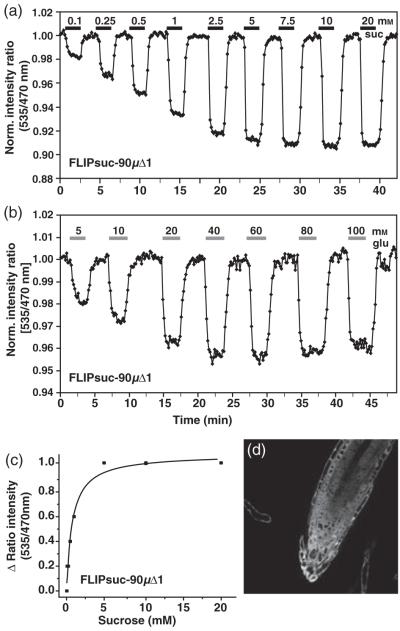
Figure 5.
Sucrose-induced FRET changes in the cytosol of intact roots.
(a.b) The FRET sensor FLIPsuc-90μΔ1 responds to sucrose perfusion (a) and glucose perfusion (b) in stably transformed rdr6-11 Arabidopsis roots. Images were acquired and data analyzed as in Figure 1. The bars above each graph represent the duration of perfusion with the indicated sucrose concentrations (black) or glucose concentrations (grey).
(c) Saturation curve for a representative in vivo sucrose titration of FLIPsuc-90μΔ1.
(d) Expression pattern (YFP channel) of FLIPsuc-90μΔ1 in root tips.
Surprisingly, slowly metabolizable 3-O-methylglucose, which is an efficient substrate for the high-affinity STP glucose transporters (Boorer et al., 1994), showed no or only marginal effects on glucose accumulation when used at 50- and 100-fold excess (Figure S2a). The ability to accumulate glucose to millimolar concentrations and the insensitivity to 3-O-methylglucose suggest dominance of unknown low-affinity transport systems.
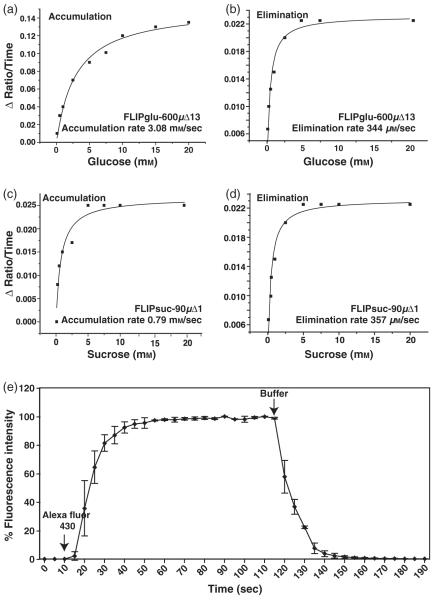
The experiments were carried out in an open-top perfusion chamber with a volume of 600 μl and high flow rates to minimize the possibility of the perfusion limiting the response kinetics. For all three sensors, FRET responses to perfusion with glucose were detectable in less than 10 sec, with the response reaching a steady state typically within less than 60 sec; complete reversion to baseline after removal of glucose occurred within <180 sec. For FLIPglu-600μΔ13, the accumulation rate, which is determined by the rate of influx, the rate of metabolism and the rate of compartmentation, calculated from the slopes of the in vivo titration data during the linear accumulation phase and plotted against the outside glucose concentration, was estimated as 3.1 ± 0.1 mm/sec (from two experiments, under the assumption that the affinity of the sensor is unaltered in vivo). The rapid elimination, which is the consequence of efflux, metabolism and fast release from transient stores, was calculated to be approximately 10 times slower than accumulation, at 0.34 mm sec-1 (Figure 2a,b). Repeated perfusion with high glucose concentrations or perfusion for extended periods of time did not lead to a change in the accumulation or elimination rates, suggesting that saturable pools such as the vacuole do not play a major role under the conditions tested (Figure 3). The accumulation rate for glucose is a minimal estimate, as perfusion kinetics were limiting even at the high flow rates and with the small dimensions of the chamber, as determined in an empty chamber using fluorescent dye (Figure 2e).
Figure 2.
In vivo accumulation and elimination rates for glucose and sucrose.
(a, b) Accumulation (a) and elimination (b) rates for glucose as measured from in vivo titration of FLIPglu-600μΔ13.
(c, d) Accumulation (c) and elimination (d) rates for sucrose as measured from in vivo titration of FLIPsuc-90μΔ1.
(e) Perfusion kinetics for the RC-26G chamber using Alexa Fluor 430. Arrows indicate the addition and removal of the fluorescent dye.
Figure 3.
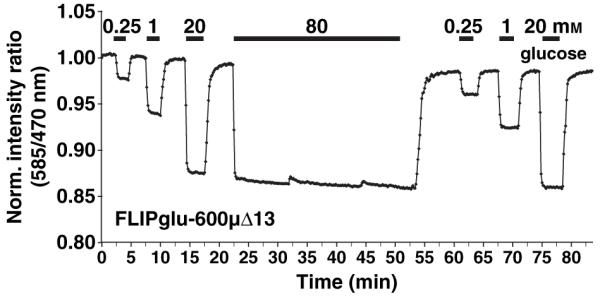
In vivo response of FLIPglu-600μΔ13 to glucose before and after exposure to saturating glucose levels.
The bars above the trace represent the duration of perfusion with the indicated sugar concentrations (see Figure 1).
In vivo response of glucose sensors to sucrose addition
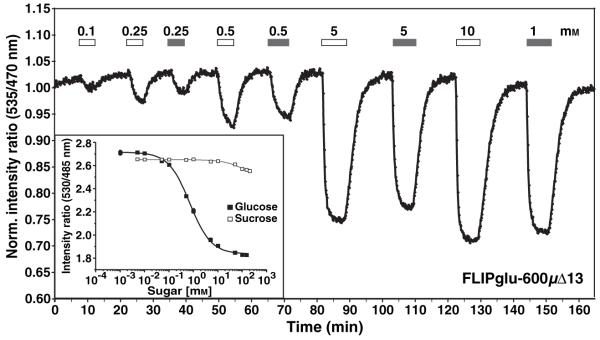
As plants can use multiple parallel pathways for sucrose catabolism, i.e. hydrolysis by extracellular, cytosolic or vacuolar invertases (Roitsch and González, 2004), or by sucrose synthase, glucose sensors may enable monitoring of sucrose metabolism in vivo. FLIPglu-600μΔ13, which does not respond to sucrose in vitro at concentrations below 20 mM, responded to perfusion with sucrose when analyzed in vivo. The response to the same molar concentration of sucrose was approximately 60-95% of the response to glucose, demonstrating rapid sucrolysis either in the apoplasm or the cytosol (Figure 4). A 10-20 sec delay in the response to sucrose relative to glucose was observed at low concentrations (<5 mm). As seen for perfusion with glucose, the accumulation of sucrose-derived glucose was reversible.
Figure 4.
Response of the glucose sensor FLIPglu-600μΔ13 to sucrose in Arabidopsis roots.
In vivo response of FLIPglu-600μΔ13 to alternating equimolar concentrations of glucose and sucrose. Images were acquired and data analyzed as in Figure 1. FLIPglu-600μΔ13 does not respond to sucrose in vitro (inset). The bars represent the duration of perfusion with glucose (white) and sucrose (grey). Sugar concentrations are indicated above each bar.
