Abstract
Chemotherapy-induced febrile neutropenia is costly in both financial and human terms. The associated costs can be reduced substantially through the development and implementation of national policies and locally agreed protocols for the prevention and management of febrile neutropenia. Patients, the NHS, healthcare professionals and the broader community all stand to benefit from a commitment to effective management of this common and predictable side effect of some chemotherapy regimens for early-stage breast cancer.
Keywords: cost, febrile neutropenia, prevention, treatment, protocols
Febrile neutropenia (FN) is a serious complication of chemotherapy for early-stage breast cancer, with significant morbidity and mortality, and important implications for patients and healthcare resources.
It is estimated that 95% of women diagnosed with breast cancer in the UK have early-stage disease (43 000 women/year) (CRUK, 2009), 13 000 (30%) of whom will be node-positive (Verschraegen et al, 2005). Around 9000 women per year with node-positive early-stage breast cancer receive chemotherapy (CRUK, 2009). On the basis of the regimens used and their reported FN rates, the incidence of FN among women receiving chemotherapy for node-positive early-stage breast cancer is estimated at around 16% (Poole et al, 2006; Roché et al, 2006; Ali et al, 2008; Head et al, 2008; Scaife et al, 2008; Zaman et al, 2008). Therefore, it is conservatively estimated that more than 1000 women each year receiving chemotherapy for node-positive breast cancer will have an episode of FN (Poole et al, 2006; Roché et al, 2006; Ali et al, 2008; Head et al, 2008; Scaife et al, 2008; Zaman et al, 2008).
FN requires hospitalisation and treatment with intravenous antibiotics, and has a negative impact on patients’ quality of life (Moore and Crom, 2006). Furthermore, the development of FN may lead to a decision to reduce or delay the patient's subsequent chemotherapy dose, which can undermine treatment outcomes, including overall survival, particularly in the adjuvant setting (Bonadonna et al, 1995; Lyman et al, 2005; Chirivella et al, 2006).
The effective management of FN embraces both prevention of the condition with prophylactic measures, such as the use of granulocyte colony-stimulating factors (G-CSF) and/or antibiotics, and the appropriate management of febrile neutropenic events, notably neutropenic sepsis.
Other articles in this supplement look in detail at FN prevention and management (Kelly and Wheatley, 2009; Cullen and Baijal, 2009). In this article, we consider the impact of prophylactic and management interventions on patients, the NHS, healthcare professionals and the broader community.
Impact of FN prophylaxis
If a patient develops FN, the direct and indirect costs to the individual, the NHS and the economy are substantial. The costs derive from a range of factors, including hospitalisation for treatment of FN, significant morbidity and mortality, financial losses for patients and their families/carers and reduced health-related quality of life (Moore and Crom, 2006; Gridelli et al, 2007). These costs also undermine public confidence in cancer services (Figure 1).
Figure 1.
National headlines reflect how public confidence in cancer services is undermined.
Furthermore, the loss of productivity associated with hospitalisation and morbidity has a detrimental impact on the economy.
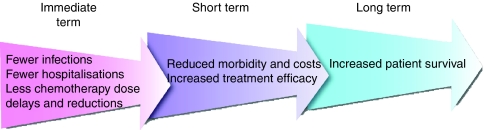
By preventing FN through the use of appropriate prophylactic measures, some of these costs may be avoided (Figure 2), leading to improved quality of life and treatment efficacy for patients, reduced healthcare costs and greater predictability of care needs (Kuderer et al, 2006).
Figure 2.
Short-term and long-term effects of FN prevention.
As discussed by Trueman (2009) in this supplement, analysis of the cost effectiveness of prophylaxis with G-CSF is fraught with difficulty, because of a lack of consistency across clinical trials and because of the problems faced when transferring pharmacoeconomic considerations from one healthcare system to another. However, various economic models suggest that primary prophylaxis with G-CSF may be cost-effective when the risk of FN exceeds specific thresholds, for example, 20% (Lyman et al, 1998), 16% (Eldar-Lissai et al, 2008) or 18% (Dale et al, 2006).
