Abstract
BACKGROUND:
The fraction of total plasma vitamin B12 bound to transcobalamin (holoTC/B12 ratio) may reflect tissue levels of the vitamin, but its clinical relevance is unclear.
METHODS:
We assessed associations between cognitive function and total B12, holoTC, and holoTC/B12 ratio in a cohort of elderly Latinos (n = 1089, age 60–101 years). We assessed cognitive function using the Modified Mini-Mental State Examination (3MSE) and a delayed recall test; we diagnosed clinical cognitive impairment by neuropsychological and clinical exam with expert adjudication; and we assessed depressive symptoms using the Center for Epidemiological Studies Depression Scale (CES-D). We measured total B12 and holoTC using radioassays.
RESULTS:
HoloTC/B12 ratio was directly associated with 3MSE score (P = 0.026) but not delayed recall score. Interactions between holoTC/B12 and CES-D score were observed for 3MSE (P = 0.026) and delayed recall scores (P = 0.013) such that associations between the ratio and cognitive function scores were confined to individuals with CES-D ≥16. For individuals with CES-D ≥16, the odds ratio for clinical cognitive impairment for the lowest holoTC/B12 tertile was 3.6 (95% CI 1.2–11.2) compared with the highest tertile (P = 0.03). We observed no associations between cognitive function and total B12 or holoTC alone, except between holoTC and 3MSE score (P = 0.021), and no interactions between holoTC or total B12 and CES-D score on cognitive function.
CONCLUSIONS:
HoloTC/B12 ratio is associated with cognitive function in elderly Latinos with depressive symptoms and may better reflect the adequacy of B12 for nervous system function than either holoTC or total B12 alone.
The clinical manifestations of vitamin B12 (B12)6 deficiency are both hematologic and neurological. Hematologic consequences occur most commonly in severely deficient individuals (1) and present primarily as macrocytic (megaloblastic) anemia. In contrast, the neurological consequences of B12 deficiency are many and varied, including subacute combined degeneration of the spinal cord, peripheral neuropathy, depression, cognitive impairment, and dementia (2, 3). Neurological complications may occur in the absence of hematologic complications (4) and may become permanent if diagnosis and treatment are delayed (5).
Vitamin B12 is transported in the circulation bound to 2 specific proteins, haptocorrin and transcobalamin (TC). The majority of total plasma B12 (approximately 70%–80%) is bound to haptocorrin, but no haptocorrin receptor has been identified on extrahepatic tissues. Vitamin B12 bound to TC, or holoTC (previously referred to as holoTC II), represents the remaining approximately 20%–30% of total plasma B12 and is responsible for cellular delivery of B12 through receptor-mediated endocytosis involving a specific TC receptor found on all tissues. Thus, TC is regarded as the primary circulating B12 binding protein for cellular delivery (6).
Although total plasma B12 is currently the standard clinical screening test for B12 deficiency, B12-responsive metabolic (7, 8) and neurological (4) abnormalities may be present when total B12 is within the reference interval (>148 pmol/L). Plasma levels of methylmalonic acid and homocysteine, both metabolic markers of B12 status, are considered to be more sensitive indicators than total plasma B12 alone. However, these metabolites are increased in renal insufficiency (8), and homocysteine concentration is also increased as the result of deficiencies of folate and vitamin B6 and in hypothyroidism and other conditions (9). HoloTC concentration is a useful measure of B12 status (10-13) and may be used most effectively in combination with total B12 (14). What has not been considered in the assessment of B12 status is the significance of the percentage of total B12 bound to TC (holoTC/B12 ratio). Although this typically ranges from 20% to 30%, it can vary among individuals from <10% to >70% (10). We hypothesized that the ratio may supply important information about the availability of B12 for delivery to the tissues and possibly provide a sensitive measure of B12 status. To date, there have been no reports on the clinical relevance of holoTC/B12 in assessing B12 status.
In this study, we assessed and compared the associations between cognitive function and holoTC, total B12, and the holoTC/B12 ratio in a representative population of community-dwelling older Latinos.
PARTICIPANTS AND METHODS
PARTICIPANTS
We carried out this analysis in a cohort of community-dwelling elderly Latinos (age 60–101 years) participating in the Sacramento Area Latino Study on Aging (SALSA) (15-18). Participants resided in Sacramento, CA, and surrounding Northern California communities. The cohort of 1789 individuals was recruited over a period of 18 months beginning in February 1998; details of sampling and recruitment have been published (15, 16). Of the original 1789 participants, 1089 had valid measurements of both holoTC and total B12 and are included in this study. Participant recruitment and study procedures for SALSA were approved by the Human Subjects Review Committee at the University of California, Davis, and written informed consent was obtained from all study participants.
