Abstract
Objective
Ileo-cecal resection (ICR) is common in Crohn’s disease (CD). Inflammation and fibrosis frequently recur at the site of anastomosis or in the small intestine (SI). No animal models of post-surgical inflammation and fibrosis exist. We developed a model of ICR in IL-10 null and wild-type (WT) mice to test the hypothesis that that ICR promotes post-surgical inflammation and fibrosis in SI or anastomosis of genetically susceptible IL-10 null, but not WT or germ free (GF)-IL-10 null mice.
Design
GF-IL-10 null mice were conventionalized (CONV) and 3 weeks later randomized to ICR, transection (T) or no treatment (NoTx). Age-matched conventionally raised (CONV) WT and GF-IL-10 null mice received ICR, T or NoTx. Animals were killed 28 days later.
Main outcome measures
Histological scoring, real-time PCR for TNFα and collagen, and immunostaining for CD3+ T cells, assessed inflammation and fibrosis.
Results
After ICR, CONV-IL-10 null, but not CONV-WT mice, developed significant inflammation and fibrosis in SI and inflammation in anastomosis compared to NoTx or T controls. Fibrosis occurred in anastomosis of both CONV-IL-10 null and CONV-WT following ICR. GF-IL-10 null mice developed little or no inflammation or fibrosis in SI or anastomosis after ICR.
Conclusions
ICR in CONV-IL-10 null mice provides a new animal model of post-surgical inflammation and fibrosis in SI and anastomosis. Absence of inflammation and fibrosis in SI of CONV-WT and GF-IL-10 null following ICR indicates that post-surgical small bowel disease occurs only in genetically susceptible IL-10 null mice and is bacteria dependent.
Keywords: Mouse model, Inflammatory Bowel Disease, ileo-cecal resection, commensal microflora, fibrosis
INTRODUCTION
Crohn’s Disease (CD) is an incurable inflammatory bowel disease (IBD) [1, 2]. CD involves chronic inflammation of the intestine, damage and distortion of tissue architecture and loss of digestive, absorptive and motility functions. Disease onset commonly occurs in the ileum and cecum. Inflammation is transmural in CD, and is associated with transmural increases in collagen deposition, thickening of the muscularis layers, and fibrosis, which can lead to stenosis and bowel obstruction [3, 4]. As many as 65% of CD patients require surgical intervention for unmanageable inflammation or fibrostenosing disease and many require repeat surgery for recurrent disease [5, 6]. Ileo-cecal resections (ICR) are common surgical interventions in CD and are associated with high rates of disease recurrence [6]. After ICR or other surgeries, recurrent inflammation or fibrosis typically occurs at the anastomosis and in small intestine (SI) immediately upstream of the anastomosis [5, 6, 7, 8, 9, 10]. Current therapies do not effectively prevent post-surgical recurrence of CD and there are no approved or effective medical therapies for fibrostenosing disease [6]. Major challenges in developing or testing new therapies, are the inherent heterogeneity of CD at surgery, variable definitions of recurrence and duration of follow-up and the fact that endoscopic evaluation of recurrent disease may not fully inform about fibrosis [4, 6, 7, 8, 9, 10]. An animal model would offer a well-controlled experimental system to address mechanisms of post-surgical inflammation and fibrosis in small bowel or anastomosis, and to test therapies.
Available evidence suggests that commensal microflora initiate and perpetuate IBD in genetically susceptible hosts [11, 12, 13]. Antibiotics have proved useful as therapies for specific complications of CD such as localized peritonitis, bacterial overgrowth due to stricture and during drainage therapy for abscesses and perianal disease [14, 15, 16, 17, 18]. More limited evidence implicates microflora in post-surgical CD. In small numbers of patients, antibiotics have reduced postoperative disease recurrence [18, 19] or promoted remission of ileocolonic anastomosis disease [20]. One study reported that ICR altered the profile of ileal microflora and that recurrent disease was associated with a high prevalence of adherent E. coli, enterococci, bacteroides, and fusobacteria [21]. Very little is known about the in vivo roles of microflora in fibrosis associated with CD or post-surgical fibrosis, despite in vitro evidence that bacterial ligands stimulate proliferation and collagen accumulation in intestinal mesenchymal cells [22].
Numerous animal models of IBD exist [11, 23]. The IL-10 null model has some features in common with CD [11, 23]. Germ free IL-10 null mice do not develop disease. Colonization of GF-IL-10 null mice with microflora from conventionally raised animals reliably initiates disease, in cecum and colon, with a highly reproducible time course and location [24]. Ciprofloxacin and metronidazole improve disease in CONV-IL-10 null mice [24], and each antibiotic preferentially affects cecal or colonic disease [24]. This is consistent with findings that different bacteria initiate inflammation in either the cecum or colon of IL-10 null mice [25]. One limitation of IL-10 null mice as a model of CD is that these animals do not typically develop small bowel disease.
Recently, we applied ICR to wild-type C57BL6 mice [26]. In the current study we applied ICR to CONV-IL-10 null mice and CONV-WT mice on the 129SvEv background. We tested the hypothesis that ICR may promote post-surgical inflammation or fibrosis in the small intestine or anastomosis of genetically susceptible IL-10 null, but not wild-type mice. We chose the IL-10 null mouse to develop a model of post-surgical inflammation and fibrosis because of its wide acceptance as an animal model with relevance to human IBD and the availability of germ-free IL-10 null mice. We successfully adapted the ICR model to germ-free IL-10 null mice to definitively test whether enteric microflora promote post-surgical inflammation or fibrosis.
METHODS
ICR in IL-10 null or WT mice
Eight week old germ free (GF) 129SvEv IL-10 null mice were obtained from the UNC gnotobiotic rodent facility. Animals were transferred to specific pathogen free housing and colonized with fecal slurries from conventionally raised mice as previously described [24]. Three weeks later, when colonization reproducibly initiates disease in the cecum and to a lesser extent colon [24], mice were randomized to sham-operation (T), ileo-cecal resection (ICR) or no surgery (NoTx) groups. Aged matched, conventionally raised WT 129SvEv (CONV-WT) mice were obtained from Jackson labs, Maine, and assigned to NoTx, T or ICR groups. All study groups were given liquid diet (Micro-Stablized Rodent Liquid Diet 101/101A, Purina Mills, MO) 2 days before and 7 days after the start of the experiment. Liquid diet minimizes complications that can occur post-operatively due to obstruction at the anastomosis by solid fecal material [26, 27]. Surgeries were performed under sterile conditions with the aid of the operating microscope (7X magnification). Sham operations (T) consisted of transection and re-anastomosis of bowel approximately 12 cm proximal to the ileocecal junction. For ICR, small bowel was divided 12 cm proximal to the ileocecal junction and at the proximal colon 2–3 cm distal to the cecum. The mesentery was ligated and the intervening SI, cecum and proximal colon removed (Figure 1). Intestinal continuity was restored using an end-to-end, single layered anastomosis with interrupted 9-0 monofilament sutures. Mice were rehydrated with 1–2 ml of warm intraperitoneal saline and the abdomen was closed with running suture. Survival of surgery was 90% for IL-10 null and 87% for WT. The few animals that died or required euthanasia did so within 2–4 days after surgery and were not included in the analyses.
