Between April 1978 and December 1979, 83 recipients of cadaveric renal homografts were submitted to prolonged thoracic duct drainage as part of the immunosuppressive treatment. Our motivation for the thoracic duct drainage trial was dissatisfaction with the so-called standard immunosuppressive regimens for cadaveric kidney transplantation (24).
From this experience (11, 20, 22, 23), it became possible to suggest that the main value of thoracic duct drainage was for pretreatment, to define the appropriate time of the conditioning period before transplantation and to relate the need for thoracic duct drainage to the pre-existing or developing antibody status of the patient. The clinical results with optimally applied thoracic duct drainage were improved over our past experience in cadaveric transplantation. However, the follow-up periods were so short (11, 22, 23) that a catch-up graft loss or a delayed patient mortality were still possibilities. Now, all of the surviving recipients to be reported upon have been observed for at least one year.
METHODS
Cervical thoracic duct drainage was established as described before (12, 20, 22, 23). The general conditions of the trial have been described earlier (22, 23). Because the cadaveric kidneys were from randomly matched donors, the antigens, when analyzed of the HLA-A, B and DR loci, were always poorly matched. There were only two instances in which there were fewer than two mismatches at the A and B loci and none in which there was a four antigen match.
Except when stipulated otherwise, the patients with thoracic duct drainage were given grafts from donors against whose tissues the recipient sera did not have warm anti-T lymphocyte antibodies. The presence or absence of recipient antibodies of several types influenced the duration of thoracic duct drainage in much of our later experience (22, 23) in that patients with preformed warm antibodies tended to be pretreated longer.
In analyzing the results of graft survival, attention was paid only to the kidney which was transplanted at the time the patient was entered into a given study series. Thus, a successful retransplantation which was common in some of the groups (22) would not be reflected in the data as presented. No patients were excluded except a recipient of a pancreas plus a kidney who died of sepsis more than two months later.
The patients were 11 to 60 years old. As described earlier (22, 23), recipients were not excluded because of high risk factors, including age, vas cular disease and myocardial dysfunction. Teṅ patients had diabetes mellitus.
Azathioprine and prednisone were begun on the day of the transplantation. The daily azathioprine dosage was designed to avoid leukopenia. The prednisone dose was 200 milligrams on the first day. Thereafter, daily reductions by 10 milligrams were made, if rejection did not supervene, until a daily dose of 40 milligrams was reached in 16 days. Further reductions were individualized, usually with monthly decrements of 5 milligrams.
Thirty-five recipients were also given short courses of an antithymocyte globulin that was thought not to be an influential factor (22). Slightly less than one-half of the patients underwent splenectomy. This incidence of splenectomy was about the same in the patients treated with thoracic duct drainage as in those in the retrospective control group. Splenectomy was usually performed because of relative leukopenia.
In the first patients, thoracic duct drainage was begun at the time of transplantation and continued for 20 to 109 days. In later patients, pretreatment with thoracic duct drainage was always used and for periods of 17 to 58 days. After transplantation, thoracic duct drainage was usually continued for three or four weeks. It was documented earlier that effective pretreatment with thoracic duct drainage for a period of four weeks or longer virtually eliminated rejection during the first two months after transplantation (23) .
Rejection was defined as a secondary rise of creatinine of more than 25 per cent above base line, along with the other biochemical findings of renal failure. The characteristic physical signs of rejection were looked for as well as the typical findings of rejection by radionuclide scanning.
The case material was divided for analysis into group 1, 25 consecutive recipients of first cadaveric kidneys had thoracic duct drainage at the same time as transplantation; and group 2, 40 consecutive recipients of first cadaveric kidneys had thoracic duct drainage for two and a half weeks to one and a half months before transplantation and for variable periods afterwards.
Retrospective controls for both groups 1 and 2 were provided by 51 consecutive recipients of first cadaveric grafts who had undergone transplantations from May 1977 to March 1978.
Group 3 consisted of 12 patients who had cadaveric retransplantation after having previously rejected from one to four kidneys. The first five of these recipients had thoracic duct drainage begun on the day of transplantation and the next seven had thoracic duct drainage pre-treatment for four weeks to two months.