Impact of management of FN events
Inadequacies in the management of FN in the UK, and several key organisational and clinical failures (Table 1), were highlighted recently by the National Confidential Enquiry into Patient Outcome and Death (NCEPOD, 2008). Such inadequacies have a negative impact on patients, and on the perception of cancer care by the broader NHS and the public. This perception leads, in turn, to reduced confidence in the NHS, which, coupled with the added stress caused by potentially avoidable additional hospital visits and extended hospitalisation, may affect patients’ willingness to undergo further treatment (Fortner et al, 2002).
Table 1. Failures in the management of patients admitted with neutropenic sepsis (NCEPOD, 2008).
| Organisational failures | Clinical failures |
|---|---|
| Lack of treatment policy in emergency departments | Delayed admission |
| Clinician unaware of treatment policy | Failure of junior doctors to make the diagnosis |
| Patient managed in an inappropriate care setting | Lack of awareness that patients without a fever may still have FN |
| Only occasional oncology visit to cancer unit in a district general hospital | Lack of early assessment by senior staff Delayed resuscitation Delayed prescription and administration of antibiotics Failure to adhere to local antibiotics policy Delayed transfer to intensive care |
In response to the findings of NCEPOD and a report from the National Cancer Peer Review Network (NCPRN, 2008), the National Chemotherapy Advisory Group has issued recommendations for improving the management of FN (NCAG, 2009). The advice is aimed not only at chemotherapy providers but also at any hospital with acute facilities to which patients with possible chemotherapy side effects may present. The implementation of the recommendations is expected to benefit patients, the NHS and healthcare professionals.
Benefits for patients
Effective management of FN events may have a significant impact on patients’ quality of life, morbidity, mortality, long-term survival and finances.
The development of FN has been shown to correlate with lower quality-of-life scores (Okon et al, 2002) and an increase in the incidence and severity of chemotherapy-related side effects such as mucositis, abdominal pain and diarrhoea, anorexia and fatigue (Glaspy et al, 2001).
The mortality rates associated with FN range from 2 to 21% (Smith et al, 2006; Herbst et al, 2008) – the higher rates are often seen in patients with comorbidities, which may be age-related, or in patients with poor performance status, including those with advanced cancer and undergoing palliative chemotherapy (Lyman et al, 2005).
FN often results in chemotherapy dose reductions and dose delays (Leonard et al, 2003), and the resulting reduction in chemotherapy dose intensity can have a significant negative impact on clinical outcome, notably survival (Bonadonna et al, 1995; Chirivella et al, 2006). Indeed, patients who receive less than 65% of their planned dose have been shown to have survival rates similar to those who receive no chemotherapy at all (Bonadonna et al, 1995). Such dose reductions are common in the absence of clear local policy on primary prevention of neutropenia in patients undergoing treatment with curative intent. However, dose reductions (and lower starting doses) may be advisable in certain high-risk patients, including those with comorbidities or poor performance status and those undergoing palliative chemotherapy, who are also susceptible to the non-haematological toxicities associated with chemotherapy (Lyman et al, 2005; NCEPOD, 2008).
FN disrupts normal life activities such as childcare and employment (Moore and Crom, 2006), and thus has financial and social implications for patients and their families.
Benefits for the NHS
FN imposes a significant burden on NHS finances and resources – a single episode is estimated to cost the NHS £3582 (Holmes et al, 2004). The major economic impact is related to hospitalisation; the average length of hospital stay is 6–8 days (Kuderer et al, 2006). Hospitalisation puts patients at risk of developing further complications, such as hospital-acquired infections and thromboembolic events, which add to the overall cost of FN.
Inadequate admission pathways and a lack of management protocols lead to inappropriate placement of patients, inefficient and inappropriate use of healthcare resources, treatment delays and prolonged hospitalisation (NCEPOD, 2008). Implementation of network policies, and locally agreed hospital admission pathways, together with the availability of clear management protocols, should lead to patients with FN being admitted efficiently, under the care of the appropriate healthcare professional and receiving the appropriate treatment in a timely manner (NCEPOD, 2008). Such practices will reduce healthcare costs, encourage appropriate and efficient use of NHS resources and reduce the costs associated with prolonged stay, morbidity and mortality.