Analysis of sucrose flux using a FRET sucrose sensor
To directly measure the flux of sucrose rather than sucrose-derived glucose in Arabidopsis roots, the newly developed sucrose sensor FLIPsuc-90μΔ1 (Lager et al., 2006) was expressed in the rdr6 background of Arabidopsis thaliana Col-0 under control of the CaMV 35S promoter. To determine the apparent in vivo K0.5 for sucrose of FLIPsuc-90μδ1, plants expressing the sensor were perfused with gradually increasing concentrations of external sucrose using the same protocol as used for the glucose sensor (Figure 5a). Cytosolic sucrose levels were almost 10 times lower relative to the perfusion medium; the in vivo K0.5 was 800 μm (Figure 5c). Minimal accumulation and elimination rates were estimated to be 0.79 ± 0.03 and 0.36 ± 0.05 mm sec-1, respectively (Figure 2c,d). The accumulation rate was lower compared to glucose, consistent with the fast rate of sucrolysis shown above. As FLIPsuc-90μΔ1 binds sucrose with a Kd of 90 μm, but can also bind glucose with a Kd of 9 mm in vitro (Lager et al., 2006), roots were perfused with increasing glucose concentrations (Figure 5b). The first response of the sensor was observed with 500 μm glucose in the perfusion medium; saturation was reached at approximately 40 mm.
Characterization of the transport mechanism for glucose and sucrose
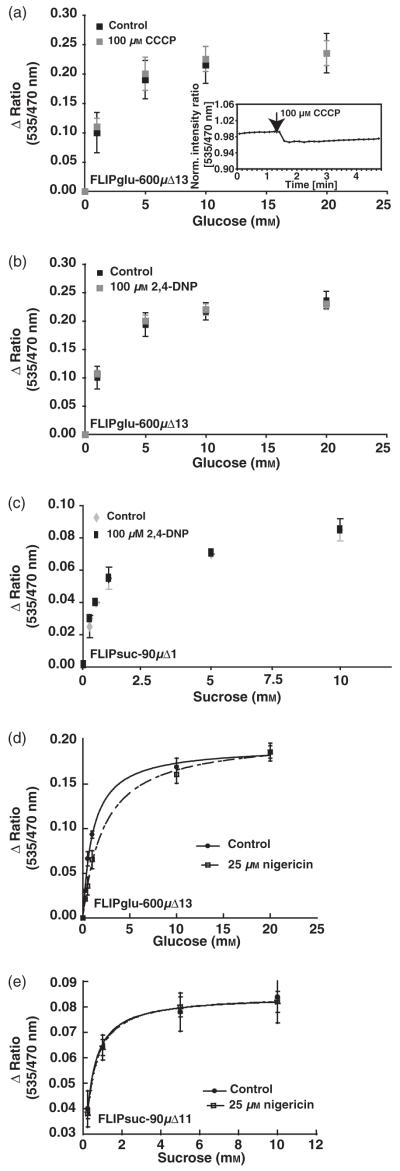
With the exception of the legume SUF sucrose transporters, the plant plasma membrane glucose and sucrose transporters characterized to date function as secondary active proton co-transporters (Zhou et al., 2007). To test whether sugar transport across the root tip plasma membranes is proton-coupled, the sensor response was analyzed in the presence of protonophores (Komor and Tanner, 1974). Individual plants expressing FLIPglu-600μΔ13 were analyzed first in the absence and then in the presence of carbonyl cyanide 3-chlorophenylhydrazone (CCCP) or 2,4-dinitrophenol (2,4-DNP) in a continuous experiment carried out with the same root tip. No significant effects of 100 μM CCCP or 2,4-DNP were observed for the maximum Δ ratio in vivo or the Δ ratio at saturating glucose concentrations (Figure 6a,b). Surprisingly, the K0.5 of the in vivo response curves was also unchanged, either suggesting that the protonophores are not effective under the conditions tested, or that the majority of the glucose uptake activity is proton-independent. The small reversible effect of CCCP treatment on the starting ratio is consistent with a small change in the cytosolic pH or ion concentrations (Figure 6a, inset) (Smith, 1986) (see below also). Experiments with FLIPsuc-90μΔ1 showed that sucrose accumulation is also insensitive to the protonophores (Figure 6c). The lack of significant effects for the glucose sensor was observed with CCCP in more than 12 independent plants, with 2,4-DNP in five plants, and for the sucrose sensor with CCCP and 2,4-DNP in seven and three independent plants, respectively (Figure 6, data for CCCP not shown). The ability of the inhibitors to block proton-coupled transport was verified by demonstrating that uptake of 35S042- into yeast cells expressing a plant sulfate transporter was sensitive to the inhibitors (data not shown). To exclude interactions of the inhibitors with the FN medium, the response was also tested in potassium phosphate buffer pH 5.8. Despite the absence of other ions in the medium, no difference in the sucrose response was observed (data not shown). The FN medium contains 1.55 mm K+; roots have a low membrane potential in this buffer. No difference in the response to glucose and no increased sensitivity to CCCP was observed when ARB medium containing 0.5 mm K+ was used (Figure S2b), which typically yields a membrane potential that is more negative than -130 mV (Ehrhardt et al., 1992). Access of CCCP to the root tips was verified by imaging movement of the YFP-labeled Golgi marker SYP32 (N. Geldner, University of Lausanne, Switzerland and J. Chory, Salk Institute, San Diego, CA, personal communication). The velocity of Golgi particles was inhibited almost completely in <80 sec after addition of CCCP under identical conditions as used for FRET analysis (Figure S3). CCCP inhibited accumulation of [14C]-glucose in intact seedlings by 30% after 30 min (Figure S4). This effect must therefore be due to a CCCP-dependent step in other tissues (Kennedy, 1977). In the presence of potassium, the ionophore nigericin dissipates the pH gradient without affecting membrane potential. Nigericin had no significant effect on sucrose accumulation in roots expressing FLIPsuc-90μΔ1, but inhibited glucose accumulation at low glucose supply (Figures 6d,e and S2c,d). Taken together, these data suggest that accumulation of glucose and sucrose is mediated by a protonophore-insensitive mechanism and that glucose and sucrose are taken up by different systems.
Figure 6.
Effect of protonophores on sugar response.
(a-c) Glucose accumulation in roots in the presence of 100 μM 2,4-DNP (a) or CCCP (b) measured using FLIPglu-600μΔ13, and sucrose accumulation in roots in the presence or absence of 2,4-DNP (c) measured using FLIPsuc-90μΔ1. Data were acquired as for Figure 1. A small dip in the baseline upon addition of CCCP to FLIPglu-600μΔ13 plants is shown as and inset in (a).
(d, e) Glucose response in the presence of 25 μm nigericin measured using FLIPglu-600μΔ13 (d) and sucrose response in the presence of 25 μm nigericin measured using FLIPsuc-90μΔ1 (e).