SAMPLE COLLECTION AND ANALYSES
Blood samples were collected from fasting participants during home visits and kept on ice until processing within 4 h at the University of California Davis Medical Center Clinical Laboratory. Plasma and serum were isolated and stored at −80 °C until analysis. Total plasma B12 was measured by radioligand binding assay (Bio-Rad Diagnostics), and plasma holoTC was measured by monoclonal antibody capture of the holoTC followed by radioligand binding assay (Axis-Shield) (12). HoloTC/B12 ratio was calculated and expressed as a percentage. Erythrocyte folate was measured by automated chemiluminescence assay (ACS 180; Chiron Diagnostics [now Siemens Diagnostics]). Total plasma homocysteine was determined by HPLC with fluorescence detection (19). Serum creatinine was analyzed by the Jaffe rate reaction method using a Synchron LX20 instrument (Beckman Coulter). CVs for each of the assays have been determined: total B12, 4.7%; holoTC, 5.7%; erythrocyte folate, 10%; total homocysteine, 4.2%; and creatinine 3.3%. Analyte cutoff values were based on standard clinical reference intervals or literature reports: total B12 <148 pmol/L (standard clinical reference interval); holoTC <35 pmol/L (13); erythrocyte folate <160 μg/L (standard clinical reference interval); homocysteine >13 μmol/L (20); creatinine >13 mg/L (standard clinical reference interval).
ASSESSMENT OF COGNITIVE FUNCTION AND DEPRESSIVE SYMPTOMS
We assessed global cognitive function, including evaluations of memory, orientation, attention, and language, using the Modified Mini-Mental State Examination (3MSE) on a scale of 0 to 100 points (21). A 3MSE score of ≤78 approximates the 20th percentile of scores for the SALSA population. We assessed the ability to learn and recall verbal information using a delayed recall test based on a 15-point scale (22). Clinical cognitive impairment was defined as either demented or cognitively impaired but not demented (CIND) and was diagnosed based on neuropsychological test scores, mental status examination, the Informant Questionnaire on Cognitive Decline in the Elderly (23), medical history, and neurologic examination. Dementia was diagnosed according to American Psychiatric Association (24) and California Alzheimer Disease Diagnostic and Treatment criteria (25). CIND was diagnosed if the person had clinically significant impairment in ≥1 cognitive domain but did not meet diagnostic thresholds for dementia because of insufficient cognitive decline or functional independence. Complete details of the diagnosis of dementia and CIND have been described (16). We assessed depressive symptoms using the Center for Epidemiological Studies Depression Scale (CES-D) (26). Total CES-D scores range from 0 to 60, with a score of ≥16 indicating a significant level of depressive symptoms (26).
ASCERTAINMENT OF DIABETES AND STROKE
We ascertained prevalence of diabetes and stroke as described (27). Briefly, participants who met one or more of the following criteria were characterized as having diabetes: (a) fasting plasma glucose ≥126 mg/dL (≥7.0 mmol/L); (b) antidiabetic medication use; (c) self-report or physician's diagnosis of diabetes. Stroke was determined by self-report in response to direct questioning. For those participants reporting a history of stroke, we confirmed 85% of reports by medical chart review; medical charts for the other 15% were unavailable for review.
STATISTICAL ANALYSES
We used multiple linear regression analyses to build statistical models to describe and compare the associations between total B12, holoTC, and holoTC/B12 ratio (independent variables) and 3MSE and delayed recall scores (dependent variables). We built a series of 3 regression models for each combination of independent and dependent variables as follows: model 1, univariate analysis; model 2, model 1 controlling for confounding by age, sex, education, diabetes, stroke, homocysteine, and creatinine, the latter variable as an indicator of renal function known to affect plasma holoTC and total B12 levels (13, 28); and model 3, model 2 with additional controlling for confounding by CES-D score (<16 or ≥16). We assessed interactions between CES-D score (<16 or ≥16) and total B12, holoTC, and holoTC/B12 ratio on 3MSE and delayed recall scores by 2-factor ANOVA. We defined an indicator variable for tertiles of holoTC/B12 ratio and then compared least square mean 3MSE and delayed recall scores, adjusted for age, sex, education, stroke, diabetes, homocysteine, and creatinine, using the Tukey-Kramer test among the holoTC/B12 ratio tertiles for individuals with low and high CES-D scores (<16 or ≥16). We used logistic regression to evaluate odds ratios (ORs) as indicators of the strength and direction of the relationship between clinical diagnosis of cognitive impairment and tertiles of total B12, holoTC, and holoTC/B12. We assessed interactions between the 3 B12 status indicators and depressive symptom score on the ORs for clinical cognitive impairment by including factors of total B12, holoTC, and holoTC/B12 times CES-D score as separate independent variables in the logistic regression analyses. Cases of dementia and CIND were combined into one group and designated as “clinical cognitive impairment” because the numbers of cases within the dementia and CIND groups were deemed too few to permit evaluation of associations within each diagnosis separately. We determined ORs (95% CIs) in adjusted models controlling for potential confounding by age, sex, education, diabetes, stroke, homocysteine, and creatinine for all participants and for participants divided according to CES-D score (<16 or ≥16). The statistical analyses were carried out using Statview for Macintosh and Windows (version 5.0.1; Abacus Concepts). Statistical significance was defined as P < 0.05.