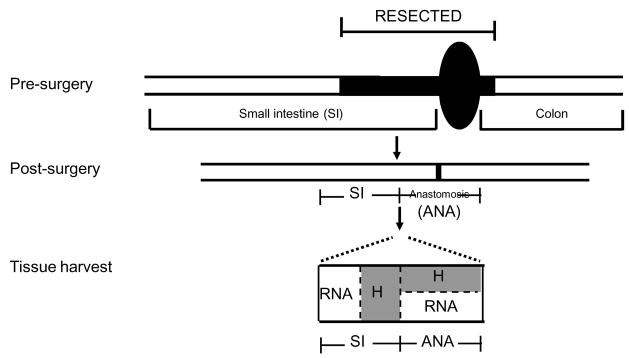
Figure 1. Schematic diagram of ileocecal resection (ICR) and tissue harvest.
A schematic to indicate the ileocecal resection model where distal ileum, cecum and a small portion of proximal colon are resected, then small intestine and colon joined at the anastomosis. Schematic of tissue harvest shows one cm segment spanning the anastomosis and two one cm segments of small intestine proximal to the anastomosis that were collected for histology (H) or RNA extraction as indicated.
GF-IL-10 null mice were obtained from the same UNC colony as animals that were conventionalized before surgery. Surgery was performed as described above but in a customized GF surgical isolator equipped with a port that permits sterile transfer of mice directly from isolator to hood. Liquid diet was sterilized by irradiation. Pilot studies in CONV-mice, verified that sterile diets result in identical weight gain as non-sterile diets. To verify GF status after surgery, fur and fecal swabs were collected at 4 and 7 days after surgery and from co-housed NoTx controls. Fecal samples were collected weekly thereafter. Aerobic and anaerobic cultures, Gram stains, and culture for molds, performed by our gnotobiotic facility [24, 25], verified lack of contamination. Enlarged cecum in GF-IL-10 null mice given T or NoTx, co-housed with ICR animals, provided an additional measure that GF status was retained after surgery. Culture and Gram stains of feces collected at tissue harvest were uniformly negative. Survival of GF-IL-10 null mice after surgery was 87%.
Tissue collection
Analyses focused on mice subject to ICR or T, and NoTx at 28 days after surgery to study the chronic responses to surgery rather than immediate, acute inflammation or wound healing responses to surgery. A subset of CONV IL-10 null and WT animals were also studied at 7 and 14 days after ICR to assess the time course of post-surgical inflammation. Animals were weighed, anesthetized and killed and entire small bowel and colon were dissected. A one cm segment spanning the anastomosis and two one cm segments of small intestine proximal to the anastomosis were collected for histology or RNA extraction (Figure 1). A segment of colon was collected from CONV-IL-10 null mice after ICR, T or NoTx for histological scoring of colitis. Histology samples were fixed in 10% formalin and paraffin embedded and samples for RNA were frozen.
Histological scoring of inflammation and fibrosis
Coded hematoxylin and eosin sections were scored for inflammation by an observer blinded to treatments. Scoring used a well-validated system developed for IL-10 null mice [25], which assigns a score of 0 – 4 for inflammation and mucosal damage based on degree and extent of transmural inflammation, goblet cell depletion and architecture distortion. Sections were stained with Sirius red to visualize collagen accumulation and scored for fibrosis using a validated scoring system as detailed in [28]. Briefly, experimental sections were compared with Sirius red stained sections of normal small intestine and scored on a scale of 0–5 for increased extent of collagen deposition throughout different layers of the bowel wall, multiplied by percent of section involved (1=< 25%; 2=<50%, 3=<75% and 4=<100%), or intensity of staining (1=no increase and 2=increased intensity). In prior studies [28], fibrosis scores showed highly significant correlation with biochemical measures of fibrosis based on collagen mRNA or protein.
Assays of TNFα and collagen mRNA abundance
TNFα and procollagen α1( I) mRNAs were quantified as independent measures of inflammation and fibrosis. Total RNA was extracted using TRIZOL (Invitrogen, CA). Four micrograms of DNAse treated (TurboDNA, Ambion, TX) RNA was reverse transcribed into cDNA using oligodT primers, reverse transcriptase (Promega, WI) and standard conditions. TNFα and collagen mRNA abundance was assessed using quantitative real-time PCR (qRT-PCR) performed on the Rotorgene (Corbett Instruments, Sydney, Australia) using Syber green reagent (Sigma-Aldrich, MO) and cycling conditions optimized for each primer and probe set. Primers were as follows: TNFα: F: 5′-CTGTCTACTGAACTTCGGGGTGAT-3′; R: 5′-GGTCTGGGCCATAGAACTGATG-3′ and procollagen α1(I): F: 5′-GGTATGCTTGATCTGTATCTGC-3′; R: 5′-AGTCCAGTTCTTCATTGCATT-3′ and Hydroxymethylbilane synthase (HMBS), F: 5′-TGTGTTGCACGATCCTGAAAC-3′ and R: 5′-CTCCTTCCAGGTGCCTCAGAA-3′ was used as a constitutively expressed housekeeping control. All test samples in given comparison groups were run in the same qRT-PCR assay, together with a standard curve comprising serial 10-fold dilutions of known concentrations of product. Cycle thresholds for each test mRNA were normalized to HMBS using Rotorgene software (Corbett, Australlia). Abundance of TNFα and collagen mRNAs were expressed as fold-change from the mean expression levels in wild type mice given no treatment.
Immunohistochemistry for CD3, to detect T-cells
5μm sections were de-paraffinized and antigen retrival performed using Tris-Triton buffer pH7.6 before overnight incubation at 4°C with rabbit anti-CD3 (A0452 Dako, Denmark) diluted 1:200 with 5% normal goat serum in Tris-Triton buffer. Samples were incubated for 60 min at room temperature with goat anti-rabbit (111-065-144, Jackson) diluted 1:500 with Tris-Triton buffer. Immunostaining was visualized using avidin-biotin DAB kit (Zymed/Invitrogen cat#00-2014).
Statistics
All data are expressed as mean ± standard error. ANOVA was used to test for statistical differences across groups. Tukeys test was used for post-hoc pair-wise comparisons and statistical significance was set at p≤0.05.