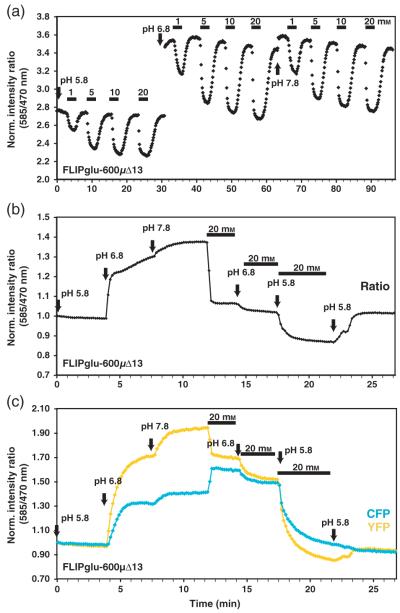
Glucose flux is insensitive to pH changes
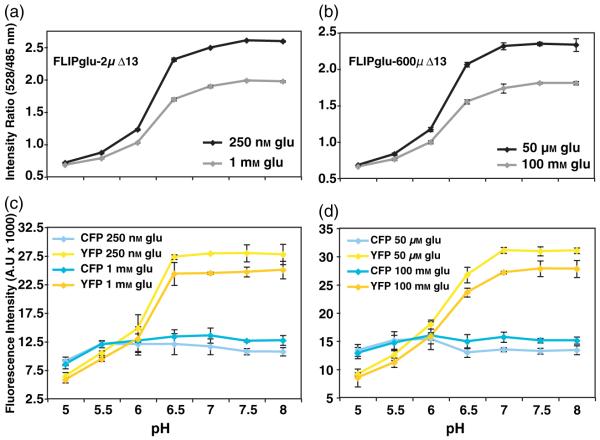
For proton-coupled transporters expressed in yeast, the uptake rate decreases with increasing pH (Riesmeier et al., 1992; Stolz et al., 1994). To test whether glucose accumulation is affected by changing the pH of the medium, the in vivo response of FLIPglu-600μΔ13 was analyzed in root tips exposed to FN medium buffered to pH 5.8, 6.8 and 7.8 (Figure 7a). The same root was first analyzed using three glucose concentrations at pH 5.8; the experiment was subsequently repeated at pH 6.8 and pH 7.8. Normalized sensor response curves were similar for all three pH conditions, with half saturation of the sensor at about 1 mm glucose. The absolute ratio and Δ ratio differed at the various pH due to effects of the pH on the starting ratio. The observed effects on the in vivo starting ratio were reproducible in vitro (Figure 8), and suggest that the cytosolic environment of the sensor changes significantly and rapidly during the pH shift. The pH changes did not lead to a FRET change, but were due to differences in the relative sensitivity of the two fluorophores to pH (Figure 7b,c). Thus, glucose responses and pH responses can be differentiated by analysis of the raw data. FLIPglu-600μΔ13 and FLIPglu-2μΔ13 were also found to be sensitive to pH below 6.5 in vitro. However, between pH 6.5 and 8, there was no significant difference in the Δratio for either of the two sensors (Figure 8a,b). The pH affected YFP fluorescence more severely than it did CFP fluorescence (Figure 8c,d).
Figure 7.
Effect of external pH on glucose accumulation in roots.
(a) Titration of FLIPglu-600μΔ13 at pH 5.8, 6.8 and 7.8. The bars above the trace show the concentration and duration of glucose treatment. The times at which pH was changed are marked with arrows.
(b) Change in FRET in response to pH in the absence and presence of glucose.
(c) eCFP (cyan) and eYFP (yellow) intensity traces in response to pH and glucose.
Figure 8.
Effect of pH on glucose sensor proteins in vitro.
(a, b) In vitro Δratio of FLIPglu-2μΔ13 (a) and FLIPglu-600μΔ13 (b) at various pH.
(c, d) eCFP (cyan) and eYFP (yellow) intensities for FLIPglu-2μΔ13 (c) and FLIPglu-600μΔ13 (d).
Expression of sugar transporter genes in root tips
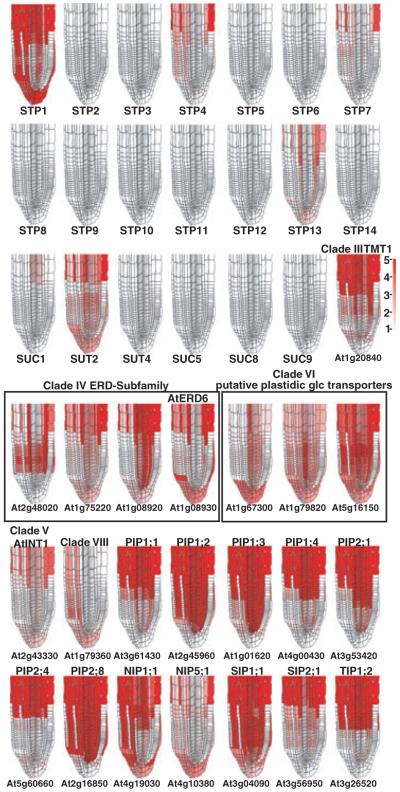
The insensitivity of glucose and sucrose steady-state levels to the presence of ions in the FN medium, to pH and to protonophores argues against a major contribution of secondary active glucose and sucrose uptake systems in root tips. The reversibility of sugar accumulation could thus also be explained by efflux via the same carrier system. Uniporters are potential candidates for sugar transport in root tips. The high-resolution spatial and temporal root expression profiles may provide a means to identify potential uniporter candidates. Existing microarray expression data cover nearly all cell types within the root via profiling of a set of fluorescently marked lines, and a set of developmental time points, but not all developmental stages of every cell type (Brady et al., 2007). A new approximation method (see Experimental procedures), which integrates both the marker line and developmental stage expression profiles were used to generate relative root expression maps of these potential uniporter candidates. Using this method, only two proton-dependent systems of the STP and SUT/SUC gene families were identified as broadly expressed in the root tip (Figure 9). Specifically, STP1 is approximated as highly expressed and SUT2 as moderately highly expressed in the lateral root cap, columella and atrichoblasts of the meristematic zone (Figure 9). Although no expression was reported in trichoblasts, expression may be present in vivo, as no current marker lines profile expression in this cell type in the meristematic zone. Among other members of the sugar transporter family, ERD6-like homologs are highly expressed (Figure 9). Of the clade IV ERD6 subfamily, At1g08920 is more highly expressed in the majority of root tissues in the meristematic zone relative to the epidermis and lateral root cap, while At1g08930 has a complementary expression pattern to that of At1g08920, with high relative expression in the lateral root cap and columella. Two clade VI plastidic glucose transporters, At1g79820 and At5g16150, are more widely expressed in the root, excluding trichoblasts, although the same caveat regarding trichoblasts still applies. Four members of the aquaporin gene family (PIP1;2, PIP1;3, PIP2;8 and SIP1;1) are quite highly expressed in nearly all root tissues relative to the lateral root cap, columella and trichoblasts. All other aquaporin gene family members are expressed in a variety of cell types or developmental stages in the root meristematic zone (Figure 9). Expression levels of all the genes shown in Figure 9 for the various marker lines and longitudinal root sections are given in Table S1.
Figure 9.
High-resolution expression mapping in Arabidopsis root tips showing the expression pattern of hexose and sucrose transporter family genes and aquaporins.
Maps for STP (rows 1 and 2) and SUT (row 3) gene families as well as the hexose transporter family and aquaporin genes (rows 4, 5 and 6) are visualized on a root-tip template and false-colored such that low expression is colored white and high expression is red. The scale is given to the right of row 3.
However, water channel blockers such as tetraethylammonium (100 μm and 1 mm) (Detmers et al., 2006) had no significant effect on glucose accumulation (Figure S5). The inhibitor mercury chloride was also tested, but affected the fluorophores of the sensor and thus could not be evaluated (data not shown).
Discussion
FRET sensors provide the opportunity to measure metabolic flux at the interface of cellular uptake and metabolism in intact organs at cellular and subcellular resolution. Root tips are actively growing apices, and thus have to be supplied with carbohydrates from the above-ground parts. Sugars are delivered via the phloem, and dye-coupling studies as well as intercellular movement of GFP have suggested that small molecules may be able to diffuse into the tip region through plasmodesmata (Stadler et al., 2005; Wright and Oparka, 1997). Moreover, root tips exude metabolites, including sugars, potentially to feed beneficial micro-organisms (Bais et al., 2006). Although the levels of soluble carbohydrates are low in soil, plants readily take up glucose and sucrose when grown in axenic culture. To characterize the mechanisms involved in sugar transport into root tips, FRET sensors for glucose and sucrose were expressed in Arabidopsis.