Group 4 was made up of six patients who had warm anti-T antibodies against all of the lymphocytes of a screening panel of 30 healthy volunteers (22). The patients were standard cross match positive against the donors. Such conditions have been shown by Terasaki and coworkers (25) to dispose to hyperacute rejection. Pretreatment with thoracic duct drainage was carried out for 25 to 54 days (22).
RESULTS
Primary Cadaveric Transplantation
Early rejection
Within the first three months, the clinical diagnosis of rejection was made in 35 of the 51 retrospective control recipients. In 20 patients, the rejections were irreversible (Table I). The incidence of early rejection was not markedly altered by beginning thoracic duct drainage at the time of transplantation, group 1, although fewer of the primary homografts were lost (Table I).
Table I.
Graft losses after primary cadaveric transplantion at three and 12 months postoperative
| No. of rejections within 3 months |
No. of grafts lost from rejection within 3 months |
No. of grafts lost to rejection in first 12 months |
Total no. of grafts lost from all causes* in first 12 months |
|
|---|---|---|---|---|
| Control, n=51 | 35 (68.6) | 20 (39.2) | 23 (45.1) | 29 (56.9) |
| Contemporaneous TTD, n=25 | 16 (64.0) | 8 (32.0) | 11 (44.0) | 11 (44.0) |
| Pretreatment TDD, Total n=40 | 9 (22.5) | 3 ( 7.5) | 8 (20.0) | 12 (30.0) |
| <28 days, n=18 | 8 (44.4) | 2(11.1) | 6 (33.3) | 6 (33.3) |
| ≥ 28 days, n=22 | 1 ( 4.5.) | 1 (4.5) | 2 ( 9.1) | 6 (27.3) |
Including death.
TDD, Thoracic duct drainage
Figures in parentheses are percentages.
In group 2, when pretreatment with thoracic duct drainage was used for less than four weeks, the incidence of rejection still was 44.4 per cent, but the acute process was seldom irreversible (Table I). With pretreatment for four weeks or longer, only one rejection occurred during the first three months for an incidence of 4.5 per cent (Table I).
Kidney survival
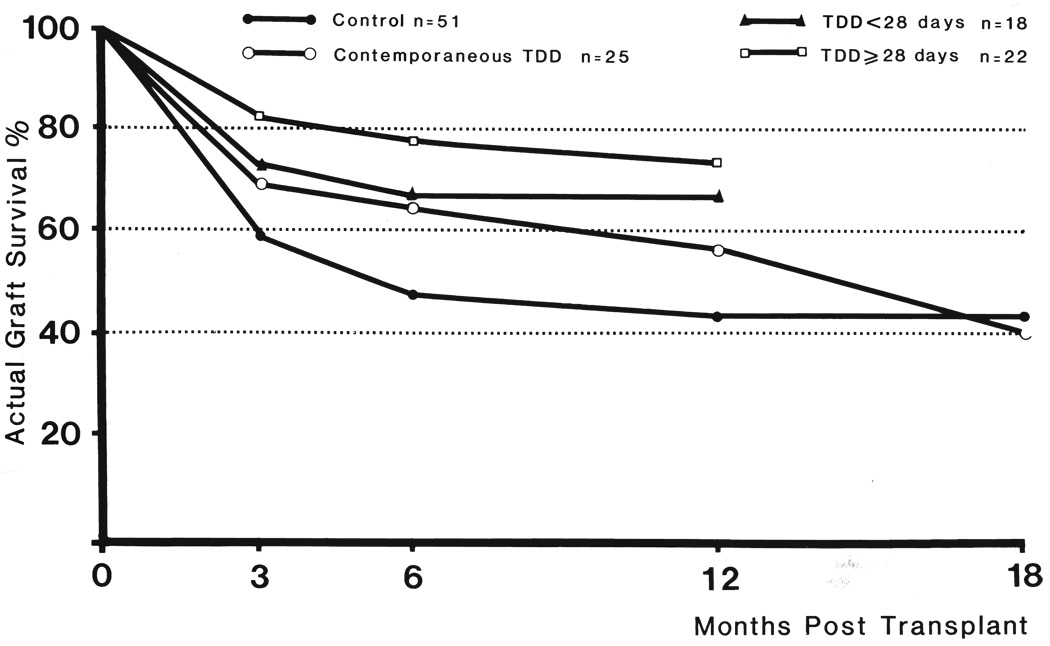
There were 30 patients of the retrospective control group who still had functioning first grafts at the end of three months. By the end of six months, five more organs had been lost, and at the end of the year, only 22 remained (Fig. 1). By the end of 12 months, these results were almost exactly the same as the national average reported in the multicenter compilation of Opelz and associates ( 17).