Results
The characteristics of the study sample are presented in Table 1. The study sample, which includes all participants with valid measures of both holoTC and total B12 (n = 1089), is closely comparable to the entire baseline cohort (n = 1789) with respect to demographic, biochemical, and neuropsychological variables (17, 18). No differences in age, sex distribution, total B12, holoTC, RBC folate, CES-D scores, or prevalence of clinical cognitive impairment (dementia and CIND), diabetes, and stroke were noted between the entire baseline cohort and our subsample. The baseline cohort had a slightly lower median education level (6.0 vs 7.5 years), a slightly lower percentage of individuals with creatinine >13 mg/L (5.7% vs 6.8%), and slightly higher percentages of individuals with 3MSE score ≤78 (22% vs 18%) and delayed recall score ≤6 (25% vs 23%).
Table 1.
SALSA study sample characteristics.a
| n | Value | Percentage abnormal (cutoff value) |
|
|---|---|---|---|
| Sex, % female | 1089 | 59 | — |
| Age, years | 1089 | 70 (60–101) | — |
| Education, years | 1083 | 7.5 (0–25) | — |
| Total B12, pmol/L | 1089 | 298 (16–1453) | 6.5 (<148) |
| HoloTC, pmol/L | 1089 | 76 (3–160) | 8.0 (<35) |
| HoloTC/B12 ratio, % | 1089 | 25.0 (2.5–75.2) | — |
| Erythrocyte folate, μg/L | 1020 | 508 (50–900) | 0.7 (<160) |
| Total homocysteine, μmol/L | 1087 | 9.8 (4.4–129) | 16.9 (>13.0) |
| Creatinine, mg/L | 1083 | 8.0 (3.0–102) | 5.7 (>13) |
| Diabetes, % | 1089 | 33 | — |
| Stroke, % | 1089 | 9.4 | — |
| CES-D score, 0–60 | 1040 | 6 (0–54) | 25 (≥16) |
| 3MSE score, 0–100 | 1071 | 89 (0–100) | 22 (≤78) |
| Delayed recall score, 0–15 | 1073 | 9 (0–15) | 23 (≤6) |
| Clinical cognitive impairment, % | 1089 | 7.9 | — |
Data are median (range) unless noted otherwise. Clinical cognitive impairment includes participants with a diagnosis of dementia or cognitively impaired but not demented (CIND). Participants with dementia diagnosis, 4.3%; CIND diagnosis, 3.6%.
Multiple regression analysis models for 3MSE score are summarized in Table 2. In the univariate model (model 1), neither total B12 nor holoTC was associated with 3MSE score. In contrast, holoTC/B12 ratio was associated with 3MSE score (P = 0.001). After controlling for confounding by age, sex, education, diabetes, stroke, homocysteine, and creatinine (model 2), holoTC/B12 remained associated with 3MSE score (P = 0.046), and this association stayed significant with additional controlling for depressive symptom score (CES-D <16 or ≥16) (model 3) (P = 0.026). HoloTC, but not total B12, became associated with 3MSE score after controlling for all confounding variables (model 3) (P = 0.021). With respect to the depressive symptom score itself, after controlling for confounding by age, sex, education, diabetes, stroke, homocysteine, and creatinine, mean (SD) 3MSE score was significantly lower in those participants with CES-D ≥16 than in those with CES-D <16 [82.9 (12.4) vs 88.1 (10.0); P < 0.001]. The strength of the association between CES-D score and 3MSE score was not significantly affected by addition of total B12, holoTC, or holoTC/B12 to the model.
Table 2.
Multiple linear regression models for global cognitive function score (3MSE).a
| Independent variable |
R2 | Coefficient (SE) |
P | R2 | Coefficient (SE) |
P | R2 | Coefficient (SE) |
P |
|---|---|---|---|---|---|---|---|---|---|
| Total B12 | 0.001 | −0.002 (0.003) | NS | 0.316 | −0.002 (0.003) | NS | 0.316 | 0.001 (0.002) | NS |
| HoloTC | 0.002 | 0.020 (0.013) | NS | 0.316 | 0.006 (0.011) | NS | 0.320 | 0.020 (0.009) | 0.021 |
| HoloTC/B12 ratio | 0.010 | 15.2 (4.60) | 0.001 | 0.318 | 7.84 (3.92) | 0.046 | 0.319 | 6.87 (3.07) | 0.026 |
Model 1, univariate analysis; model 2, model 1 plus age, sex, years of education, diabetes, stroke, homocysteine, and creatinine; model 3, model 2 plus CES-D score (< or ≥16). R2 values are for the entire model including the B12 status measure and all covariates. Coefficients (SE) and P values are for each B12 status measure within the model. Sample sizes are smaller in models 2 and 3 due to missing values among the covariates. NS, not significant (P > 0.05).