In a previous study, glucose accumulation had been analyzed in Arabidopsis roots grown in soil using FRET glucose sensors. As the roots had to be removed from soil and washed, there was the potential for injuring the roots. To overcome this issue, roots of intact seedlings grown on plates in a complex FN medium supplemented with 1-2% sucrose were imaged in this study. At the same time, significant improvements regarding sensitivity and dynamic range were made compared to the initial experiments (Deuschle et al., 2006) by using a more sensitive CCD camera and by improvements of the perfusion system, allowing better quantitative analysis of accumulation and removal rates. However, glucose accumulation rates are extremely fast, so the rates shown here represent only a minimal estimate. Using transgenic lines expressing three nanosensors with various affinities for glucose (Deuschle et al., 2006) and a high-affinity sucrose sensor (Lager et al., 2006), steady-state levels were determined in the tips of intact roots. Glucose uptake appears to be more rapid than the reverse phase after withdrawal from the medium, and the gradient across the plasma membrane is much steeper than anticipated from non-aqueous fractionation studies (Farré et al., 2001; Heineke et al., 1994). The accumulation rates for glucose and sucrose derived from the slope of the response curves after adding or removing the sugars (for a detailed description of the analysis, see Okumoto et al., 2008) and the levels accumulating in the cytosol are striking, and probably relate to the small volume of the actual cytosol (cell volume minus organelles, vacuole and endomembrane system). Despite metabolic activity, glucose accumulates to levels in the millimolar range. The affinities of the plant hexose transporters characterized so far, e.g. STP1, are in the micromolar range, and thus these are probably not major players for root tip uptake (Williams et al., 2000). These findings may suggest that the root tip expresses low-affinity transporters for glucose and sucrose, the molecular nature of which needs to be determined.
Efficient sucrolysis during import
The glucose sensor, which does not bind sucrose to a measurable extent in vitro, responded to sucrose in vivo in a concentration-dependent manner. Also, the in vivo K0.5 for sucrose as measured using the sucrose sensor was 800 μm; approximately nine times lower than the in vitro Kd, suggesting that a significant proportion of sucrose must be hydrolyzed either extracellularly or in the cytosol. To obtain a more detailed understanding of the individual flux components, it would be interesting to determine the relative rates of sucrolysis in the apoplasm versus the cytosol by targeting the sucrose and glucose sensors to the extracellular face of the plasma membrane as has been done for the glutamate FRET sensor in hippocampal neurons (Okumoto et al., 2005) or to study the effect of invertase inhibitors or RNAi inhibition of invertase expression in root tips. However, without knowledge of the transport mechanism and the contribution of intra- versus extracellular hydrolysis of sucrose, it is not yet possible to model sugar flux in the tip system.
Uniport as the dominating import mechanism in root tips?
The flux studies in Arabidopsis root tips showed that protonophores such as CCCP or 2,4-DNP had no discernable effect on glucose or sucrose accumulation, suggesting the dominance of proton-independent sugar transport systems in root tips under the conditions tested. Nigericin had no effect on steady-state levels of sucrose, but reduced cytosolic glucose levels by about 30% (Figure 6d,e). CCCP was functional, as shown by control experiments in yeast and plants. Movement of Golgi particles is rapidly inhibited after addition of CCCP, probably due to changes in the pH of vesicles and a reduction in ATP availability. In mammalian cells, addition of 100 μm CCCP for 1 h was not lethal and reduced cellular ATP levels by only 40% (Burkhardt and Argon, 1989; Burkhardt et al., 1989). Mitochondrial pH decreases by at least one unit in response to CCCP treatment (Llopis et al., 1998).
The finding that steady-state levels of both glucose and sucrose, which are a result of influx rate and metabolic rate as well as compartmentalization, are unaffected by CCCP, 2,4-DNP and nigericin (affecting glucose only) is surprising because the drugs have a variety of side effects, e.g. they can inhibit adenylate cyclase in Escherichia coli (Peterkofsky and Gazdar, 1979), stimulate the overall activity mediated by the facilitative glucose transporter GLUT1 (Hamrahian et al., 1999), and inhibit the overall activity mediated by GLUT4 (Chu et al., 2002). The effects on GLUT activity are probably due to effects on targeting to the plasma membrane as shown for other proteins (Burkhardt and Argon, 1989), and may be due to the effect of protonophores on Golgi pH (Llopis et al., 1998). The findings are also surprising as CCCP has been shown to increase sugar efflux from corn roots (Mühling et al., 1993).
As accumulation of sugars is readily reversible after removal of glucose or sucrose from the medium, uptake and release may be mediated by uniporters for glucose (Lalonde et al., 2004). Alternatively, accumulation may be mediated by CCCP-insensitive endocytosis. Receptor-mediated internalization of [125I]-transferrin was not sensitive to CCCP (Burkhardt et al., 1989). The difference in nigericin sensitivity of glucose and sucrose accumulation suggests the involvement of different uptake mechanisms. Consistent with a proton-independent uptake of glucose into root tip cells, no membrane depolarization was detected in cells in the elongation zone of Arabidopsis roots upon perfusion with 10 mm glucose in 4 out of 5 independent experiments using microelectrodes (David W. Ehrhardt, FH and WBF, Carnegie Institution, unpublished results).
As root tips exude soluble sugars, a potential function of uniporters could be the feeding of beneficial micro-organisms in soil (Figure 10). Sugar transport in plants, especially the transport of glucose and sucrose, has been studied extensively over the last 25 years, and evidence for energy-independent transport of both sucrose and glucose has been observed previously. Radioactive uptake studies into plant tissues typically show multi-phase kinetics. Typically, at least two components are discernable: a linear low-affinity, high-capacity component and a saturable high-affinity, low-capacity component (Delrot and Bonnemain, 1981; Komor and Tanner, 1974; Maynard and Lucas, 1982). The saturable component of glucose and sucrose uptake in sugar beet leaf disks was sensitive to protonophores, which was taken as evidence of proton-coupled transport, while the linear component was found to be insensitive to protonophores, suggesting the involvement of uniport transport mechanisms (Maynard and Lucas, 1982). Olive suspension cells were also shown to take up glucose by a diffusion-like mechanism, with a linear dependence on external glucose concentration up to 100 mm (Conde et al., 2007). Uptake of glucose into root border cells could only be inhibited by phlorizin at external glucose concentrations below 2 mm, but not at external concentrations between 2 and 20 mm (Stubbs et al., 2004). For sucrose, transport across the plasma membrane of sugar beet root was CCCP-insensitive and pH-independent (Saftner and Wyse, 1980). Interestingly, symplasmic movement of fluorescent dyes between cells in the root tip was also unaffected by protonophores (Wright and Oparka, 1997).
Figure 10.
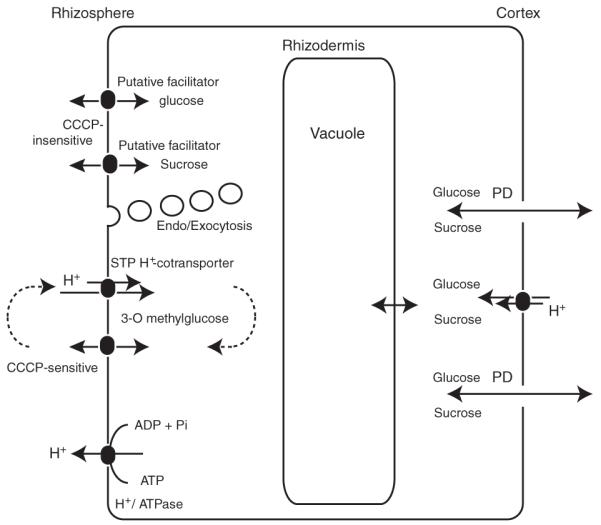
Hypothetical model for sugar uptake and efflux in the rhizodermis of Arabidopsis root tips.