FIG. 1.
Actual graft survival in primary cadaveric renal transplant. Thoracic duct drainage, TDD.
The one year results in the 25 patients in group 1 who had contemporaneous thoracic duct drainage were slightly better. By the end of the year, 14 of the original grafts were still functioning. However, during the ensuing few months, four more kidneys were lost. Thus, the actual 18 month graft survival rate was 40 per cent, slightly lower than that for the retrospective control patients (Fig. 1).
The patients in group 2 who had pretreatment with thoracic duct drainage for less than 28 days also had an improved one year survival time (Fig. 1). Twelve of the 18 still had, and still have, viable grafts. However, two of these recipients who are now 17 a nd 18 months postoperative have serum creatinine levels of 4 to 6 milligrams per cent. They are expected to lose the grafts, and if they do, the graft survival rate will fall to 50 per cent.
Sixteen of the 22 patients in group 2 who had pretreatment with thoracic duct drainage for 28 days or longer had one year graft survival (Fig. 1). This early improvement in results was eroded by the loss of a kidney each in the 13th and 16th months, for a current 12 to 18 month follow-up organ survival rate of 63.6 per cent, 14 in 22. If an additional kidney is rejected by a patient with a serum creatinine level that is now 5 milligrams per cent in the 17th postoperative month, the kidney survival rate will drop to 59.1 per cent.
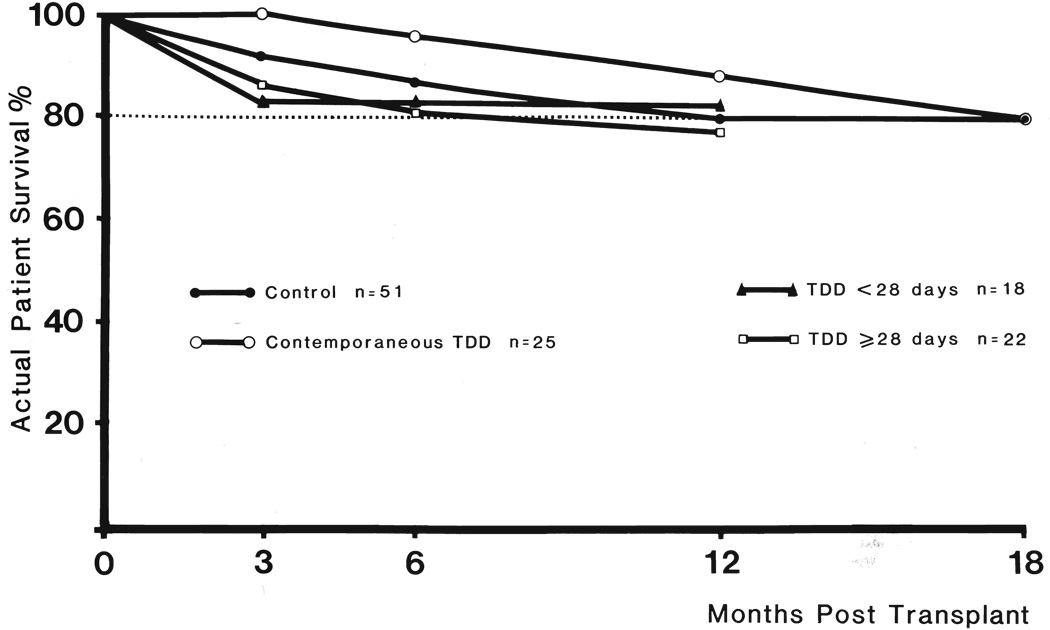
Patient survival
In the study groups shown in Figure 1, the mortality during the first postoperative year ranged from 12 to 22.7 per cent (Fig. 2). The lowest early death rate was in those patients having contemporaneous thoracic duct drainage, but two additional deaths that occurred shortly after the end of the first year eliminated this advantage.
