Abstract
Background
Cardiovascular factors are associated with cognitive decline. Antioxidants may be beneficial.
Methods
The Women’s Antioxidant Cardiovascular Study was a trial of vitamin E (402 mg every other day), β-carotene (50 mg every other day) and vitamin C (500 mg daily) for the secondary prevention of cardiovascular disease (CVD). From 1995–1996, women 40+ years, with CVD or ≥3 coronary risk factors were randomized. From 1998–1999, a cognitive function substudy was initiated among 2824 participants aged 65+ years. With 5 cognitive tests, cognition was assessed by telephone four times over 5.4 years. The primary outcome was a global composite score averaging all scores; repeated measures analyses were used to examine cognitive change over time.
Results
Vitamin E and β-carotene supplementation were not associated with slower rates of cognitive change (mean difference in change for vitamin E versus placebo = −0.01, 95% CI −0.05, 0.04, p=0.78; for β-carotene=0.03, 95% CI −0.02, 0.07, p=0.28). Although vitamin C supplementation was associated with better performance at the last assessment (mean difference = 0.13, 95% CI 0.06, 0.20, p=0.0005), it was not associated with cognitive change over time (mean difference in change = 0.02, 95% CI −0.03, 0.07, p=0.39). Vitamin C was more protective against cognitive change among those with new cardiovascular events during the trial (p-interaction= 0.009).
Conclusions
Antioxidant supplementation did not slow cognitive change among women with preexisting CVD or CVD risk factors. A possible late effect of vitamin C or of β-carotene among those with low dietary intake on cognition warrant further study.
INTRODUCTION
Growing evidence supports the role of vascular disease and vascular risk factors in cognitive decline and Alzheimer’s dementia (AD).1 Given the high prevalence of vascular conditions in older persons, identifying modifiable approaches to prevent cognitive decline in this population is of vital importance.
Oxidative damage may play a key role in the neuropathology of dementia,2 even in the earliest stages of cognitive impairments2, 3. Several clinical trials of antioxidants and cognitive function have been published to date in generally healthy participants.4–6 Little data is available on the effect of antioxidant supplementation on populations with existing vascular disease or vascular risk factors, a growing segment of our aging population. In the only previous study of antioxidant intervention among those with vascular conditions7, no effects were found. However, the findings are difficult to interpret, because only a single cognitive test was administered at the end of the follow-up, and the treatment group was randomized to receive all three antioxidants combined, such that the specific effects of individual antioxidants are unknown.
Therefore, we conducted a cognitive ancillary study within the Women’s Antioxidant and Cardiovascular Study (WACS), a 2×2×2 factorial, randomized placebo-controlled trial of supplementation with vitamin E, vitamin C, and β-carotene in the secondary prevention of cardiovascular disease among older women.
METHODS
The Women’s Antioxidant Cardiovascular Study (WACS) began in 1995 – 1996. WACS was a 2×2×2 randomized placebo-controlled trial of 3 antioxidants: 402 mg (600 IU) of vitamin E every other day, 500 mg of vitamin C daily, and 50 mg of β-carotene every other day for the secondary prevention of cardiovascular disease (CVD). Eligible participants were female health professionals, 40+ years, with at least three coronary risk factors or prevalent cardiovascular disease (CVD). The women were 94.0% Caucasian, 3.3% African-American, 0.9% Latino-American, 0.7% Asian-American and 1.1% of other / multiple race. Coronary risk factors included parental history of premature MI, diabetes, hypertension, high cholesterol, obesity (BMI ≥ 30 kg/m2). CVD included myocardial infarction, stroke, revascularization procedures (percutaneous transluminal angioplasty, coronary artery bypass graft, carotid endarterectomy, or peripheral artery surgery), and symptomatic angina pectoris or transient cerebral ischemia. In a three-month run-in phase to assess compliance, women received placebo caplets. Women (n= 8,171) who reported good compliance, had no history of cancer in the past 10 years, active liver disease, chronic kidney failure, or use of anticoagulants, and who expressed willingness to forgo the use of out-of-study vitamin supplements beyond the recommended daily allowance were randomized.
Every year during follow-up, the women were sent a 12-months’ supply of calendar packs containing active agents or placebo. Women completed annual mailed questionnaires on compliance, side effects, health and lifestyle characteristics and clinical endpoints. Participants were followed through the scheduled end (January 31, 2005).8 When assessed on annual questionnaires, participants’ compliance to assigned study agents was high and comparable between the active and placebo groups: average compliance (defined as taking at least two-thirds of assigned study medications) during follow-up was 83% and did not differ significantly between the two groups.8 Participants provided written informed consent; the trial was approved by the institutional review board of Brigham and Women’s Hospital, Boston and was monitored by an external data and safety monitoring board.