Sugars derived from photosynthesis are imported into the cells either symplasmically or apoplasmically. High-affinity influx is mediated by STP H+ co-transporters, which also mediate 3-O-methylglucose uptake. Efflux of 3-O-methylglucose occurs through an unknown pathway. Low-affinity uptake and release of sucrose and glucose are possibly mediated by facilitators or endo/exocytosis.
pH independence of root sugar accumulation
As expected for a uniport system, the glucose flux appears to be largely independent of pH over a range from pH 5.8 (the typical pH of plant growth media) to pH 7.8. The saturation curves obtained at pH 5.8, 6.8 and 7.8 are comparable: the in vivo K0.5 was approximately 1 mm for all three conditions. A significant shift in the baseline was observed when the pH of the medium was changed. The most dramatic change occurred between pH 5.8 and 6.8 (a baseline shift of almost 0.8), corresponding to a shift of the cytosolic pH from 6 to 6.5 (as suggested from in vitro pH titration of the sensor), while the change from 6.8 to 7.8 caused only a small increase in the baseline of 0.1. Many other studies using pH-sensitive electrodes or dyes do not support such a dramatic change in cytosolic pH. An alternative explanation for the significant change in the ratio may be that other ion concentrations change in addition to pH, and the sensors report the simultaneous change in pH and ion concentrations. Apparently, the pH change may be distinguished from a response to sugars, as the pH change does not cause a FRET change but is instead due to differential sensitivity of the two fluorophores to pH. The protonophore treatment also caused a reversible baseline shift, but this shift was comparatively small. Apparently, plant cells are capable of buffering the protonophore-induced change in cytosolic pH, as shown using pH-sensitive electrodes (Smith, 1986).
Candidates for root tip sugar uniporters
The independence from pH and the insensitivity to inhibitors suggest that uptake and release of glucose and sucrose are mediated by an unknown class of transporters expressed in the root tip of Arabidopsis. Recently, evidence for proton-independent transport of sucrose was obtained for SUF transporters, a subclade of the SUT sucrose transporter family (Zhou et al., 2007). Both the human and yeast hexose transporter homologs function as uniporters. Therefore, it is conceivable that, among the 54 members of the Arabidopsis hexose transporter family, members of subclades other than STP that have not been functionally characterized so far may function as uniporters (Lalonde et al., 2004). Another possibility could be facilitation through aquaporins. In Arabidopsis, there are 38 aquaporins in four subclasses (Kaldenhoff et al., 2007). Functional analyses have shown that aquaporins transport not only water but a variety of other substrates such as glycerol, urea, NH3, CO2, silicon, boron, formamide, malate, glycine and hydrogen peroxide (Kaldenhoff et al., 2007). It may therefore be speculated that members of the aquaporin family function in facilitation of sugar transport. Systematic analysis of the high-resolution expression map of Arabidopsis root tips indicates that only STP1 and SUT2 are highly expressed in root tips. Candidates for a function in facilitative sugar transport include members of the ERD6-like family of hexose transporters and members of the aquaporin family, although several of the aquaporin candidates are localized in intracellular compartments. Further functional analysis will be required to identify the uniporters. In addition, the genome of Arabidopsis contains a large number of unknown transporters that may serve as uniporters (Schwacke et al., 2003).
Potential role of sugar uniport in root tips
The independence from pH and the insensitivity to inhibitors suggest that, under certain conditions, roots express facilitators that can release glucose into the soil solution, potentially to feed beneficial micro-organisms, or, as work by Jones and Darrah (1993) suggests, even resorb sugars from the soil. Microbial biomass and activity in the vicinity of plant roots are usually higher than in bulk soil, a phenomenon known as the rhizosphere effect (Bürgmann et al., 2005). Plants can release up to 20% of carbon assimilated by photosynthesis by exuding metabolites mucilage and by releasing cells. Plant roots exude a complex mixture of sugars, organic acids, phytosiderophores, vitamins, amino acids, nucleotides and nucleosides, inorganic ions and they release root border cells (Dakora and Phillips, 2002). A large proportion of the carbohydrates released by roots is respired by micro-organisms, providing a basis for the up to 100-fold increase in the size of the microbial population compared to surrounding bulk soil (Weller and Thomashow, 1994). Glucose from exudates apparently plays an important role for certain soil bacteria (Kuiper et al., 2002) Sucrose is thought to play a less important role as it is probably hydrolyzed in the apoplasmic space or the soil solution.
Conclusions
FRET sensors enable monitoring of flux into intact organs. The results obtained for Arabidopsis root tips provide evidence that uptake is dominated by high-capacity systems that appear to be pH-independent and protonophore-insensitive. FRET sensors can provide quantitative data for modeling of carbohydrate flux in roots. However, before a detailed flux model can be generated, we require information on the nature and kinetics of the contributing transporters as well as additional information such as apoplasmic sugar levels and the contribution of vacuolar and organellar levels and fluxes.
The next steps will be to directly measure efflux with apoplasmically anchored FRET sensors and to test Arabidopsis T-DNA insertion lines for various transporters expressed in roots, including aquaporins, as well as evolutionary diverse members of the glucose transporter family for effects on sugar accumulation (Lalonde et al., 2004).
Experimental procedures
Plant material and FLIP constructs
Homozygous Arabidopsis lines (T3) expressing FLIPglu-2μΔ13 (line 1), FLIPglu-600μΔ13 (line 2) or FLIPglu-3.2mΔ13 (line 3) were used for the glucose flux analyses (Deuschle et al., 2006). To generate lines expressing the sucrose sensor, the FLIPsuc-90μΔ1 insert was excised from pRSETB (Lager et al., 2006) using BamHI/HindIII and cloned into the XhoI/BamHI sites of pRT101 (Töpfer et al., 1987). The XhoI overhang of pRT101 and the HindIII overhang from FLIPsuc-90μΔ1 were made compatible to each other by partially filling in using DNA polymerase I (Klenow fragment). The CaMV 35S-Sensor-Terminator cassette was then excised from pRT101 using HindIII and cloned into pPZP312 (Hajdukiewicz et al., 1994). The presence of intact inserts was verified by DNA sequencing. Binary plasmids were introduced into Agrobacterium strain GV3101 and used for transformation of homozygous Arabidopsis rdr6 lines (Peragine et al., 2004) (a generous gift from Scott Poethig, University of Pennsylvania) using the flower-dip method (Clough and Bent, 1998).
Plant growth conditions
For imaging, plants were germinated in full nutrient (FN) medium (1 mm KH2PO4, 1 mm MgSO4, 0.25 mm K2SO4, 0.25 mm CaCl2, 2 mm NH4NO3, 0.1 mm Na-Fe-EDTA, 50 μm KCl, 30 μm H3BO3, 5 μm MnSO4, 1 μm ZnSO4, 1 μm CuSO4, 0.7 μm NaMoO4, pH 5.8, adjusted with KOH) (Loqué et al., 2005) with 1-2% sucrose, buffered with 20 mm MES and solidified with 0.7% agar in a growth chamber with 16 h light at 190 μmol m-2 sec-1, 50% humidity, 22 °C for 7 days. Before imaging, the plants were transferred to the same medium lacking sucrose for 6 h to 5 days depending on the affinity of the sensor expressed in the plants. Where indicated, plants were imaged in potassium phosphate buffer or in ARB medium (2 mm MES, 2 mm CaSO4, 0.5 mm KH2PO4 and 0.5 mm MgSO4, pH 5.8) (Ehrhardt et al., 1992). For pH experiments, the pH of FN medium was adjusted to 5.8, 6.8 or 7.8 using 10 m KOH.