FIG. 2.
Actual patient survival in primary cadaveric transplant. Thoracic duct drainage, TDD.
The most common reason for death was infection, myocardial infarction being about a fourth as frequent a cause. In the group receiving thoracic duct drainage pretreatment for more than 28 days, five patients died within one year. Three of the deaths were unrelated to immunosuppression therapy. There were two myocardial infarctions and one pulmonary emboli. One patient with diabetes died of septicemia at 11 months after undergoing a hip replacement.
CADAVERIC RETRANSPLANTATION
The five patients in group 3 who had begun thoracic duct drainage on the day they were given second to fifth kidneys had unacceptable results previously shown by Ascher and colleagues (2) and by us (8) to be characteristic of cadaveric retransplantation. Four of the five grafts were lost after three to nine months, and in the process, two of these patients died. The fifth patient died of infection just after one year with a well functioning kidney.
The seven patients who had pretreatment with thoracic duct drainage for 28 to 58 days fared slightly better. One patient suffered an irreversible rejection after one month. Two patients died of infection after four and seven months with well functioning kidneys. Three others are in excellent health from 13 to 21 months postoperative. The seventh patient had slow rejection of his kidney after a year and has been returned to dialysis therapy.
TRANSPLANTATION TO PRESENSITIZED RECIPIENT
As reported before (20, 22), three of the six patients with broadly reacting warm anti-T lymphocyte antibodies escaped hyperacute rejection. Unfortunately, their painfully won kidneys were slowly rejected after eight, 11 and 13 months. One of the latter patients died of infection. The five other patients are back on dialysis.
DISCUSSION
The results of these investigations, as well as other recent studies carried out by Johnson and colleagues (9), Kaplan (10), Niblack (16), Traeger (26) and Walker (27) and their associates, have supported the value of thoracic duct drainage as an immunosuppressive adjunct, thus confirming the expectations of earlier workers, including those of Archimbaud (1), Fish (5), Frankson (6, 7), Murray (14), Newton (15) and Sarles (18) and their associates. In earlier publications (11, 23), as well as in the present report, the conditions were defined whereby maximum benefit of thoracic duct drainage could be expected. The most important factor was lymphoid depletion for at least four weeks prior to transplantation. The results using early rejection as an immunologic barometer showed the same one month time curve of thoracic duct drainage effectiveness that had been defined earlier with classical immunologic tests by Machleder and Paulus (13) in patients with autoimmune disease and by us (22, 23) with serologic and other tests in transplant recipients.
The most significant data were obtained in patients undergoing cadaveric renal transplantation for the first time. As reported earlier (23), the conclusion was inescapable that pretreatment with thoracic duct drainage should be provided for at least four weeks. When this was done, rejection in primary cadaveric graft recipients was almost eliminated during the first three months and also for significant subsequent times. Treatment for 17 to 27 days was demonstrably less effective. Finally, when thoracic duct drainage was begun on the day of transplantation, there was little if any tangible benefit for the first homograft, although subsequent retransplantation was seemingly expedited by continuation of lymph drainage (20, 22).
In all the series, the incidence of renal function at one year was increased and with a patient mortality that was not higher than had been observed by us with conventional immunosuppression. However, the significance of these findings was diminished by subsequent graft losses which brought the renal survival curves toward, but not down to, those attainable by conventional immunosuppression. In the patients given optimal pretreatment for more than four weeks, the quality of results was even better than expressed by the statistics since grafts were lost late from deaths which were not obviously related to the transplantation.
If there were an enduring influence of the temporary therapeutic maneuver of thoracic duct drainage, it would have to be explained by the various and not mutually exclusive hypotheses of graft acceptance that have been discussed else-where (19). In such hypotheses, the continuing presence of homograft antigen plus nonspecific immunosuppression has been envisioned as causing specific clone depletion and then tolerance, or alternatively, as producing enhancing antibodies. Cicciarelli and co-authors (4) have shown drastic changes in the residual thoracic duct cell population of patients pretreated only with thoracic duct drainage for a month or longer. A large per cent of these remaining cells have had features of both T cells and B cells. Cicciarelli and associates (4) have speculated that the presence of such presumably pluripotential lymphocytes may signal that conditions are propitious for tolerance induction.