Benefits for healthcare professionals
As a result of locally agreed and implemented policies for the management of FN, healthcare professionals will become more confident in their ability to manage patients with the condition, and the public's confidence in healthcare services will be enhanced. Achieving this goal will require education of all healthcare professionals, including those not directly involved in frontline cancer care, both at a national and local level (NCEPOD, 2008). It will also require a regular, systematic audit of the complications of chemotherapy, with review at a local and network level forming part of the appraisal and education of healthcare professionals (NCEPOD, 2008).
Impact of change
Several groups have conducted audits in patients receiving chemotherapy, looking at the rates of FN, dose delays and dose reductions, and the effects of adding prophylactic G-CSF and/or antibiotics (Ali et al, 2008; Head et al, 2008; Scaife et al, 2008). The results of three audits of patients receiving FEC-T (fluorouracil, epirubicin and cyclophosphamide followed by docetaxel) or TAC (docetaxel, doxorubicin and cyclophosphamide) were presented at the 2008 National Cancer Research Institute Cancer Conference, which took place in Birmingham. The findings are summarised in Tables 2,3,4. All three groups plan to re-audit their practice following the introduction of primary prophylaxis with G-CSF with or without antibiotics in the same groups of patients. The findings of these re-audits may prove useful in guiding the management of FN in the future.
Table 2. Rates of FN associated with the use of adjuvant FEC-T chemotherapy in high-risk node-positive patients with early breast cancer: a UK perspective (Head et al, 2008).
| Data audited | Patients audited | Audit findings | Recommendation | Plan to re-audit |
|---|---|---|---|---|
| FN rate | 137 | 25% | Primary G-CSF should be used throughout treatment with FEC-T | Following introduction of primary prophylaxis with G-CSF |
| Dose delays | 25/137 (18.2%) | |||
| Dose reductions | 27/137 (19.7%) | |||
| Use of primary G-CSF | 30 patients | |||
| FN rate after primary G-CSF | 2/30 (8.5%) | |||
| Use of secondary G-CSF | 25% | |||
| FN rate after secondary G-CSF | 0 |
Table 3. Experience of FN and secondary G-CSF prophylaxis during FEC-T chemotherapy in the Merseyside and Cheshire Cancer Network (Ali et al, 2008).
| Data audited | Patients audited | Audit findings | Recommendation | Plan to re-audit |
|---|---|---|---|---|
| FN rate | 123 | 33/123 (27%) | Primary or secondary prophylaxis may be indicated with FEC-T | Following the introduction of primary prophylaxis with antibiotics |
| Cycles complicated by FN | 39/728 (5.36%) | |||
| Use of primary G-CSF | 3 patients | |||
| FN after primary G-CSF | 2 patients | |||
| Use of primary prophylactic antibiotics | 3 patients | |||
| FN after primary prophylactic antibiotics | 0 patients | |||
| Use of secondary G-CSF | 24 patients | |||
| Episodes of FN after secondary G-CSF | 2/24 (8%) |
Table 4. FN in patients receiving TAC chemotherapy for breast cancer (Scaife et al, 2008).
| Data audited | Patients audited | Audit findings | Recommendation | Plan to re-audit |
|---|---|---|---|---|
| One or more episodes of FN | 49 | 16/49 (33%) | Quinolone antibiotics should be commenced 5 days post-chemotherapy | Re-audit following the introduction of primary prophylaxis with both G-CSF and antibiotics |
| Episodes of FN in cycle 1 | 8/16 (50%) | |||
| Dose delay or reduction | 13/16 (81%) | |||
| Death due to sepsis | 1 patient | |||
| Median duration of inpatient stay for FN | 4 days |
Conclusion
FN is a significant complication of chemotherapy treatment from the point of view of patients, healthcare professionals and the NHS. Reports of serious inadequacies in the management of FN in the UK (NCEPOD, 2008; NCPRN, 2008) have led to recommendations (NCAG, 2009) for robust systems to be put in place to admit and manage patients with FN. The implementation and audit of such systems will have an impact not only on individual patients but also on healthcare professionals and the wider NHS.