RESULTS
Ileo-cecal resection (ICR) results in inflammation in the small intestine and anastomosis of CONV IL-10 null, but not WT mice
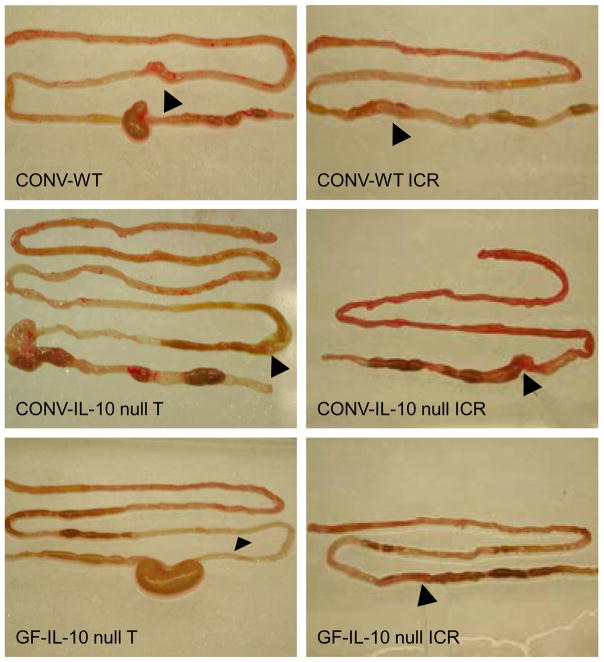
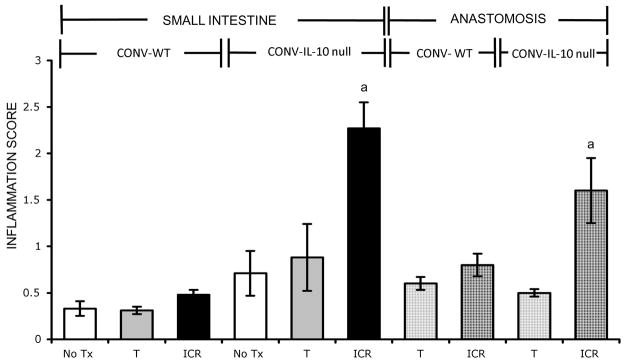
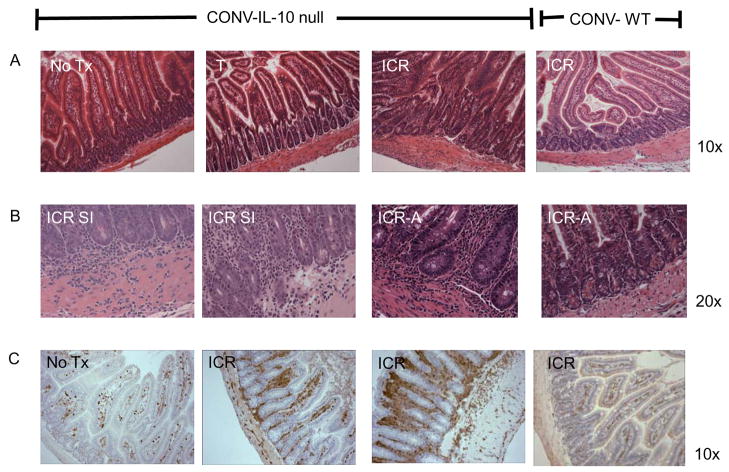
Figure 2 shows representative photographs of entire bowel of CONV-WT or CONV-IL-10 null mice after T or ICR. Note the evident cecal and colonic inflammation in CONV-IL-10 null mice given T but normal small bowel. Note the evident inflammation in small intestine and at anastomosis of CONV-IL-10 null mice but not WT after ICR. Figure 3 shows histological inflammation scores for small intestine and anastomosis of CONV-WT and CONV-IL-10 null mice given NoTx, T or ICR. In WT mice at 28 days following ICR, inflammation scores in small intestine and anastomosis were low and did not differ significantly from T or NoTx controls. IL-10 null mice showed significantly increased inflammation scores in both the small intestine and anastomosis compared with IL-10 null given T or NoTx. Inflammation scores for small intestine and anastomosis of un-operated IL-10 null mice, or animals given transection, did not differ significantly from WT mice, consistent with previous studies demonstrating lack of small bowel disease in un-operated CONV IL-10 null mice [29]. Thus, development of inflammation in small intestine and anastomosis after ICR occurs only in genetically susceptible IL-10 null mice. Scoring of inflammation in colon confirmed colitis in CONV-IL-10 null mice and revealed that ICR or T did not affect disease severity in colon (colitis scores: NoTx 2.8 ± 0.4; T 2.4 ± 0.5; ICR 2.5 ± 0.4). Photomicrographs of representative H&E stained sections in small intestine of CONV-IL-10 null mice after ICR (Figure 4) illustrate transmural inflammation (Fig. 4A and 4B) and infiltration of small intestine with CD3+ T cells ICR (Fig. 4C). Representative photomicrographs of the anastomosis demonstrate inflammation in IL-10 null and not WT mice after ICR (Figure 4B). Figure 5 shows low and high power histology in small intestine of CONV-IL-10 null mice at 7, 14 and 28 days after ICR compared with WT at 7 days after ICR. Note the transmural inflammation in CONV-IL-10 null mice at 7 and 14 as well as 28 days after ICR with no evidence for inflammation in WT at 7 days (Figure 5) or other times. Subsequent studies focused on the 28 day time point.
Figure 2. Photographs of dissected small and large bowel from CONV-WT, CONV-IL-10 null or GF-IL-10 null given transection (T) or ICR.
Arrows indicate anastomosis. Note the significant small bowel and anastomosis inflammation in CONV-IL-10 null given ICR compared with WT or GF-IL-10 null.
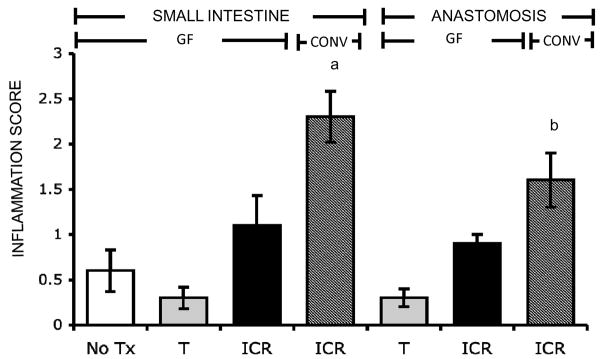
Figure 3. Inflammation in small intestine and anastomosis of IL-10 null, but not WT, after ICR.