Our experience with late graft failures has suggested that, if such an advantage is created by thoracic duct drainage, it may ultimately be diminished. It is probable that the same applies to other lymphoid depleting procedures, such as antilymphocyte globulin and lymphapheresis which are used temporarily. The lesson has been relearned that more effective early therapy is only part of the answer which, for full exploitation, must be succeeded by improved chronic immunosuppression. It is possible that this can be achieved with the new immunosuppressive agent, cyclosporin A, which was introduced by Caine and colleagues (3) and tested extensively by us (21).
In spite of evidence that effective and safe recipient conditioning can be achieved with thoracic duct drainage, the extent to which this procedure will be used in the future remains unclear. Aside from its personal inconvenience to the patients, the necessity for a month or more hospitalization period before transplantation has engendered substantial hospitalization costs. In the United States, funding for thoracic duct drainage has been difficult to assure because of the unclassified position of this procedure between patient service and research. It is possible that the main use of thoracic duct drainage will be in complicated instances in which the patients are undergoing retransplantation or possess widely reactive anti-T warm antibodies. Thoracic duct drainage apparently helped the avoidance of hyperacute rejection in one-half of our six patients (22) with such warm antibodies, but the grafts were slowly rejected months later. Although the experience of Niblack and co-workers (16) in these difficult instances has been considerably more encouraging, the mere avoidance of an early immunologic calamity will not be a definitive achievement until the succeeding treatment can be perfected. Here also, cyclosporin A may play a role. The combination of thoracic duct drainage, cyclosporin A and low dosages of prednisone has been shown to be safe and efficient (21).
SUMMARY
Thoracic duct drainage was added to conventional immunosuppression with azathioprine, prednisone and, sometimes, antilymphoctye globulin in 83 patients given cadaveric kidneys, including 65 primary graft recipients. The most effective use of thoracic duct drainage was for pretreatment. Optimal conditioning was at least four weeks duration, and when lymph drainage was this long, the incidence of rejection during the first three postoperative months was reduced to 4.5 per cent. Shorter pretreatment or institution of thoracic duct drainage contemporaneous with transplantation were less effective, but the one year results were still better than those with conventional immunosuppression alone. However, the advantage gained with thoracic duct drainage during the first year was diminished in all the treatment groups by graft losses in the second postoperative year. It was concluded that, without better maintenance therapy, the full value of temporary early lymphoid depletion procedures cannot be fully exploited
Acknowledgments
This work was supported by research projects from the Veterans Administration; by U. S. Public Health Service Grant Nos. Am-17260 and AM-07772, and by Grant Nos. RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institute of Health.
REFERENCES
- 1.Archimbaud JP, Banssillon V, Banssillon G, et al. Technique, surveillance et interet de drainage du canal thoracique, effectue en vue d’une transplantation renal. J. Chir. Paris. 1969;98:211. [PubMed] [Google Scholar]
- 2.Ascher NL, Ahrenholz DH, Simmonds RL, Najarian JS. 100 second renal allografts from a single transplantation institution. Transplantation. 1979;27:30. doi: 10.1097/00007890-197901000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Calne RY, Rolles K, White DJG, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs; 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 4.Cicciarelli JC, Iwaki Y, Terasaki PI, et al. Preliminary evidence of dual-marked lymphocytes in thoracic duct lymph fluid. Transplant. Proc. 1980;12:490. [PMC free article] [PubMed] [Google Scholar]