In vivo imaging
Roots of intact seedlings were immobilized on cover slips (24 × 50 mm, VWR, http://www.vwr.com) using medical adhesive (stock number 7730, Hollister, http://www.hollister.com). For screening the sensor response, five or six plants were mounted onto cover slips, and a chamber was created using polymer clay (Sculpey, http://www.sculpey.com) and filled with FN medium. The plants were then imaged during perfusion of a near-saturating concentration of sugar to determine the maximal response. Slides with plants that showed a response were then mounted on a P-1 or RC-26G perfusion chamber (Warner Instruments, http://www.warneronline.com) mounted on a stage adapter for a Leica SA-20L3P inverted microscope (Leica, http://www.leica.com). The volume of the chambers was 0.4-0.7 ml. Ratio imaging was performed on an inverted fluorescence microscope (DM IRE2, Leica) using a QuantEM digital camera and a 20× oil objective (HC PL APO 20x/0.7IMM CORR, Leica). Dual emission intensities were simultaneously recorded using a DualView with a dual CFP/YFP-ET filter set (ET470/24m; ET535/3, Chroma, http://www.chroma.com) and Slidebook software (Intelligent Imaging Innovations Inc., http://www.intelligent-imaging.com). Excitation (filter ET430/24x, Chroma) was provided by a Lambda DG4 light source (Sutter Instruments, http://www.sutter.com). Images were acquired within the linear detection range of the camera, and exposure times varied between 400 and 600 msec depending on the expression level, with binning 2 and an Electron Multiplying gain of 300. Fluorescence intensities for eCFP and eYFP were typically in the range of 2000-8000 and 6000-14 000, respectively. Regions outside the root were used for background subtraction (background value 1000-1300; 300 msec exposure time). Perfusions were performed with FN medium buffered with 20 mm MES pH 5.8 at 3 ml min-1. The baselines throughout were corrected using second- or third-order polynomial fits of the ratios measured in the absence of glucose. The obtained function describes the baseline aberration (photobleaching) as a function of time during the perfusion. To correct for this effect, the difference between the ratio at the beginning of the experiment, r(0), and the baseline aberration, f(t), were calculated at each time point of the measurement and added to the value of the measured ratio at the respective time point, r(t), such that rcorr(t) = r(t) + r(0)) f(t). Accumulation and elimination rates for glucose and sucrose were calculated by determining Δratio/time at various external sugar concentrations (for a detailed description of the analysis, see Okumoto et al., 2008). The data fitted well to Michaelis-Menten kinetics. However, it would be more accurate to use a model that incorporates all contributing fluxes (e.g. Fehr et al., 2005).
Imaging in the presence of inhibitors
CCCP and 2,4-DNP (both Sigma, http://www.sigmaaldrich.com/) were prepared as 250 mm stock in 100% DMSO and frozen; nigericin (EMD Biosciences, http://www.emdbiosciences.com) was prepared as 5 mm stock in 95% ethanol and frozen. Before use, CCCP and 2,4-DNP stocks were diluted to 100 μm in FN medium (final DMSO concentration 0.04%); nigericin was diluted to 25 μm. All experiments were repeated at least three times. Controls were performed with solutions containing 0.04% DMSO or 0.5% ethanol, respectively. Tetraethylammonium was prepared as a 100 mm stock solution in H2O and was diluted prior to use. Plants were incubated with the indicated inhibitor for 15 min before resuming perfusion with either sugar. 3-O-methylglucose was prepared as a 1 m stock in H2O.
Estimate of exchange rates for the RC-26G perfusion chamber
Exchange rates were determined on the same perfusion set-up as used for the imaging experiments using fluorescent dye Alexa Fluor 430 (Invitrogen, http://www.invitrogen.com/) at 3 ml min-1. After the fluorescence intensity reached saturation, perfusion was switched back to the buffer. The rates were calculated by taking the maximum fluorescence intensity as 100% and determining the time that it takes to reach 85% or 15% fluorescence, i.e. the linear uptake or efflux phases, respectively. The addition and removal rates are the mean of two experiments.
Confocal microscopy
For analysis of Golgi movement, Arabidopsis thaliana seedlings expressing YFP:SYP32 were grown for 6 days on FN medium and root tips were imaged on a spinning-disk confocal microscope as described by Takanaga et al. (2008). Plants were mounted and treated as for imaging experiments. Images were acquired every 4 sec for 2 min before adding 100 μm CCCP and for 8 min thereafter. Image processing was performed using Metamorph (Molecular Devices, http://www.moleculardevices.com) and ImageJ software (W. Rasband, National Institutes of Health, Bethesda, MD). Two images (20 sec apart) were merged after false coloring in red for the first frame and green for the second. YFP:SYP32 wave lines were a generous gift from Niko Geldner and Joanne Chory. More information about these lines can be found at http://www.unil.ch/dbmv/page49637_en.html. The expression of the nanosensors expressed under the control of the CaMV 35S promoter was analyzed by collecting an image series for root tips expressing either FLIPglu-600μΔ13 or FLIPsuc-90μΔ1 as z-stacks using a Leica confocal SP5 microscope equipped with an argon laser (514 nm excitation for YFP) and a 442 diode laser (442 nm excitation for CFP). The emission signal was captured from 525-560 nm for YFP and 460-500 nm for CFP. The pinhole was adjusted to an airy disk size of 0.5, and images were captured at 1.5 μm intervals.
In vitro titration of glucose sensor proteins under various pH conditions
FLIPglu-2μΔ13 and FLIPglu-600μΔ13 were expressed in E. coli (BL21 DE3 gold) and grown for 3 days at room temperature. Protein was isolated as described previously (Deuschle et al., 2005). Substrate titration curves and substrate specificity analysis were performed using a monochromator microplate reader (Safire; excitation 433/12 nm; emission 485/12 and 528/12 nm; gain 80, http://www.tecan.com). In vitro analyses at various pH were performed in MES/Tris buffer; pH was adjusted by mixing 40 mm MES and 40 mm Tris buffers. Proteins were titrated using various glucose concentrations in each of the pH buffers. FRET was approximated as the peak emission intensity ratio at 480 nm (first eCFP peak) and 528 nm (eYFP peak). Measurements were performed on two independent protein extracts.
Approximation of cell-type specific expression at developmental stage resolution
High-resolution genome-wide expression data at the level of each cell type at all developmental stages in the Arabidopsis root are not available, due to the technical limitations regarding the amount of material required for microarray measurements. However, using the large microarray dataset profiling 19 semi-overlapping marker lines and 13 developmental sections published recently (Brady et al., 2007), a reasonable approximation can be made. Expression of a given gene within a particular tissue or cell type and section was approximated as the mean expression from all marker lines covering that tissue in that section, and scaling that mean by a factor equal to the expression of that gene in that developmental section relative to its mean expression over all longitudinal sections. Thus, expression of gene g within tissue t in developmental section s, denoted Gts, can be approximated as:
where Mg is the expression of gene g in marker line m, Cmts is a 1/0 indicator variable depending on whether marker m covers tissue t in section s, Sg is the expression of gene g in section s, and Ag is the mean expression of gene g over all sections. Expression within a region that is not covered by any marker line (e.g. trichoblasts in meristematic zone sections) is reported as 0. Table S2 and Figure S6 show the marker line × developmental section, marker line × cell type and developmental section × cell type matrices that were used to approximate expression.
Approximating cell type-/developmental stage-specific resolution by this method has some restrictions, such as treating expression within a marker line as constant throughout all developmental stages, the inability to approximate expression in areas not covered by any marker line, and the inability to guarantee that the approximated data exactly reproduce the observed measurements. Methods that are not subject to such restrictions would be very useful for more detailed studies, but, for the purposes of identifying candidate genes, our simplistic approximation is sufficient. The scale was set to a maximum of 5; thus some of the transporters are more highly expressed than shown here (see Table S2 for detailed quantification).
Supplementary Material
Figure S1. Comparison of FRET sensor responses in various regions of the root tip.
Movie S1. Cellular expression of the sucrose nanosensor in root tips of Arabidopsis.