Acknowledgments
My thanks to Succinct Healthcare Communications and Consultancy for editorial support. This supplement was sponsored by an educational grant from sanofi-aventis. The company has checked the factual and medical content but final editorial control resides with the author and editor.
Footnotes
Conflict of interest
D Krell has declared no financial interests. A Jones has received consulting fees from sanofi-aventis and lecture fees from Roche.
References
- Ali Z, O’Reilly S, Zahoor T, Schofield P, Malik Z (2008) Experience of febrile neutropaenia and secondary G-CSF prophylaxis during FEC_D chemotherapy in Merseyside and Cheshire Cancer Network. National Cancer Research Institute Cancer Conference; Abstract B67
- Bonadonna G, Valagussa P, Moliternni A, Zambetti M, Brambilla C (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node positive breast cancer: the results of 20 years of follow-up. N Engl J Med 332: 901–906 [DOI] [PubMed] [Google Scholar]
- Cancer Research UK (2009) UK breast cancer incidence statistics. Available at: http://info.cancerresearchuk.org/cancerstats/types/breast/incidence/ (accessed May 2009)
- Chirivella I, Bermejo B, Insa A, Perez-Fidalgo A, Magro A, Rosello S, Garcia-Garre E, Martin P, Bosch A, Lluch A (2006) Impact of chemotherapy dose-related factors on survival in breast cancer patients treated with adjuvant anthracycline-based chemotherapy. J Clin Oncol 24: Abstract 668 [Google Scholar]
- Cullen M, Baijal S (2009) Prevention of febrile neutropenia: use of prophylactic antibiotics. Br J Cancer 101(Suppl 1): S11–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale DC, Cosler LE, Wolff DA, Culakova E, Poniwierski MS, Crawford J, Lyman GH, for the ANC study group (2006) Economic analysis of prophylactic granulocyte colony-stimulating factor (G-CSF) use based on a risk model for neutropenic complications in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 24: Abstract 6107 [Google Scholar]
- Eldar-Lissai A, Cosler LE, Culakova E, Lyman GH (2008) Economic analysis of prophylactic pegfilgrastim in adult cancer patients receiving chemotherapy. Value Health 11: 172–179 [DOI] [PubMed] [Google Scholar]
- Fortner BV, Tauer K, Zhu L, Okon TA, Moore K, Templeton D, Schwartzberg L (2002) Medical visits for chemotherapy and chemotherapy-induced neutropenia: a survey of the impact on patient time and activities. BMC Cancer 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaspy J, Hackett J, Flyer P, Dunford D, Liang B (2001) Febrile neutropaenia is associated with an increase in the incidence, duration and severity of chemotherapy toxicities. American Society of Hematology Annual Meeting
- Gridelli C, Aapro MS, Barni S, Beretta GD, Colucci G, Daniele B, Del Mastro L, Di Maio M, De Petris L, Perrone F, Thatcher N, De Marinis F (2007) Role of colony stimulating factors (CSFs) in solid tumours: results of an expert panel. Crit Rev Oncol Hematol 63: 53–64 [DOI] [PubMed] [Google Scholar]
- Head J, Archer C, Harper-Wynne C, Sinha R, Ring A, Banner R, Sutherland S, Johnston S (2008) Rates of neutrpaenic sepsis with the use of adjuvant FEC100-Docetaxel (FEC100-T) chemotherapy in high-risk node-positive patients with early breast cancer; a UK perspective. National Cancer Research Institute Cancer Conference; Abstract B64
- Herbst C, Naumann F, Kruse E, Knauel I, Schulz H, Bohlius J, Engert A (2008) Prophylactic antibiotics and G-CSF for the prevention of infections and improvement of survival in cancer patients undergoing chemotherapy (Protocol). Cochrane Database Syst Rev 4: CD007107. [DOI] [PubMed] [Google Scholar]
- Holmes J, Dunlop D, Hemmett L, Sharplin P, Bose U (2004) A cost-effectiveness analysis of docetaxel in the second-line treatment of non-small cell lung cancer. Pharmacoeconomics 22: 581–589 [DOI] [PubMed] [Google Scholar]