- 5.Fish JC, Sarles HE, Tyson KR, et al. The immunologic consequences of lymph lymphocyte depletion. Surg. Forum. 1969;20:268. [PubMed] [Google Scholar]
- 6.Frankson C. Survival of homografts of skin in rats depleted of lymphocytes by chronic drainage from the thoracic duct. Lancet. 1964;1:1331. doi: 10.1016/s0140-6736(64)91451-5. [DOI] [PubMed] [Google Scholar]
- 7.Frankson C, Lundgren G, Magnusson E, Ringden O. Drainage of thoracic duct lymph in renal transplant patients. Transplantation. 1976;21:133. doi: 10.1097/00007890-197602000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Husberg BS, Starzl TE. The outcome of kidney retransplantation. Arch. Surg. 1974;108:584. doi: 10.1001/archsurg.1974.01350280184030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson HK, Niblack GD, Tallent MB, Richie RE. Immunologic preparation for cadaveric renal transplant by thoracic duct drainage. Transplant. Proc. 1977;9:1499. [PubMed] [Google Scholar]
- 10.Kaplan MP. Thoracic duct drainage; an overview. Dial Transplant. 1979;8:781. [Google Scholar]
- 11.Klintmalm G, Iwatsuki S, Kano T, et al. Determinants of effectiveness of thoracic duct drainage for primary kidney transplantation. Transpl. Proc. 1981;13:537. [PMC free article] [PubMed] [Google Scholar]
- 12.Koep LJ, weil R, starzl TE. The technique of prolonged thoracic duct drainage in transplantation. Surg. Gynecol. Obstet. 1980;151:61. [PMC free article] [PubMed] [Google Scholar]
- 13.Machleder HI, Paulus H. Clinical and immunological alterations observed in patients undergoing long-term thoracic duct drainage. Surgery. 1978;84:157. [PubMed] [Google Scholar]
- 14.Murray JE, Wilson RE, Tilney NL, et al. Five years’ experience in renal transplantation with immunosuppressive drugs; survival, function, complications, and the role of lymphocyte depletion by thoracic duct fistula. Ann. Surg. 1968;168:416. doi: 10.1097/00000658-196809000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton WT. The biologic basis of tissue transplantation. Surg. Clin. North Am. 1965;45:393. doi: 10.1016/s0039-6109(16)37539-9. [DOI] [PubMed] [Google Scholar]
- 16.Niblack GD, Johnson HK, Richie RE, et al. Preformed cyctotoxic antibody in patients subjected to thoracic duct drainage. Proc. Clin. Dial. Transplant. Forum. 1975;5:146. [PubMed] [Google Scholar]
- 17.Opelz G, Mickey MR, Terasaki PI. HLA matching and cadaver transplant survival in North America. Transplantation. 1977;23:490. doi: 10.1097/00007890-197706000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Sarles HE, Remmers AR, Jr, Fish PI, et al. Depletion of lymphocytes for the protection of renal allografts. Arch. Intern. Med. 1970;125:443. [PubMed] [Google Scholar]
- 19.Starzl TE. Experience in hepatic transplantation. With the assistance of C. W. Putnam. Philadelphia: W. B. Saunders Co; 1969. [Google Scholar]
- 20.Starzl TE, Koep LJ, Weil R, et al. Thoracic duct drainage in organ transplantation; will it permit immunosupression? Transplant. Proc. 1979;11:276. [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Weil R, Iwatsuki S, et al. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg. Gynecol. Obstet. 1980;151:17. [PMC free article] [PubMed] [Google Scholar]
- 22.Starzl TE, Weil R, Koep LJ, et al. Thoracic duct fistula and renal transplantation. Ann. Surg. 1979;190:474. doi: 10.1097/00000658-197910000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Idem. Thoracic duct drainage before and after cadaveric kidney transplantation. Surg. Gynecol. Obstet. 1979;149:815. [PMC free article] [PubMed] [Google Scholar]
- 24.Starzl TE, Weil R, Putnam CW. Modern trends in kidney transplantation. Transplant. Proc. 1977;9:1. [PMC free article] [PubMed] [Google Scholar]
- 25.Terasaki PI, Bernoco D, Parks MS, et al. Microdroplet testing for HLA-A-B-C, and -D antigens. Am. J. Clin. Pathol. 1978;69:103. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]
- 26.Traeger J, Touraine JL, Archimbaud JP, et al. Thoracic duct drainage and antilymphocyte globulin for renal transplantation in man. Kidney Int. 1978;13 Suppl. 8:103. [PubMed] [Google Scholar]
- 27.Walker WE, Niblack GD, Richie RE, et al. Use of thoracic duct drainage in human renal transplantation. Surg. Forum. 1977;28:316. [PubMed] [Google Scholar]