Histograms show mean ± SEM for inflammation scores in the small intestine and anastomosis of CONV-WT or CONV-IL-10 null mice after 28 days of no treatment (NoTx; WT: n=3; IL-10 null: n= 6), transection (T; WT: n=8; IL-10 null: n=6) or ileocecal resection (ICR; WT: n=10; IL-10 null: n=11). a= p<0.05 compared with all other groups.
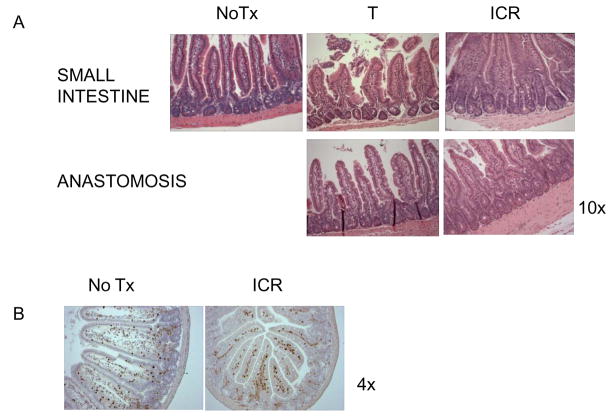
Figure 4. Representative photomicrographs of H & E stained sections of IL-10 null or WT small intestine to illustrate inflammation in CONV-IL-10 null mice after ICR.
A) Small intestine of CONV-IL-10 null after NoTx, T or ICR and WT after ICR. Magnification is shown at 10x to show inflammation in IL-10 mice following ICR, which is absent in NoTx, T and WT controls. B) High power (20x) of small intestine (SI) or anastomosis (A) of IL-10 null mice after ICR to demonstrate transmural inflammation spanning the mucosa and muscle layers. High power of anastomosis from WT shows little inflammation. C) Immunohistochemical staining for CD3 in small intestine and colon of IL-10 null mice after NoTx or ICR to demonstrate increased infiltration of T-cells into the lamina propria of small intestine after ICR and T cell infiltration in colon. Small intestine of WT after ICR is also shown to demonstrate no increase in T cells.
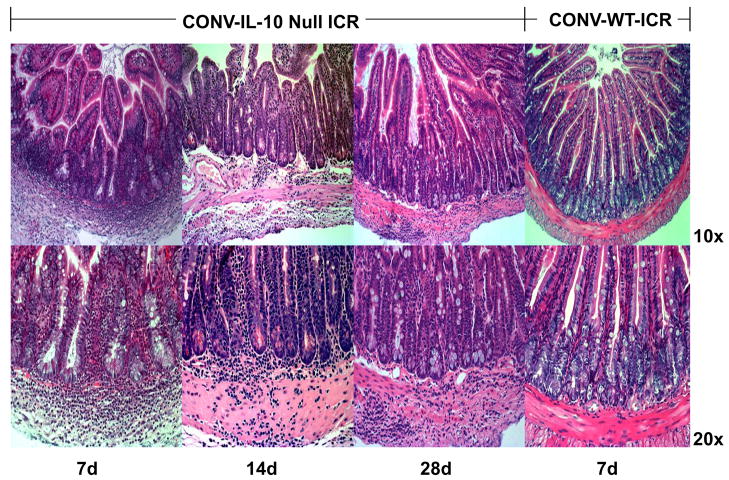
Figure 5. Serial analyses of inflammation in CONV-IL-10 null mice at 7, 14, and 28 days after ICR.
Top: Low power 10x magnification and bottom high power 20x magnification of small intestine of CONV-IL-10 null mice at 7, 14, 28 days after ICR compared with CONV-WT- at 7 days to illustrate that post-surgical small intestine inflammation in CONV-IL-10 null mice is transmural even at early times after ICR and is sustained to 28 days, while WT show no inflammation even at 7 days. Mean inflammation scores after ICR were: CONV-IL-10 null at 7 days 1.8±0.7 n=6; 14 days 2.3±0.7 n=4; and 28 days 2.3±0.2 n=6; WT at 7 days 0.5±0.1 n=8.
TNFα mRNA is up-regulated in SI and anastomosis of CONV IL-10 null, but not WT mice after ICR
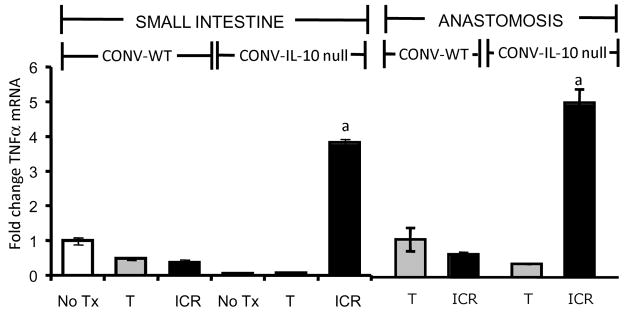
In small intestine and anastomosis real-time qRT-PCR revealed significantly increased TNFα mRNA abundance in IL-10 null mice after ICR compared with all other IL-10 null or WT groups (Figure 6). These biochemical data verify histological inflammation scores.
Figure 6. Increased expression of TNF α mRNA in small intestine and anastomosis of CONV IL-10 null and not WT following ICR.
Histograms show the mean ± SEM for TNF α mRNA abundance in CONV-WT or CONV-IL-10 null small intestine after ICR or T compared with TNFα mRNA in a corresponding region of SI from NoTx controls. Values are expressed as fold change vs. the mean value in WT, NoTx. TNF α mRNA was significantly increased in both the small intestine and anastomosis of IL-10 null mice after ICR when compared with all other groups (a= p ≤ 0.05). N=3 or more animals in each group.