Multiple regression analysis models for delayed recall score are summarized in Table 3. None of the B12 status measures was associated with delayed recall score in any of the models. With respect to depressive symptom score, after controlling for confounding by age, sex, education, diabetes, stroke, homocysteine, and creatinine, mean delayed recall score was significantly lower in those participants with CES-D ≥16 than in those with CES-D <16 [8.0 (3.0) vs 9.0 (2.8); P < 0.001]. This association between CES-D score and delayed recall score was not significantly affected by addition of total B12, holoTC, or holoTC/B12 to the model.
Table 3.
Multiple linear regression models for delayed recall score.a
| Independent variable |
R2 | Coefficient (SE) |
P | R2 | Coefficient (SE) |
P | R2 | Coefficient (SE) |
P |
|---|---|---|---|---|---|---|---|---|---|
| Total B12 | <0.001 | 0.0003 (0.001) | NS | 0.316 | −0.001 (0.001) | NS | 0.276 | −0.001 (0.001) | NS |
| HoloTC | 0.003 | 0.005 (0.003) | NS | 0.315 | 0.0001 (0.002) | NS | 0.276 | 0.001 (0.002) | NS |
| HoloTC/B12 | 0.003 | 7.97 (0.29) | NS | 0.316 | 1.24 (0.88) | NS | 0.276 | 0.921 (0.85) | NS |
Model 1, univariate analysis; model 2, model 1 plus age, sex, years of education, diabetes, stroke, homocysteine, and creatinine; model 3, model 2 plus CES-D score (< or ≥16). R2 values are for the entire model including the B12 status measure and all covariates. Coefficients (SE) and P values are for each B12 status measure within the model. Sample sizes are smaller in models 2 and 3 due to missing values among the covariates. NS, not significant (P > 0.05).
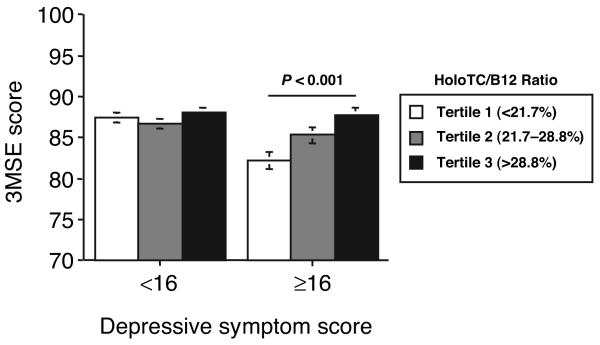
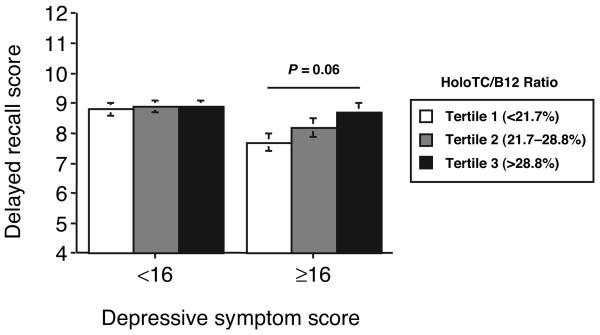
Two-factor ANOVA revealed significant interactions between holoTC/B12 and depressive symptom score on both the 3MSE (P = 0.026) and delayed recall (P = 0.013) tests. By multiple regression analysis, we then assessed separately the associations between holoTC/B12 and 3MSE and delayed recall scores for participants with low and high CES-D scores (<16 and ≥16), controlling for confounding by age, sex, education, diabetes, stroke, homocysteine, and creatinine. HoloTC/B12 was associated with 3MSE score in those participants with high CES-D scores [coefficient (SE) 20.7 (7.5); P = 0.006], but not in those with low CES-D scores [2.0 (3.3); not significant]. Likewise, holoTC/B12 was associated with delayed recall score in those participants with high CES-D scores [4.6 (1.9); P = 0.016], but not in those with low CES-D scores [−0.17 (0.95); not significant]. Secondary analyses comparing mean 3MSE and delayed recall scores among tertiles of holoTC/B12 are summarized in Figs. 1 and 2. For participants with high CES-D scores, mean 3MSE score was 5.6 points higher (P < 0.001) and mean delayed recall score was 1.1 point higher (P = 0.06) in the highest holoTC/B12 ratio tertile compared with the lowest tertile. No significant interactions were observed between CES-D score and either holoTC or total B12 alone with respect to 3MSE or delayed recall scores (data not shown).
Fig. 1. Mean (SE) scores (0–100 scale) on the 3MSE for participants divided by depressive symptom score (CES-D score < or ≥16) and holoTC/B12 tertile.