Figure S2. Glucose and sucrose response curves in the absence and presence of various inhibitors.
Figure S3. Effect of CCCP on Golgi movement.
Figure S4. [14C]-glucose uptake into intact seedlings.
Figure S5. Insensitivity of responses to tetraethylammonium.
Figure S6. Marker line coverage.
Table S1 Expression levels for the transporter genes as shown in Figure 9.
Table S2 Marker line coverage.
Appendix S1. Radioactive glucose uptake in intact seedlings of Arabidopsis thaliana.
Acknowledgements
We are very grateful to Nakako Shibagaki (Carnegie Institution for Science) for testing the efficacy of the protonophores in yeast uptake experiments, Victor Kirik for help with the Golgi movement experiments, and Guillaume Pilot (Carnegie Institute for Science, Stanford, CA) for help with radioactive glucose uptake in intact seedlings. This work was made possible by a grant from the Department of Energy to W.B.F. (DE-FG02-04ER15542) and grants from the National Science Foundation Arabidopsis 2010 program to P.N.B.
References
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- Boorer KJ, Loo DD, Wright EM. Steady-state and presteady-state kinetics of the H+/hexose cotransporter (STP1) from Arabidopsis thaliana expressed in Xenopus oocytes. J. Biol. Chem. 1994;269:20417–20424. [PubMed] [Google Scholar]
- Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science. 2007;318:801–806. doi: 10.1126/science.1146265. [DOI] [PubMed] [Google Scholar]
- Bürgmann H, Meier S, Bunge M, Widmer F, Zeyer J. Effects of model root exudates on structure and activity of a soil diazotroph community. Environ. Microbiol. 2005;7:1711–1724. doi: 10.1111/j.1462-2920.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Argon Y. Intracellular transport of the glycoprotein of VSV is inhibited by CCCP at a late stage of post-translational processing. J. Cell Sci. 1989;92:633–642. doi: 10.1242/jcs.92.4.633. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Hester S, Argon Y. The glycoprotein of VSV accumulates in a distal Golgi compartment in the presence of CCCP. J. Cell Sci. 1989;92:643–654. doi: 10.1242/jcs.92.4.643. [DOI] [PubMed] [Google Scholar]
- Chaudhuri B, Niittylä T, Hörmann F, Frommer WB. Fluxomics with ratiometric metabolic dyes. Plant Signal Behav. 2007;2:120–122. doi: 10.4161/psb.2.2.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CY, Kao YS, Fong JC. Nigericin inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes. J. Cell. Biochem. 2002;85:83–91. [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Conde C, Silva P, Agasse A, Tavares RM, Delrot S, Geros H. An Hg-sensitive channel mediates the diffusional component of glucose transport in olive cells. Biochim. Biophys. Acta. 2007;1768:2801–2811. doi: 10.1016/j.bbamem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Dakora FD, Phillips DA. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil. 2002;245:35–47. [Google Scholar]
- Delrot S, Bonnemain JL. Involvement of protons as a substrate for the sucrose carrier during phloem loading in Vicia faba leaves. Plant Physiol. 1981;67:560–564. doi: 10.1104/pp.67.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmers FJ, de Groot BL, Muller EM, Hinton A, Konings IB, Sze M, Flitsch SL, Grubmuller H, Deen PM. Quaternary ammonium compounds as water channel blockers. Specificity, potency, and site of action. J. Biol. Chem. 2006;281:14207–14214. doi: 10.1074/jbc.M513072200. [DOI] [PubMed] [Google Scholar]
- Deuschle K, Okumoto S, Fehr M, Looger LL, Kozhukh L, Frommer WB. Construction and optimization of a family of genetically encoded metabolite sensors by semirational protein engineering. Protein Sci. 2005;14:2304–2314. doi: 10.1110/ps.051508105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle K, Chaudhuri B, Okumoto S, Lager I, Lalonde S, Frommer WB. Rapid metabolism of glucose detected with FRET glucose nanosensors in epidermal cells and intact roots of Arabidopsis RNA-silencing mutants. Plant Cell. 2006;18:2314–2325. doi: 10.1105/tpc.106.044073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt DW, Atkinson EM, Long SR. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science. 1992;256:998–1000. doi: 10.1126/science.10744524. [DOI] [PubMed] [Google Scholar]
- Farrar J, Hawes M, Jones D, Lindow S. How roots control the flux of carbon to the rhizosphere. Ecology. 2003;84:827–837. [Google Scholar]
- Farré EM, Tiessen A, Roessner U, Geigenberger P, Trethewey RN, Willmitzer L. Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiol. 2001;127:685–700. doi: 10.1104/pp.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr M, Frommer WB, Lalonde S. Visualization of maltose uptake in living yeast cells by fluorescent nanosensors. Proc. Natl Acad. Sci. USA. 2002;99:9846–9851. doi: 10.1073/pnas.142089199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr M, Lalonde S, Lager I, Wolff MW, Frommer WB. In vivo imaging of the dynamics of glucose uptake in the cytosol of COS-7 cells by fluorescent nanosensors. J. Biol. Chem. 2003;278:19127–19133. doi: 10.1074/jbc.M301333200. [DOI] [PubMed] [Google Scholar]
- Fehr M, Lalonde S, Ehrhardt DW, Frommer WB. Live imaging of glucose homeostasis in nuclei of COS-7 cells. J. Fluoresc. 2004;14:603–609. doi: 10.1023/b:jofl.0000039347.94943.99. [DOI] [PubMed] [Google Scholar]
- Fehr M, Takanaga H, Ehrhardt DW, Frommer WB. Evidence for high-capacity bidirectional glucose transport across the endoplasmic reticulum membrane by genetically encoded fluorescence resonance energy transfer nanosensors. Mol. Cell. Biol. 2005;25:11102–11112. doi: 10.1128/MCB.25.24.11102-11112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 1994;25:989–994. doi: 10.1007/BF00014672. [DOI] [PubMed] [Google Scholar]
- Hamrahian AH, Zhang JZ, Elkhairi FS, Prasad R, IsmailBeigi F. Activation of GLUT1 glucose transporter in response to inhibition of oxidative phosphorylation. Arch. Biochem. Biophys. 1999;368:375–379. doi: 10.1006/abbi.1999.1320. [DOI] [PubMed] [Google Scholar]
- Heineke D, Wildenberger K, Sonnewald U, Willmitzer L, Heldt HW. Accumulation of hexoses in leaf vacuoles - studies with transgenic tobacco plants expressing yeast-derived invertase in the cytosol, vacuole or apoplasm. Planta. 1994;194:29–33. [Google Scholar]
- Jaeger CH, III, Lindow SE, Miller W, Clark E, Firestone MK. Mapping of sugar and amino acid availability in soil around roots with bacterial sensors of sucrose and tryptophan. Appl. Environ. Microbiol. 1999;65:2685–2690. doi: 10.1128/aem.65.6.2685-2690.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali B, Suryanarayana D. Shift in the carbohydrate spectrum of root exudates of wheat in relation to its root-rot disease. Plant Soil. 1971;34:261–267. [Google Scholar]
- Javelle A, Thomas G, Marini AM, Kramer R, Merrick M. In vivo functional characterization of the Escherichia coli ammonium channel AmtB: evidence for metabolic coupling of AmtB to glutamine synthetase. Biochem. J. 2005;390:215–222. doi: 10.1042/BJ20042094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, Darrah P. Re-sorption of organic compounds by roots of Zea mays L. and its consequences in the rhizosphere. Plant Soil. 1993;153:47–59. [Google Scholar]
- Kaldenhoff R, Bertl A, Otto B, Moshelion M, Uehlein N. Characterization of plant aquaporins. Methods Enzymol. 2007;428:505–531. doi: 10.1016/S0076-6879(07)28028-0. [DOI] [PubMed] [Google Scholar]