ICR-induced fibrosis of small intestine, but not anastomosis, is restricted to CONV-IL-10 null mice
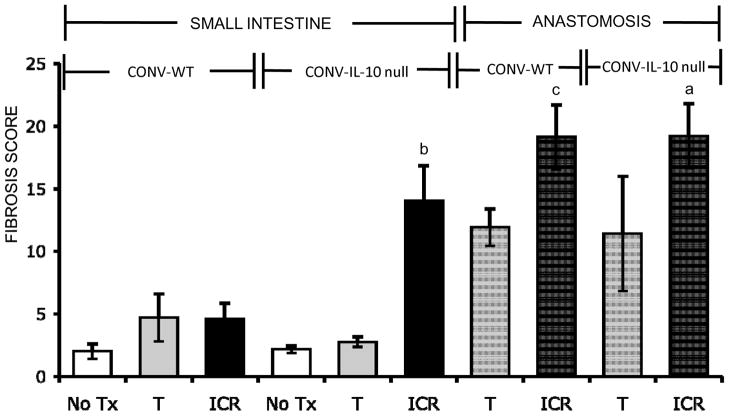
In small intestine of CONV-WT mice, neither ICR nor T significantly increased fibrosis scores compared with NoTx controls (Figure 7). CONV-IL-10 null mice showed major increases in fibrosis scores in small intestine after ICR whereas control transection did not result in fibrosis of small intestine. The anastomosis showed a different pattern. After ICR, significant fibrosis was observed at the anastomosis of both CONV-WT and CONV-IL-10 null mice (Figure 7). In mice given T, mean anastomosis fibrosis scores in both IL-10 null and WT were higher than but not significantly different than scores in untreated small intestine which reflected high inter-animal variability. These results indicate that fibrosis at the anastomosis is likely a consequence of ICR or surgical manipulation and is not influenced by IL-10 deficiency. Figure 8 shows representative Sirius red stained sections to illustrate fibrosis in small intestine of only CONV-IL-10 null mice after ICR, but fibrosis at anastomosis of CONV-WT and CONV-IL-10 null mice after ICR or T.
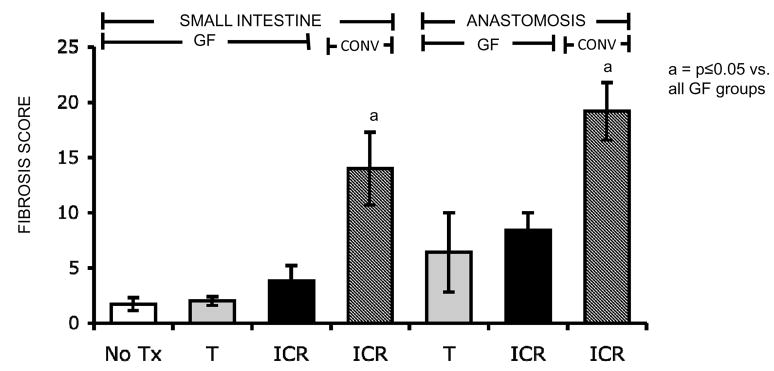
Figure 7. Fibrosis scores in small intestine and anastomosis of CONV-IL-10 null and WT mice.
Histograms show mean ± SEM for fibrosis scores in the small intestine and anastomosis of WT and IL-10 null mice, 28 days after NoTx, T, or ICR (N=as in Figure 3). In small intestine ICR caused significant fibrosis in IL-10 null, but not WT. Fibrosis at the anastamosis was not restricted to IL-10 null mice, as WT mice also had significantly higher fibrosis scores than No Tx controls (a= p ≤ 0.05 vs. all other groups; b= p ≤ 0.05 vs. all other SI groups; c= p ≤ 0.05 vs. NoTx).
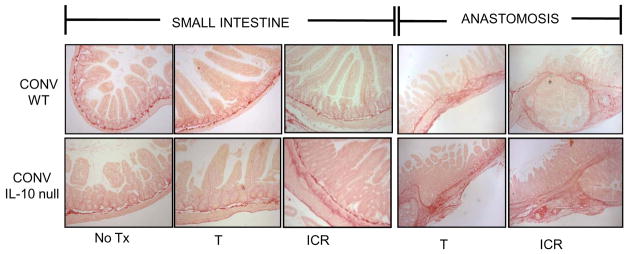
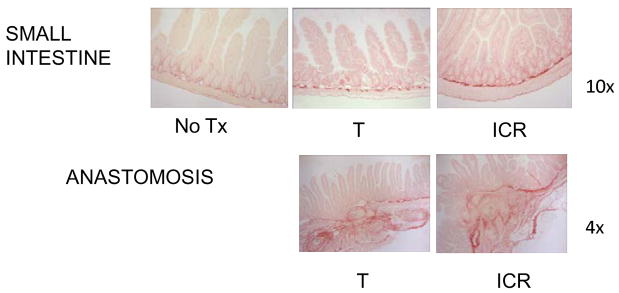
Figure 8. Representative photomicrographs of Sirius red stained sections of small intestine and anastomosis of CONV IL-10 null and WT mice given NoTx, T or ICR.
Photomicrographs of Sirius red stained sections of small intestine (10x magnification) or anastomosis (4x magnification) to demonstrate the increased collagen deposition (stained red) in small intestine of IL-10 null, but not WT after ICR. Fibrosis at the anastomosis is observed in both IL-10 null and WT control mice after ICR, and in a subset of mice given T.
Collagen mRNAs are up-regulated in SI of CONV-IL-10 null, but not WT mice after ICR
In small intestine RT-qPCR revealed significantly increased levels of procollagen α1(I) mRNAs in IL-10 null mice after ICR compared with all other groups (Figure 9). These results provide important biochemical confirmation of histological scoring data. At the anastomosis RT-qPCR did not reveal an increase in collagen mRNA in WT or IL-10 null mice after T or ICR compared with NoTx controls (Figure 9). IGF-I and TGFβ mRNA assays revealed no close correlation between expression levels of these fibrogenic mediators and collagen mRNA or fibrosis scores within small intestine or anastomosis (supplemental Figure 1).
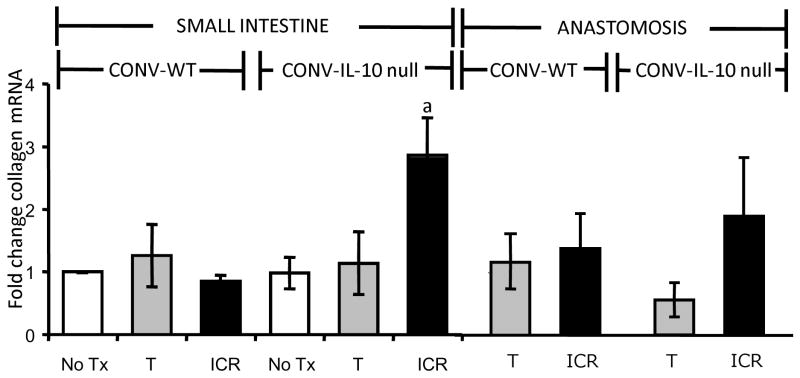
Figure 9. Increased expression of collagen mRNA in small intestine and anastomosis of CONV IL-10 null and not WT following ICR.
Histograms show collagen mRNA abundance, expressed as fold change (mean ± SEM), in IL-10 null and WT small intestine and anastomosis compared with small intestine of WT-NoTx controls (N=3 or more per group). Consistent with histological scoring, collagen mRNA abundance in CONV IL-10 null small intestine was significantly higher following ICR than in NoTx, T or WT controls (a=p<0.05). Neither ICR nor T in IL-10 null or WT was associated with significant increases in collagen mRNA at the anastomosis.