The results of the primary trial have been published;8 briefly, antioxidant supplementation did not protect against cardiovascular disease, and it did not cause any major adverse side effects.8
Cognitive Cohort
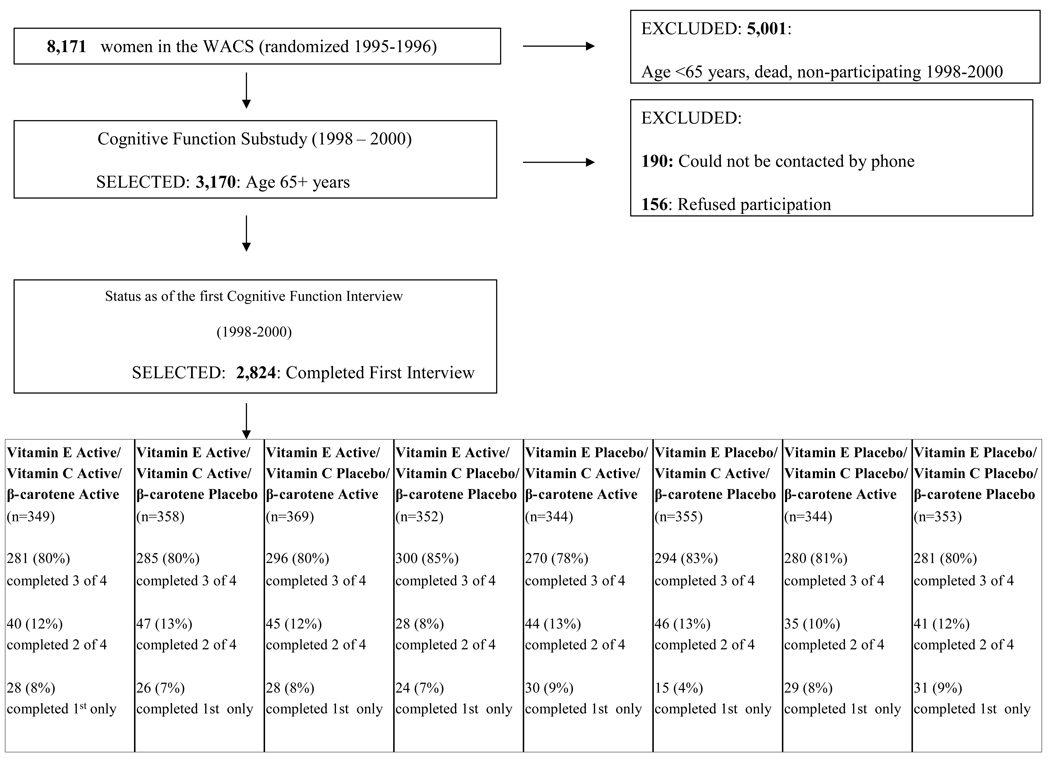
After a mean 3.5 years after randomization, from December 1998-July 2000, we initiated a substudy of cognitive function. The substudy was focused on the oldest women: all active participants aged 65 years or older (n=3170). Of these women, we could not contact 190 by telephone; of the 2980 women we contacted, 156 (5%) declined participation and 2824 (95%) completed the initial telephone cognitive assessment (Figure 1). Participation in our initial cognitive interview was virtually identical across all the treatment and placebo groups (range 94–95%).
FIGURE 1.
Flow chart of participation in the Cognitive Cohort of Women's Antioxidant Cardiovascular Study (WACS)
Participants received three follow-up cognitive assessments approximately every two years. High follow-up was maintained across the groups (Figure 1): 93 % completed at least one follow-up assessment, and 81 % completed at least 3. In the fourth assessment, 24% of participants were not contacted for their interview, as only a short interval had passed between their third interview and the end of the trial in January 2005. Follow-up rates were nearly identical across treatment groups at each assessment.
Cognitive Function Assessment
Substantial research implicates vascular factors in cognitive health—including cognitive outcomes not traditionally associated with vascular health, such as general cognition, episodic memory, and Alzheimer dementia (AD).1 Thus, the emphasis of this study was not on executive function measures, but on general cognition. We hypothesized that if cardiovascular disease and cognitive decline share similar pathways of development, then antioxidants that may protect against the development of cardiovascular disease may also confer benefits for the maintenance of general cognitive function.