Scores are adjusted for age, sex, education, stroke, diabetes, homocysteine, and creatinine. Differences among the tertiles were assessed by Tukey-Kramer multiple-comparisons test. Sample sizes for low and high CES-D score groups were n = 771 and n = 251, respectively.
Fig. 2. Mean (SE) scores (0–15 scale) on the delayed recall test for participants divided by depressive symptom score (CES-D score < or ≥16) and holoTC/B12 tertile.
Scores are adjusted for age, sex, education, stroke, diabetes, homocysteine, and creatinine. Differences among the tertiles were assessed by Tukey-Kramer multiple-comparisons test. Sample sizes for low and high CES-D score groups were n = 774 and n = 250, respectively.
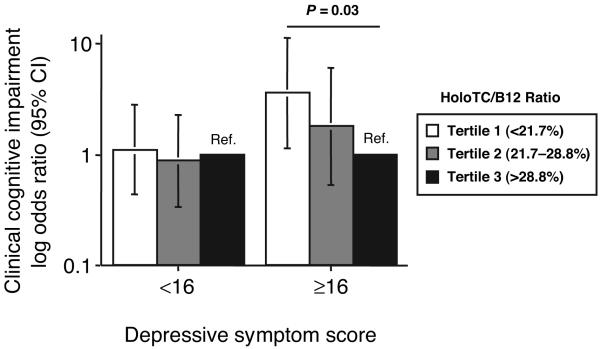
For analyses of the associations between indicators of B12 status, CES-D score, and clinical cognitive impairment, we combined diagnoses of dementia and CIND into 1 group as the dependent variable. By logistic regression analysis, after controlling for confounding by age, sex, education, CES-D score, diabetes, stroke, homocysteine, and creatinine, the OR (95% CI) for clinical cognitive impairment for the lowest tertile of holoTC/B12 was 1.9 (0.94–3.8) compared with the highest (reference) tertile (P = 0.07). In addition, there was a significant interaction between holoTC/B12 and CES-D score on the risk of clinical cognitive impairment (P = 0.014). We then calculated separately the ORs for clinical cognitive impairment by tertiles of holoTC/B12 for individuals with low and high depressive symptom scores. For those individuals with a CES-D score ≥16, the OR for clinical cognitive impairment for the lowest tertile of holoTC/B12 was >3-fold higher (OR 3.6; 95% CI 1.2-11.2) than the highest tertile (P = 0.03) (Fig. 3). In contrast, for those with a CES-D score <16, the OR for clinical cognitive impairment for the lowest tertile of holoTC/B12 was not significantly different from unity (Fig. 3). No significant associations between holoTC or total B12 and clinical cognitive impairment were observed, nor were significant interactions between CES-D score and holoTC or total B12 on clinical cognitive impairment observed (data not shown). With respect to depressive symptom score itself, after controlling for confounding by age, sex, education, diabetes, stroke, and creatinine, the OR for clinical cognitive impairment for those participants with CES-D ≥16 compared with those with CES-D <16 was 3.0 (1.7–5.6) (P < 0.001).
Fig. 3. ORs (95% CIs) for clinical cognitive impairment (diagnosis of CIND or dementia) for participants divided by depressive symptom score (CES-D score < or ≥16) and holoTC/B12 tertile.
HoloTC/B12 tertile 3 serves as the reference tertile (Ref.). ORs are adjusted for age, sex, education, stroke, diabetes, homocysteine, and creatinine. Differences among the tertiles were assessed by logistic regression analysis. Sample sizes for low and high CES-D score groups were n = 780 and n = 254, respectively.
Discussion
In this elderly Latino population, a low ratio of holoTC to total B12 was associated with low 3MSE and low delayed recall scores and an increased odds ratio for clinical cognitive impairment (diagnosis of dementia or CIND) among those who also had increased depressive symptom scores. In contrast, neither holoTC nor total B12, when considered separately, were significantly associated with global cognitive function or clinical cognitive impairment regardless of depressive symptom score. These results suggest that the proportion of total B12 on TC may better reflect B12 status in the brain than either holoTC or total B12 alone. One possible interpretation of these findings is that a low holoTC/B12 ratio is indicative of suboptimal supply of B12 to the brain, which results in a global effect characterized by both depressive symptoms and cognitive impairment. However, because this is a cross-sectional study, it is not possible to draw any conclusions about cause and effect, and the possibility of reverse causation cannot be dismissed.