- Kaper T, Lager I, Looger LL, Chermak D, Frommer WB. Fluorescence resonance energy transfer sensors for quantitative monitoring of pentose and disaccharide accumulation in bacteria. Biotechnol. Biofuels. 2008;1:11. doi: 10.1186/1754-6834-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CD. Effect of d-glucose and other sugars on transroot potential of Zea mays. J. Exp. Bot. 1977;28:903–908. [Google Scholar]
- Komor E, Tanner W. The hexose-proton cotransport system of Chlorella. pH-dependent change in Km values and translocation constants of the uptake system. J. Gen. Physiol. 1974;64:568–581. doi: 10.1085/jgp.64.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper I, Kravchenko LV, Bloemberg GV, Lugtenberg BJ. Pseudomonas putida strain PCL1444, selected for efficient root colonization and naphthalene degradation, effectively utilizes root exudate components. Mol. Plant-Microbe Interact. 2002;15:734–741. doi: 10.1094/MPMI.2002.15.7.734. [DOI] [PubMed] [Google Scholar]
- Lager I, Fehr M, Frommer WB, Lalonde S. Development of a fluorescent nanosensor for ribose. FEBS Lett. 2003;553:85–89. doi: 10.1016/s0014-5793(03)00976-1. [DOI] [PubMed] [Google Scholar]
- Lager I, Looger LL, Hilpert M, Lalonde S, Frommer WB. Conversion of a putative Agrobacterium sugar-binding protein into a FRET sensor with high selectivity for sucrose. J. Biol. Chem. 2006;281:30875–30883. doi: 10.1074/jbc.M605257200. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Wipf D, Frommer WB. Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu. Rev. Plant Biol. 2004;55:341–372. doi: 10.1146/annurev.arplant.55.031903.141758. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Ehrhardt DW, Frommer WB. Shining light on signaling and metabolic networks by genetically encoded biosensors. Curr. Opin. Plant Biol. 2005;8:574–581. doi: 10.1016/j.pbi.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl Acad. Sci. USA. 1998;95:6803–6808. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loqué D, Ludewig U, Yuan L, von Wirén N. Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol. 2005;137:671–680. doi: 10.1104/pp.104.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. Academic Press/Harcourt Brace & Co; London: 1996. [Google Scholar]
- Maynard JW, Lucas WJ. Sucrose and glucose uptake into Beta vulgaris leaf tissues: a case for general (apoplastic) retrieval systems. Plant Physiol. 1982;70:1436–1443. doi: 10.1104/pp.70.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Mühling KH, Schubert S, Mengel K. Mechanism of sugar retention by roots of intact maize and field bean plants. Plant Soil. 1993;156:99–102. [Google Scholar]
- Okumoto S, Looger LL, Micheva KD, Reimer RJ, Smith SJ, Frommer WB. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc. Natl Acad. Sci. USA. 2005;102:8740–8745. doi: 10.1073/pnas.0503274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumoto S, Takanaga H, Frommer WB. Tansley review: quantitative imaging for discovery and assembly of the metabo-regulome. New Phytol. 2008 doi: 10.1111/j.1469-8137.2008.02611.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peragine A, Yoshikawa M, Wu G, Albrecht HL, Poethig RS. SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes Dev. 2004;18:2368–2379. doi: 10.1101/gad.1231804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A, Gazdar C. Escherichia coli adenylate cyclase complex: regulation by the proton electrochemical gradient. Proc. Natl Acad. Sci. USA. 1979;76:1099–1103. doi: 10.1073/pnas.76.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992;11:4705–4713. doi: 10.1002/j.1460-2075.1992.tb05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, González M-C. Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. 2004;9:606–613. doi: 10.1016/j.tplants.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Saftner RA, Wyse RE. Alkali cation/sucrose co-transport in the root sink of sugar beet. Plant Physiol. 1980;66:884–889. doi: 10.1104/pp.66.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacke R, Schneider A, van der Graaff E, Fischer K, Catoni E, Desimone M, Frommer WB, Flügge UI, Kunze R. ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol. 2003;131:16–26. doi: 10.1104/pp.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F. Short-term measurements of the cytoplasmic pH of Chara corallina derived from the intracellular equilibration of 5,5-dimethyloxazolidine-2,4-dione (DMO) J. Exp. Bot. 1986;37:1733–1745. [Google Scholar]
- Stadler R, Wright KM, Lauterbach C, Amon G, Gahrtz M, Feuerstein A, Oparka KJ, Sauer N. Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel postphloem domain in roots. Plant J. 2005;41:319–331. doi: 10.1111/j.1365-313X.2004.02298.x. [DOI] [PubMed] [Google Scholar]
- Stolz J, Stadler R, Opekarova M, Sauer N. Functional reconstitution of the solubilized Arabidopsis thaliana STP1 monosaccharide-H+ symporter in lipid vesicles and purification of the histidine tagged protein from transgenic Saccharomyces cerevisiae. Plant J. 1994;6:225–233. doi: 10.1046/j.1365-313x.1994.6020225.x. [DOI] [PubMed] [Google Scholar]
- Stubbs VEC, Standing D, Knox OGG, Killham K, Bengough AG, Griffiths B. Root border cells take up and release glucose-C. Ann. Bot. 2004;93:221–224. doi: 10.1093/aob/mch019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanaga H, Chaudhuri B, Frommer WB. GLUT1 and GLUT9 as major contributors to glucose influx in HepG2 cells identified by a high sensitivity intramolecular FRET glucose sensor. Biochim. Biophys. Acta. 2008;1778:1091–1099. doi: 10.1016/j.bbamem.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam R, Saier MH., Jr Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol. Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer R, Matzeit V, Gronenborn B, Schell J, Steinbiss HH. A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res. 1987;15:5890. doi: 10.1093/nar/15.14.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TS, Bais HP, Grotewold E, Vivanco JM. Root exudation and rhizosphere biology. Plant Physiol. 2003;132:44–51. doi: 10.1104/pp.102.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller DM, Thomashow LS. Current challenges in introducing beneficial microorganisms into the rhizosphere. In: O’Gara F, Dowling DN, Boesten B, editors. Molecular Ecology of Rhizosphere Microorganisms. VCH; Weinheim, Germany: 1994. pp. 1–18. [Google Scholar]
- Williams LE, Lemoine R, Sauer N. Sugar transporters in higher plants - a diversity of roles and complex regulation. Trends Plant Sci. 2000;5:283–290. doi: 10.1016/s1360-1385(00)01681-2. [DOI] [PubMed] [Google Scholar]
- Wright KM, Oparka KJ. Metabolic inhibitors induce symplastic movement of solutes from the transport phloem of Arabidopsis roots. J. Exp. Bot. 1997;48:1807–1814. [Google Scholar]
- Zhou Y, Qu H, Dibley KE, Offler CE, Patrick JW. A suite of sucrose transporters expressed in coats of developing legume seeds includes novel pH-independent facilitators. Plant J. 2007;49:750–764. doi: 10.1111/j.1365-313X.2006.03000.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison of FRET sensor responses in various regions of the root tip.
Movie S1. Cellular expression of the sucrose nanosensor in root tips of Arabidopsis.
Figure S2. Glucose and sucrose response curves in the absence and presence of various inhibitors.
Figure S3. Effect of CCCP on Golgi movement.
Figure S4. [14C]-glucose uptake into intact seedlings.
Figure S5. Insensitivity of responses to tetraethylammonium.
Figure S6. Marker line coverage.
Table S1 Expression levels for the transporter genes as shown in Figure 9.
Table S2 Marker line coverage.
Appendix S1. Radioactive glucose uptake in intact seedlings of Arabidopsis thaliana.