We assessed cognitive function by telephone and administered 5 tests measuring general cognition, verbal memory and category fluency. For general cognition, we used the Telephone Interview of Cognitive Status (TICS)9 a telephone adaptation of the Mini-Mental State Examination (MMSE). For verbal memory, we administered the delayed recall of the TICS 10-word list, and the immediate and delayed recalls of the East Boston Memory Test,10 in which a short paragraph is read and 12 key elements are repeated immediately and 15 minutes later. Finally, in a test of category fluency (used to measure executive retrieval functions),11 women were asked to name as many animals as possible in one minute.
The primary, pre-specified outcome of this trial was the change from baseline of the global composite score, which is an average of all five cognitive tests made into z-scores. In addition, because verbal memory is strongly associated with risk of Alzheimer disease,12 our key secondary outcome was the change from baseline of the verbal memory composite score; this composite score was calculated by averaging scores across four measures of verbal memory (the immediate and delayed recalls of both the East Boston Memory Test and 10 word list). To calculate the composite scores for participants who did not complete all tests (only 0.5% for both the global composite score and the verbal memory score), we used the mean of the z-scores of the tests that were completed.
The telephone cognitive interviews were administered by trained interviewers, who were masked to the participants’ randomized treatment assignment. There was high reliability and validity of our telephone cognitive test battery. In a test-retest reliability study of the TICS, administered twice 31 days apart, we found a correlation of 0.7 (p<0.001) among 35 high-functioning, educated women. In a validation study of our telephone instrument, 61 women who had completed an extensive in-person interview were administered our brief telephone-administered assessment; we found a correlation of 0.81 comparing the global composite scores on those two measures, demonstrating high validity of our telephone method. Importantly, among 88 older female health professionals, cognitive impairment as determined by our telephone assessment was strongly associated with dementia diagnosis after three years; poor performance in the TICS and in verbal memory were both associated with significant 8 and 12 fold increases, respectively, of dementia.
Statistical Analysis
Characteristics at baseline between randomized groups were compared using Wilcoxon rank sum tests and chi-square tests for proportions. Mean performance at each assessment by treatment assignment was evaluated using repeated measures analysis of means, which takes into account correlations between assessments. The mean for each intervention group at each time was estimated, allowing for an interaction of group and time, and modeling the correlation of measures over time with an unstructured covariance matrix. Such general linear models of response profiles address the non-linearity of scores and impose minimal structure on outcome trends over time.13 Second, the primary analytic outcome was the mean difference in cognitive change from the initial to the second through fourth assessments. The mean difference in change was basically calculated by subtracting the baseline score from follow-up scores and then taking the difference of cognitive change between the treatment and placebo groups. Thus, a negative value for mean difference in cognitive change indicates an adverse effect of treatment. The mean differences in cognitive change were evaluated by treatment assignment in a repeated measures model. This included fixed effects for time and a common intervention effect over time for each group, reflecting the average difference between groups over time. All models were fitted by maximum likelihood, incorporating the longitudinal correlation within study subjects using unstructured covariance structures; for statistical testing, we used Wald tests.13 For statistical analyses, Proc Mixed in SAS (SAS release 9.1, SAS Institute Inc., Cary, NC) was used.
We also evaluated the differences in cognitive change between those assigned to any of the 3 antioxidants compared with those assigned to all placebos. We further evaluated taking various combinations of antioxidants (e.g. vitamin E and vitamin C versus placebos for both).
We examined effect modification by key risk factors for cognitive change at randomization as well as by incident cardiovascular disease during the trial. We also selected factors that may affect the metabolism of antioxidants (e.g., smoking). Tests of effect modification were performed by evaluating interaction terms in models of mean change in cognition.
In secondary analyses, we examined the influence of non-compliance by repeating the main analyses after excluding women who were taking less than two-thirds of their assigned study medications.
We also constructed models adjusting for assignment to other antioxidant agents or B vitamins, but results were essentially unchanged (data not shown); thus we did not include assignment to other supplements as covariates in models. Also, effect modification by assignment to other trial agents was not observed.
Finally, to assess the impact of antioxidant supplementation on the risk of substantial cognitive change, we fitted logistic regression models adjusting for follow-up time between the first and last assessments, defining the outcome as those in the worst 10% of the distribution of cognitive change from the initial to the final cognitive assessment.