- Kelly S, Wheatley D (2009) Prevention of febrile neutropenia: use of granulocyte colony-stimulating factors. Br J Cancer 101(Suppl 1): S6–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderer NM, Dale DS, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106: 2258–2266 [DOI] [PubMed] [Google Scholar]
- Leonard RCF, Miles D, Thomas R, on behalf of the UK Breast Cancer Neutropenia Audit Group (2003) Impact of neutropenia on delivering planned adjuvant chemotherapy: UK audit of primary breast cancer patients. Br J Cancer 89: 2062–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman GH, Kuderer N, Green J, Balducci L (1998) The economics of febrile neutropaenia: implications for the use of colony-stimulating factors. Eur J Cancer 34: 1857–1864 [DOI] [PubMed] [Google Scholar]
- Lyman GH, Lyman CH, Agboola O, for the ANC study group (2005) Risk models for predicting chemotherapy-induced neutropenia. Oncologist 10: 427–437 [DOI] [PubMed] [Google Scholar]
- Moore K, Crom D (2006) Hematopoietic support with moderately myelosuppressive chemotherapy regimens: a nursing perspective. Clin J Oncol Nurs 10: 383–388 [DOI] [PubMed] [Google Scholar]
- National Cancer Peer Review Programme 2004–2007 (2008) National Report: an Overview of the Findings from the Second National Round of Peer Reviews of Cancer Services in England. NCPRN: London [Google Scholar]
- National Chemotherapy Advisory Group (2009) Chemotherapy Services in England: Ensuring Quality and Safety. NCAG: London [Google Scholar]
- National Confidential Enquiry into Patient Outcome and Death (2008) For Better, for Worse?. NCEPOD: London [Google Scholar]
- Okon TA, Fortner BV, Schwartzberg I, Tauer KT, Durrence H, Kovacs A, Wheetly K, Woods C, Taylor D, Houts AC (2002) Quality of life (QOL) in patients with grade IV chemotherapy induced neutropenia (CIN). Proc Am Soc Clin Oncol 21: Abstract 2920 [Google Scholar]
- Poole CJ, Earl HM, Hiller L, Dunn JA, Bathers S, Grieve RJ, Spooner DA, Agrawal RK, Fernando IN, Brunt AM, O’Reilly SM, Crawford SM, Rea DW, Simmonds P, Mansi JL, Stanley A, Harvey P, McAdam K, Foster L, Leonard RC, Twelves CJ, NEAT Investigators and the SCTBG (2006) Epirubicin and cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy for early breast cancer. N Engl J Med 355: 1851–1862 [DOI] [PubMed] [Google Scholar]
- Roché H, Fumoleau P, Spielmann M, Canon JL, Delozier T, Serin D, Symann M, Kerbrat P, Soulié P, Eichler F, Viens P, Monnier A, Vindevoghel A, Campone M, Goudier MJ, Bonneterre J, Ferrero JM, Martin AL, Genève J, Asselain B (2006) Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol 24: 5664–5671 [DOI] [PubMed] [Google Scholar]
- Scaife J, Matthews R, Jenkins P (2008) Febrile neutropaenia in patients receiving TAC chemotherapy for breast cancer. National Cancer Research Institute Cancer Conference; Abstract BOA14
- Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC (2006) 2006 Update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol 24: 3187–3205 [DOI] [PubMed] [Google Scholar]
- Trueman P (2009) Prophylactic G-CSF in patients with early-stage breast cancer: a health economic review. Br J Cancer 101(Suppl 1): S15–S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschraegen C, Vinh-Hung V, Cserni G, Gordon R, Royce ME, Vlastos G, Tai P, Storme G (2005) Modeling the effect of tumour size in early breast cancer. Ann Surg 241: 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman AAL, McGhie A, Shaikh G, Canney P, Fraser J (2008) DGH Experience of Adjuvant FE[100]C-D Chemotherapy in Node Positive Breast Cancer. NHS Greater Glasgow and Clyde: Glasgow [Google Scholar]