Clinically, vitamin B12 deficiency is known to affect the nervous system, resulting in peripheral neuropathy, gait ataxia, depression, cognitive impairment, and dementia (2, 3). Although low plasma B12 status has been associated with cognitive impairment and dementia in case-control studies (29, 30), both cross-sectional and prospective cohort studies have shown mixed associations of plasma B12 concentration with specific measures of cognitive function. In a cross-sectional assessment of 1000 free-living adults age 75 years and older, low serum B12 concentrations (≤133 pmol/L) were associated with a 2- to 4-fold increased risk of cognitive impairment as indicated by low Mini-Mental State Examination (MMSE) scores (31). In contrast, no correlation between serum B12 concentrations and cognitive performance was observed on several tests including the MMSE in cross-sectional (n = 559) analyses of participants of the Leiden 85-Plus Study (32). In addition, in a subsample of this same cohort (n = 341), serum B12 levels did not predict cognitive decline longitudinally (32). To further complicate the issue, in some studies, high rather than low plasma B12 concentrations were associated with poor cognitive function. One study, using cross-sectional data from 818 individuals 50–70 years old, found plasma B12 concentrations to be inversely associated with specific functions of memory and sensori-motor speed (33). However, this study excluded individuals with B12 deficiency, which may explain, in part, the inverse associations observed. In a case-control study of 30 primary Alzheimer-type dementia patients, a significant association was observed between declining Cambridge Cognitive Examination scores and increasing total serum B12 concentrations (34). The authors suggested that these paradoxical findings might be explained by the fact that total B12 consists of B12 bound in serum to both haptocorrin and TC, implying that an increased total B12 may be due to an increase in haptocorrin and therefore not all the B12 would be available to extrahepatic tissues.
One possible explanation for the disparate findings among epidemiological studies is that depressive symptoms generally have not been taken into consideration as a potential effect modifier of the association between B12 and cognitive function. Though limited, there is evidence that the associations between plasma vitamin B12 and cognitive function test scores are stronger in depressive patients than in nondepressive patients (35). This is consistent with the present study in which the significant association between holoTC/B12 and cognitive function was confined to those participants with depressive symptoms. Thus, we hypothesize that patients exhibiting concurrently depressive symptoms and cognitive impairment may be those most likely to respond to B12 supplementation, particularly if the holoTC/B12 ratio is low. However, it cannot be ruled out that cognitive impairment causes both depressive symptoms and reduced holoTC/B12 ratio by unrecognized pathophysiological mechanisms or by behavioral modifications affecting dietary intake of B12.
The disparate findings among epidemiological studies may also be explained by variations in sensitivity and accuracy of assessments used to determine the degree of cognitive function in individuals, or by difficulties in accurately assessing B12 status. Typically, B12 status is assessed using a single measurement of total plasma or serum B12. However, the use of only 1 measure to establish a deficient or suboptimal state allows for potential misclassification. Other assays used in conjunction with total B12, such as methylmalonic acid, homocysteine, and holoTC, may allow for better discrimination of B12 status than total B12 alone (11, 14, 36, 37), but inherent limitations in these assays may still lead to misclassification of B12 status. It remains to be seen if various combinations of metabolic assays, using consistent cutoff values, will further improve the ability to detect an association between B12 status and cognitive function in epidemiological studies.
In this study, we have introduced the concept of combining holoTC and total B12 measurements in a ratio. We propose that this ratio may provide a better reflection of cellular delivery and tissue B12 status, at least for the brain, than any of the current measures used, including holoTC and total B12 alone. The use of holoTC in a ratio to assess B12 status has been reported, but usually in the context of the percentage of total TC that has bound B12 or percent TC saturation (a calculated value that is distinct from the holoTC/B12 ratio). Percent TC saturation, in combination with holoTC, has been suggested as a better measure of change in B12 status than holoTC alone (10, 11, 38). Whereas this information may be useful in diagnosing malabsorption, holoTC/B12 may better reflect the availability of circulating B12 to tissues, and therefore intracellular B12 status. The use of TC saturation as a measure of B12 status may be analogous to what has been reported for circulating iron in which the ratio of serum iron to total iron binding capacity (transferrin saturation) provides informative evidence of tissue iron supply (39). However, because there are 2 distinct plasma B12 transport proteins, TC and haptocorrin, a measure of the distribution of total B12 between these proteins, i.e., the holoTC/B12 ratio, may better reflect the functional efficiency of B12 delivery to extrahepatic tissues than does TC saturation. Moreover, there is some evidence to suggest that plasma concentrations of TC (but not holoTC) rise as an acute phase reactant in association with infection or inflammatory disease (40). The usefulness of TC saturation might therefore be compromised in individuals with inflammatory conditions.
HoloTC/B12 ratio is associated with cognitive function in elderly Latinos with increased depressive symptoms. Thus, the ratio may serve as a convenient biomarker of global brain function in older adults and may be more useful to identify individuals at risk for combined cognitive impairment and depression than simple measurement of total B12 or holoTC alone. This should be confirmed in other populations and in longitudinal and intervention studies to apply this ratio for diagnostic purposes.
Acknowledgments
We thank Teresa Ortiz and the staff of the SALSA study for participant recruitment, phlebotomy, data collection, and data management; Rebecca Cotterman, Jennifer Linfor, and the UC Davis Clinical Laboratory for blood sample processing and biochemical assessments; Janet Peerson for statistical consultation; and the Axis-Shield corporation (Norway) for supplying the kits used to measure holoTC.