ICR does not induce post-surgical inflammation in small intestine or anastomosis of GF-IL-10 null mice
In small intestine of GF-IL-10 null mice, in contrast to small intestine of CONV-IL-10 null mice, ICR did not lead to a significant increase in inflammation scores (Figure 10). While GF IL-10 null mice showed higher mean inflammation scores at the anastomosis following ICR, this was not statistically significant. Inflammation scores in small intestine of GF-IL-10 null mice after ICR were significantly lower than in small intestine of CONV-IL-10 null mice after ICR, and a similar pattern was observed at the anastomosis (Figure 10). Photomicrographs of representative H&E stained sections are shown in Figure 11A. CD3 staining for T-cells showed no evidence of increased T-cell infiltration into small intestine of GF-IL-10 null mice after ICR (Figure 11B). Together these data demonstrate that commensal microflora is required to initiate post-surgical small bowel inflammation in IL-10 null mice.
Figure 10. No post-surgical inflammation in small intestine and anastomosis of GF IL-10 null mice after ICR.
Histograms show mean ± SEM for inflammation in the small intestine and anastomosis of GF- IL-10 null after NoTx (n=6), T (n=6) or ICR (n=8). Inflammation score for CONV-IL-10 null mice after ICR is shown (hashed bars) for comparison. Histological scoring revealed significantly (p=0.009) reduced inflammation in GF-IL-10 null small intestine. Inflammation also tended to be scored lower at the GF-IL-10 null anastomosis.
Figure 11. Representative photomicrographs of H & E stained sections of small intestine and anastomosis of GF IL-10 null mice.
A) Photomicrographs of small intestine of GF-IL-10 null mice after NoTx, T or ICR and of anastomosis after T or ICR to demonstrate little or no inflammation in GF-IL-10 null small intestine and anastomosis following ICR (magnification at 10x). B) Immunohistochemical staining for CD3 to demonstrate similar numbers of T-cells infiltrating into GF small intestine following ICR, compared with NoTx.
Reduced post-surgical fibrosis in small intestine and anastomosis of GF compared with CONV IL-10 null mice
In GF-IL-10 null mice neither ICR nor T induced significant increases in fibrosis scores in small intestine, and fibrosis scores were significantly higher in small intestine of CONV versus GF-IL-10 null mice after ICR (Figure 12). Fibrosis scores at the anastomosis of a few GF animals receiving either T (3 out of 8 animals) or ICR (1 out of 7 animals) were higher than mean NoTx fibrosis scores. However, overall mean anastomosis fibrosis scores in GF- IL-10 null mice given ICR or T were significantly lower than in CONV IL-10 null mice given ICR (Figure 12) suggesting that bacteria promote fibrotic responses at the site of surgery. Figure 13 shows representative photomicrographs of Sirius red stained sections to illustrate little or no fibrosis in small intestine of GF IL-10 null mice following ICR, compared with NoTx, or T, and mild fibrosis at anastomosis in a subset of GF-IL-10 null mice given T or ICR. However comparisons of Figure 8 and 13 demonstrate much more dramatic fibrosis in CONV IL-10 null mice after ICR.
Figure 12. No significant fibrosis in small intestine or anastomosis of GF IL-10 null mice after ICR.
Histograms show mean ± SEM for fibrosis scoring of the SI and anastomosis in GF- IL-10 null after NoTx, T or ICR (N=as for Figure 9). Fibrosis score for CONV-IL-10 null mice after ICR is shown for comparison (hashed bars). Histological scoring revealed significantly lower fibrosis in GF-IL-10 null small intestine and anastomosis (a= p<0.05).
Figure 13. Representative photomicrographs of Sirius red stained sections of small intestine and anastomosis of GF-IL-10 null mice given NoTx, T or ICR.
Photomicrographs of Sirius red stained sections of small intestine (10x magnification) or anastomosis (4x magnification) to demonstrate low levels of collagen deposition (red stain) in GF-IL-10 null mice following ICR.
Discussion
The primary goal of this study was to develop an animal model of post-surgical small bowel inflammation and fibrosis. Using three independent measures, histological scoring, TNFα mRNA abundance and T cell infiltration, we provide evidence that ICR results in sustained post-surgical inflammation in the small intestine and anastomosis of CONV-IL-10 null mice but not CONV-WT at 28 days after ICR. Un-operated CONV-IL-10 null mice do not typically develop small bowel disease [24, 25]. While we do not yet know the precise mechanisms that elicit post-surgical inflammation in the small intestine and anastomosis of CONV-IL-10 null mice after ICR, a likely contributing factor is exposure of the anastomosis or small intestine to microflora or luminal contents from the colon due to loss of ileo-cecal valve. A recent review highlighted exposure to luminal agents including bacteria, as causative factors in post-surgical disease in CD patients, particularly those with ileocolonic anastomosis [30]. The fact that GF-IL-10 null mice do not develop inflammation after ICR supports this concept. However, findings that inflammation develops in small intestine and anastomosis of CONV-IL-10 null, but not CONV-WT mice, demonstrate that post-surgical small bowel inflammation occurs only in IL-10 null mice with genetic susceptibility to IBD or ongoing IBD. SI and anastomosis are typical sites of inflammation and fibrosis in CD patients after ileo-colonic resection [5, 6, 7, 8]. While we must be cautious in extrapolating the findings in our animal model to post-surgical disease in CD, ICR in CONV and GF-IL-10 null mice provides a new and unique model to pursue future studies of the mechanisms of post-surgical inflammation including the role of specific commensal microflora. It will be of significant interest for example to assess if specific bacteria linked to recurrent disease in CD patients [21] induce post-surgical small bowel disease in GF-IL-10 null mice after ICR.
There is no consensus that typically employed post-surgical therapies such as immunosuppressives or antibiotics reduce post-surgical disease recurrence in CD patients [6, 7] and nor is it clear whether newer therapies such as anti-TNFα impact on post-surgical disease [31]. Our animal model of post-surgical inflammation provides a well-controlled experimental model to test the effects of anti- TNFα and other emerging therapies such as probiotics [2, 32, 33]. Modulation of luminal flora by probiotics [12, 34, 35, 36, 37, 38] is a potential new therapy for CD or post-surgical CD. Trials of single probiotics in CD [39, 40] or post-surgical CD [41] did not improve disease, leading to a consensus that more information is needed before probiotics are used more widely in CD [15, 34, 38, 42]. The animal models developed in our study should prove particularly useful for future preclinical testing of probiotics during post-surgical inflammation.
A common cause of surgery or repeat surgery in CD patients is stricture, caused by fibrostenosing disease [5, 6, 7, 8]. Much less is known about mechanisms of fibrosis or stricture in CD than inflammation, and even less is known about mechanisms of post-surgical fibrosis and stricture. Histological scoring and assays of collagen mRNA abundance demonstrate fibrosis as well as inflammation in small intestine and anastomosis of IL-10 null mice after ICR. Only IL-10 null and not WT mice given ICR developed post-surgical fibrosis in small intestine distant from the site of surgery. However, WT given ICR and a subset of IL-10 null and WT given transection, develop fibrosis at the anastomosis in the absence of detectable inflammation. This suggests that development of post-surgical fibrosis in small bowel during active inflammation may have distinct mechanisms than fibrosis at the anastomosis. Consistent with this concept, post surgical fibrosis of small intestine, but not anastomosis, was associated with increases in procollagen α1(I) mRNA indicative of ongoing collagen synthesis. A recent review suggested that strictures which are inflammatory, fibrotic or both, may respond differently to therapies such as anti-TNFα [31]. Testing of anti-TNFα or other therapies in IL-10 null versus WT mice given ICR or control transection could therefore be very valuable in differentiating effects of existing or new therapies on surgery-induced fibrogenic responses at anastomosis versus fibrosis associated with active inflammation in small intestine. Our findings that GF-IL-10 null mice given ICR exhibit only a low incidence and much less severe fibrosis in small intestine or anastomosis, provide new in vivo evidence to support findings in cultured cells that bacteria or bacterial products can drive fibrosis [22]. This sets the stage for future studies where GF IL-10 null or WT mice given ICR or transection can be used as models to evaluate the effects of specific bacteria on surgery or inflammation associated fibrosis.
Future studies are needed to fully define the time course and the mechanisms of post-surgical inflammation and fibrosis in our IL-10 null ICR model. Initial examination of CONV-IL-10 null mice at 7 and 14 days after ICR demonstrates inflammation within submucosal as well as mucosal layers in genetically susceptible IL-10 null mice but not WT early after ICR, indicating that even early post-surgical inflammation is transmural in nature and occurs only in genetically susceptible IL-10 null mice. Future studies aim to better characterize the cellular nature of inflammatory infiltrate at different times after ICR and assess if fibrosis progresses to stricture. It will also be of interest to study the consequences of ICR or ileal resection in spontaneous ileitis models such as the SAMP1/Yit [43, 44] and TNFΔARE [45] mice. Accumulating evidence suggests that fibrostenosing disease has a genetic component with recent links to CARD15/NOD2 or fractalkine receptor mutations [46, 47]. Evaluation of fibrosis or inflammation after ICR in mouse models carrying similar mutations could provide a useful experimental system to directly define the roles of these genes in post-surgical inflammation or fibrosis.
Acknowledgments
We would like to thank Dr. Sandra Kim for assistance and guidance in inflammation scoring, and thank Kirk McNaughton and Carolyn Suitt for histological sectioning and staining.
FUNDING
Support for these studies was provided by a post-doctoral fellowship from the Crohns’ and Colitis Foundation of America (R.J. Rigby), NIH (DK067395 M.A. Helmrath; DK080283 P.K. Lund and M.A. Helmrath) and a pilot feasibility study from the UNC Center for Gastrointestinal Biology and Disease (P30DK34987). This study used core facilities of the National Gnotobiotic Rodent Resource Center (P40RR018603).
Footnotes
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article to be published in Gut editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our license http://gut.bmjjournals.com/ifora/licence.pdf.
COMPETING INTERESTS
None declared.
References
- 1.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12 (Suppl 1):S3–9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 3.Theiss AL, Fruchtman S, Lund PK. Growth factors in inflammatory bowel disease: the actions and interactions of growth hormone and insulin-like growth factor-I. Inflamm Bowel Dis. 2004;10:871–80. doi: 10.1097/00054725-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann EM, Lund PK. Fibrogenesis. Kirsner’s Inflammatory Bowel Disease. (6) 2004:219–29. [Google Scholar]
- 5.Fichera A, Lovadina S, Rubin M, et al. Patterns and operative treatment of recurrent Crohn’s disease: a prospective longitudinal study. Surgery. 2006;140:649–54. doi: 10.1016/j.surg.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Penner RM, Madsen KL, Fedorak RN. Postoperative Crohn’s disease. Inflamm Bowel Dis. 2005;11:765–77. doi: 10.1097/01.mib.0000171273.09757.f2. [DOI] [PubMed] [Google Scholar]
- 7.Froehlich F, Juillerat P, Felley C, et al. Treatment of postoperative Crohn’s disease. Digestion. 2005;71:49–53. doi: 10.1159/000083873. [DOI] [PubMed] [Google Scholar]
- 8.Froehlich F, Juillerat P, Mottet C, et al. Obstructive fibrostenotic Crohn’s disease. Digestion. 2005;71:29–30. doi: 10.1159/000083869. [DOI] [PubMed] [Google Scholar]
- 9.Sorrentino D, Avellini C, Beltrami CA, et al. Selective effect of infliximab on the inflammatory component of a colonic stricture in Crohn’s disease. Int J Colorectal Dis. 2006;21:276–81. doi: 10.1007/s00384-005-0739-0. [DOI] [PubMed] [Google Scholar]
- 10.Thompson JS, DiBaise JK, Iyer KR, et al. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201:85–9. doi: 10.1016/j.jamcollsurg.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Elson CO, Cong Y, McCracken VJ, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–76. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 12.Sartor RB. Role of commensal enteric bacteria in the pathogenesis of immune-mediated intestinal inflammation: lessons from animal models and implications for translational research. J Pediatr Gastroenterol Nutr. 2005;40 (Suppl 1):S30–1. doi: 10.1097/00005176-200504001-00018. [DOI] [PubMed] [Google Scholar]
- 13.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–94. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs KL, Sartor RB. Treatment of inflammatory bowel disease with antibiotics. Gastroenterol Clin North Am. 2004;33:335–45. x. doi: 10.1016/j.gtc.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–33. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Thukral C, Travassos WJ, Peppercorn MA. The Role of Antibiotics in Inflammatory Bowel Disease. Curr Treat Options Gastroenterol. 2005;8:223–8. doi: 10.1007/s11938-005-0014-z. [DOI] [PubMed] [Google Scholar]