Grant/Funding Support: This study was supported financially by the National Institutes of Health (grants AG12975, AG10220, AG10129, and DK60753), the U.S. Department of Agriculture (grant 00-35200-9073), and Axis-Shield ASA (Oslo, Norway).
Footnotes
Presented in part at the Experimental Biology annual meeting (Washington, DC, May 1, 2007) and published in abstract form (FASEB J 2007;21:350.4).
6 Nonstandard abbreviations: B12, vitamin B12; TC, transcobalamin; holoTC, holotranscobalamin; SALSA, Sacramento Area Latino Study on Aging; 3MSE, Modified Mini-Mental State Examination; CIND, cognitively impaired but not demented; CES-D, Center for Epidemiological Studies Depression scale; OR, odds ratio.
Financial Disclosures: One of the authors (R. Green) declares a prior financial interest in a company (Axis-Shield ASA) whose product was studied in the present work.
References
- 1.Marcus DL, Shadick N, Crantz J, Gray M, Hernandez F, Freedman ML. Low serum B12 levels in a hematologically normal elderly subpopulation. J Am Geriatr Soc. 1987;35:635–8. doi: 10.1111/j.1532-5415.1987.tb04339.x. [DOI] [PubMed] [Google Scholar]
- 2.Savage DG, Lindenbaum J. Neurological complications of acquired cobalamin deficiency: clinical aspects. Baillieres Clin Haematol. 1995;8:657–78. doi: 10.1016/s0950-3536(05)80225-2. [DOI] [PubMed] [Google Scholar]
- 3.Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45:1435–40. doi: 10.1212/wnl.45.8.1435. [DOI] [PubMed] [Google Scholar]
- 4.Lindenbaum J, Healton EB, Savage DG, Brust JC, Garrett TJ, Podell ER, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318:1720–8. doi: 10.1056/NEJM198806303182604. [DOI] [PubMed] [Google Scholar]
- 5.Hoffbrand AV, Green R. Megaloblastic anaemia. In: Hoffbrand AV, Catovsky D, Tuddenham EG, editors. Postgraduate Haematology. 5th ed. Blackwell Publishing; Oxford: 2005. [Google Scholar]
- 6.Green R, Miller JW. Vitamin B12. In: Zempleni J, Rucker RB, McCormick DB, Suttie JW, editors. Handbook of Vitamins. 4th ed. Taylor and Francis; Boca Raton, FL: 2007. [Google Scholar]
- 7.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J. 1993;7:1344–53. doi: 10.1096/fasebj.7.14.7901104. [DOI] [PubMed] [Google Scholar]
- 8.Green R. Metabolite assays in cobalamin and folate deficiency. Baillieres Clin Haematol. 1995;8:533–66. doi: 10.1016/s0950-3536(05)80220-3. [DOI] [PubMed] [Google Scholar]
- 9.Green R, Jacobsen DW. Clinical implications of homocysteinemia. In: Bailey LB, editor. Folate in Health and Disease. Marcel Dekker; New York: 1994. [Google Scholar]
- 10.Refsum H, Johnston C, Guttormsen AB, Nexo E. Holotranscobalamin and total transcobalamin in human plasma: determination, determinants, and reference values in healthy adults. Clin Chem. 2006;52:129–37. doi: 10.1373/clinchem.2005.054619. [DOI] [PubMed] [Google Scholar]
- 11.Nexo E, Hvas AM, Bleie Ø, Refsum H, Fedosov SN, Vollset SE, et al. Holo-transcobalamin is an early marker of changes in cobalamin homeostasis: a randomized placebo-controlled study. Clin Chem. 2002;48:1768–71. [PubMed] [Google Scholar]
- 12.Ulleland M, Eilertsen I, Quadros EV, et al. Direct assay for cobalamin bound to transcobalamin (holo-transcobalamin) in serum. Clin Chem. 2002;48:526–32. [PubMed] [Google Scholar]
- 13.Herrmann W, Obeid R, Schorr H, Geisel J. Functional vitamin B12 deficiency and determination of holotranscobalamin in populations at risk. Clin Chem Lab Med. 2003;41:1478–88. doi: 10.1515/CCLM.2003.227. [DOI] [PubMed] [Google Scholar]
- 14.Miller JW, Garrod MG, Rockwood AL, Kushnir MM, Allen LH, Haan MN, Green R. Measurement of total vitamin B12 and holotranscobalamin, singly and in combination, in screening for metabolic vitamin B12 deficiency. Clin Chem. 2006;52:278–85. doi: 10.1373/clinchem.2005.061382. [DOI] [PubMed] [Google Scholar]
- 15.Wu CC, Mungas D, Petkov CI, Eberling JL, Zrelak PA, Buonocore MH, et al. Brain structure and cognition in a community sample of elderly Latinos. Neurology. 2002;59:383–91. doi: 10.1212/wnl.59.3.383. [DOI] [PubMed] [Google Scholar]
- 16.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–77. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 17.Miller JW, Green R, Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN. Homocysteine and cognitive function in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2003;78:441–7. doi: 10.1093/ajcn/78.3.441. [DOI] [PubMed] [Google Scholar]
- 18.Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN, Green R, Miller JW. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2005;82:1346–52. doi: 10.1093/ajcn/82.6.1346. [DOI] [PubMed] [Google Scholar]
- 19.Gilfix BM, Blank DW, Rosenblatt DS. Novel reductant for determination of total plasma homo-cysteine. Clin Chem. 1997;43:687–8. [PubMed] [Google Scholar]
- 20.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–54. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 21.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 22.Mungas DM, Reed BR, Marshall SC, Gonzalez HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older people. Neuropsychology. 2000;14:1–15. doi: 10.1037//0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- 23.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–22. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed., revised American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- 25.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–80. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 26.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 27.Wu J, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH. Impact of diabetes on cognitive function among older Latinos: a population-based cohort study. J Clin Epidemiol. 2003;56:686–93. doi: 10.1016/s0895-4356(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 28.Carmel R, Vasireddy H, Aurangzeb I, George K. High serum cobalamin levels in the clinical setting: clinical associations and holo-transcobalamin changes. Clin Lab Haematol. 2001;23:365–71. doi: 10.1046/j.1365-2257.2001.00134.x. [DOI] [PubMed] [Google Scholar]
- 29.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–55. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 30.Malaguarnera M, Ferri R, Bella R, Alagona G, Carnemolla A, Pennisi G. Homocysteine, vitamin B12 and folate in vascular dementia and in Alzheimer disease. Clin Chem Lab Med. 2004;42:1032–5. doi: 10.1515/CCLM.2004.208. [DOI] [PubMed] [Google Scholar]
- 31.Hin H, Clarke R, Sherliker P, Atoyebi W, Emmens K, Birks J, et al. Clinical relevance of low serum vitamin B12 concentrations in older people: the Banbury B12 study. Age Ageing. 2006;35:416–22. doi: 10.1093/ageing/afl033. [DOI] [PubMed] [Google Scholar]
- 32.Mooijaart SP, Gussekloo J, Frölich M, Jolles J, Stott DJ, Westendorp RG, de Craen AJ. Homocysteine, vitamin B-12, and folic acid and the risk of cognitive decline in old age: the Leiden 85-Plus study. Am J Clin Nutr. 2005;82:866–71. doi: 10.1093/ajcn/82.4.866. [DOI] [PubMed] [Google Scholar]
- 33.Durga J, van Boxtel MP, Schouten EG, Bots ML, Kok FJ, Verhoef P. Folate and the methylenetet-rahydrofolate reductase 677C->T mutation correlate with cognitive performance. Neurobiol Aging. 2006;27:334–43. doi: 10.1016/j.neurobiolaging.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 34.McCaddon A, Davies G, Hudson P, Tandy S, Cattell H. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry. 1998;13:235–9. doi: 10.1002/(sici)1099-1166(199804)13:4<235::aid-gps761>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.van Goor LP, Woiski MD, Lagaay AM, Meinders AE, Tak PP. Review: cobalamin deficiency and mental impairment in elderly people. Age Ageing. 1995;24:536–42. doi: 10.1093/ageing/24.6.536. [DOI] [PubMed] [Google Scholar]
- 36.Clarke R, Refsum H, Birks J, Evans JG, Johnston C, Sherliker P, et al. Screening for vitamin B-12 and folate deficiency in older persons. Am J Clin Nutr. 2003;77:1241–7. doi: 10.1093/ajcn/77.5.1241. [DOI] [PubMed] [Google Scholar]
- 37.Obeid R, Schorr H, Eckert R, Herrmann W. Vitamin B12 status in the elderly as judged by available biochemical markers. Clin Chem. 2004;50:238–41. doi: 10.1373/clinchem.2003.021717. [DOI] [PubMed] [Google Scholar]
- 38.Bor MV, Nexo E, Hvas AM. Holo-transcobalamin concentration and transcobalamin saturation reflect recent vitamin B12 absorption better than does serum vitamin B12. Clin Chem. 2004;50:1043–9. doi: 10.1373/clinchem.2003.027458. [DOI] [PubMed] [Google Scholar]
- 39.Huebers HA, Finch CA. The physiology of transferrin and transferrin receptors. Physiol Rev. 1987;67:520–82. doi: 10.1152/physrev.1987.67.2.520. [DOI] [PubMed] [Google Scholar]
- 40.Frater-Schroder M, Hitzig WH, Grob PJ, Kenny AB. Increased unsaturated transcobalamin II in active autoimmune disease. Lancet. 1978;2:238–9. doi: 10.1016/s0140-6736(78)91747-6. [DOI] [PubMed] [Google Scholar]