- 17.Prantera C, Lochs H, Campieri M, et al. Antibiotic treatment of Crohn’s disease: results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther. 2006;23:1117–25. doi: 10.1111/j.1365-2036.2006.02879.x. [DOI] [PubMed] [Google Scholar]
- 18.Rutgeerts P, Van Assche G, Vermeire S, et al. Ornidazole for prophylaxis of postoperative Crohn’s disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2005;128:856–61. doi: 10.1053/j.gastro.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology. 1995;108:1617–21. doi: 10.1016/0016-5085(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 20.Elliott PR, Moore GT, Bell SJ, et al. Severe recurrent Crohn’s disease of the ileocolonic anastomosis disappearing completely with antibacterial therapy. Gut. 2005;54:1818–9. doi: 10.1136/gut.2005.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neut C, Bulois P, Desreumaux P, et al. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. Am J Gastroenterol. 2002;97:939–46. doi: 10.1111/j.1572-0241.2002.05613.x. [DOI] [PubMed] [Google Scholar]
- 22.van Tol EA, Holt L, Li FL, et al. Bacterial cell wall polymers promote intestinal fibrosis by direct stimulation of myofibroblasts. Am J Physiol. 1999;277:G245–55. doi: 10.1152/ajpgi.1999.277.1.G245. [DOI] [PubMed] [Google Scholar]
- 23.Byrne FR, Viney JL. Mouse models of inflammatory bowel disease. Curr Opin Drug Discov Devel. 2006;9:207–17. [PubMed] [Google Scholar]
- 24.Hoentjen F, Harmsen HJ, Braat H, et al. Antibiotics with a selective aerobic or anaerobic spectrum have different therapeutic activities in various regions of the colon in interleukin 10 gene deficient mice. Gut. 2003;52:1721–7. doi: 10.1136/gut.52.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SC, Tonkonogy SL, Albright CA, et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Dekaney CM, Fong JJ, Rigby RJ, et al. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1013–22. doi: 10.1152/ajpgi.00218.2007. [DOI] [PubMed] [Google Scholar]
- 27.Helmrath MA, Shin CE, Erwin CR, et al. Epidermal growth factor upregulates the expression of its own intestinal receptor after small bowel resection. J Pediatr Surg. 1998;33:229–34. doi: 10.1016/s0022-3468(98)90437-7. [DOI] [PubMed] [Google Scholar]
- 28.Theiss AL, Fuller CR, Simmons JG, et al. Growth hormone reduces the severity of fibrosis associated with chronic intestinal inflammation. Gastroenterology. 2005;129:204–19. doi: 10.1053/j.gastro.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Sydora BC, Tavernini MM, Wessler A, et al. Lack of interleukin-10 leads to intestinal inflammation, independent of the time at which luminal microbial colonization occurs. Inflamm Bowel Dis. 2003;9:87–97. doi: 10.1097/00054725-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Colombel JF, Watson AJ, Neurath MF. The 10 remaining mysteries of inflammatory bowel disease. Gut. 2008;57:429–33. doi: 10.1136/gut.2007.122192. [DOI] [PubMed] [Google Scholar]
- 31.Sorrentino D. Role of biologics and other therapies in stricturing Crohn’s disease: what have we learnt so far? Digestion. 2008;77:38–47. doi: 10.1159/000117306. [DOI] [PubMed] [Google Scholar]
- 32.Isaacs KL, Lewis JD, Sandborn WJ, et al. State of the art: IBD therapy and clinical trials in IBD. Inflamm Bowel Dis. 2005;11 (Suppl 1):S3–12. doi: 10.1097/01.mib.0000184852.84558.b2. [DOI] [PubMed] [Google Scholar]
- 33.Korzenik JR, Podolsky DK. Evolving knowledge and therapy of inflammatory bowel disease. Nat Rev Drug Discov. 2006;5:197–209. doi: 10.1038/nrd1986. [DOI] [PubMed] [Google Scholar]
- 34.Floch MH, Madsen KK, Jenkins DJ, et al. Recommendations for probiotic use. J Clin Gastroenterol. 2006;40:275–8. doi: 10.1097/00004836-200603000-00022. [DOI] [PubMed] [Google Scholar]
- 35.Lepage P, Seksik P, Sutren M, et al. Biodiversity of the mucosa-associated microbiota is stable along the distal digestive tract in healthy individuals and patients with IBD. Inflamm Bowel Dis. 2005;11:473–80. doi: 10.1097/01.mib.0000159662.62651.06. [DOI] [PubMed] [Google Scholar]
- 36.Marteau P, Lepage P, Mangin I, et al. Review article: gut flora and inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20 (Suppl 4):18–23. doi: 10.1111/j.1365-2036.2004.02062.x. [DOI] [PubMed] [Google Scholar]
- 37.Paton AW, Morona R, Paton JC. Designer probiotics for prevention of enteric infections. Nat Rev Microbiol. 2006;4:193–200. doi: 10.1038/nrmicro1349. [DOI] [PubMed] [Google Scholar]
- 38.Rioux KP, Fedorak RN. Probiotics in the treatment of inflammatory bowel disease. J Clin Gastroenterol. 2006;40:260–3. doi: 10.1097/00004836-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Prantera C, Scribano ML, Falasco G, et al. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: a randomised controlled trial with Lactobacillus GG. Gut. 2002;51:405–9. doi: 10.1136/gut.51.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz M, Timmer A, Herfarth HH, et al. Lactobacillus GG in inducing and maintaining remission of Crohn’s disease. BMC Gastroenterol. 2004;4:5. doi: 10.1186/1471-230X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marteau P, Lemann M, Seksik P, et al. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn’s disease: a randomised, double blind, placebo controlled GETAID trial. Gut. 2006;55:842–7. doi: 10.1136/gut.2005.076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier R, Steuerwald M. Place of probiotics. Curr Opin Crit Care. 2005;11:318–25. doi: 10.1097/01.ccx.0000166396.42894.60. [DOI] [PubMed] [Google Scholar]
- 43.Kosiewicz MM, Nast CC, Krishnan A, et al. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn’s disease. J Clin Invest. 2001;107:695–702. doi: 10.1172/JCI10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsumoto S, Okabe Y, Setoyama H, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–8. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kontoyiannis D, Boulougouris G, Manoloukos M, et al. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn’s-like inflammatory bowel disease. J Exp Med. 2002;196:1563–74. doi: 10.1084/jem.20020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crawford NP, Colliver DW, Eichenberger MR, et al. CARD15 genotype-phenotype relationships in a small inflammatory bowel disease population with severe disease affection status. Dig Dis Sci. 2007;52:2716–24. doi: 10.1007/s10620-006-9208-z. [DOI] [PubMed] [Google Scholar]
- 47.Brand S, Hofbauer K, Dambacher J, et al. Increased expression of the chemokine fractalkine in Crohn’s disease and association of the fractalkine receptor T280M polymorphism with a fibrostenosing disease Phenotype. Am J Gastroenterol. 2006;101:99–106. doi: 10.1111/j.1572-0241.2005.00361.x. [DOI] [PubMed] [Google Scholar]