RESULTS
The average time from randomization to the initial cognitive assessment was 3.5 years (range 3.1 – 4.7), and from randomization to the last assessment was 8.9 years (range 7.8 – 9.6). At the end of the study, compliance (defined as taking at least two-thirds of study pills) was comparable across all groups (range 64–68%). No racial/ethnic-based differences were present. Other demographic and health characteristics at randomization were similar between all treatment and placebo groups, with a few minor exceptions (Table 1).
Table 1.
Baseline Characteristics of Participants in the WACS Cognitive Cohort
| Vitamin E | Vitamin C | β-Carotene | ||||
|---|---|---|---|---|---|---|
| Active | Placebo | Active | Placebo | Active | Placebo | |
| (n=1428) | (n=1396) | (n=1406) | (n=1418) | (n=1406) | (n=1418) | |
| Characteristics * | Mean (SD; range) | Mean (SD; range) | Mean (SD; range) | Mean (SD; range) | Mean (SD; range) | Mean (SD; range) |
| 69.1 | 69.0 | 69.0 | 69.1 | 69.1 | 69.0 | |
| Age at randomization, years | ||||||
| (4.3; 62.6–87.4) | (4.2; 62.6–87.9) | (4.2; 62.6–86.9) | (4.3; 62.6–87.9) | (4.3; 62.6–87.9) | (4.2; 62.6–87.6) | |
| 72.6 | 72.5 | 72.5 | 72.6 | 72.6 | 72.5 | |
| Age at initial cognitive assessment, years | ||||||
| (4.3; 66.1–90.9) | (4.2; 66.1–91.3) | (4.2; 66.1–90.5) | (4.3; 66.1–91.3) | (4.3; 66.2–91.3) | (4.2; 66.1–91.2) | |
| 132.7 | 117.2 | 123.2 | 126.9 | 129.2 | 121.0 | |
| Total vitamin E intake (mg/day) | ||||||
| (204.6; 1.5–864.8) | (184.1; 1.24–884.1.5) | (193.8; 1.24–864.8) | (195.9; 1.39–884.1) | (198.3; 1.4–864.8) | (191.4; 1.2–884.1) | |
| 245.9 | 248.7 | 247.6 | 247.0 | 248.1 | 246.6 | |
| Total vitamin C intake (mg/day) | ||||||
| (214.2; 34.1–1793.9) | (229.8; 25.1–1890.9) | (228.1; 25.1–1890.9) | (215.88; 28.1–1793.8) | (218.8; 25.1–1775.3) | (225.2; 27.9–1890.9) | |
| 6.8 | 6.6 | 6.8 | 6.6 | 6.8 | 6.6 | |
| Total carotenoid intake (mg/day) | ||||||
| (5.1; 0.4 –74.5) | (4.6; 0.1 –35.4) | (5.2; 0.4 –74.5) | (4.5; 0.1 –35.4) | (5.0; 0.4 –74.5) | (4.8; 0.1 –41.8) | |
| 3.7 | 3.8 | 3.7 | 3.8 | 3.8 | 3.7 | |
| Total Alcohol intake (g/day) | ||||||
| (8.1; 0 – 66.7) | (8.7; 0 – 85.7) | (8.5; 0 – 83.9) | (8.3; 0 – 85.7) | (8.3; 0 – 85.7) | (8.5; 0 –72.4) | |
| 862.9 | 938.4 | 890.5 | 909.8 | 901.8 | 898.6 | |
| Total Physical Activity (kcal/week) | ||||||
| (1086.4; 0–14,669.3) | (1240.0; 0 – 19017.4) | (1109.2; 0–14669.3) | (1218.5; 0 – 19017.4) | (1208.4; 0 – 19017.4) | (1121.1; 0 – 14669.3) | |
| 28.6 | 28.7 | 28.7 | 28.7 | 28.6 | 28.8 | |
| Body mass index (kg/m2) | ||||||
| (5.6; 15.9 – 56.7) | (5.7; 15.4 – 58.6) | (5.6; 15.4 – 56.7) | (5.7; 15.5 – 58.6) | (5.8; 15.4 – 54.9) | (5.6; 16.0–58.6) | |
| Percent | Percent | Percent | Percent | Percent | Percent | |
| Highest attained education | ||||||
| LPVN/AD/RN † | 71.1 | 69.4 | 69.9 | 70.6 | 70.7 | 69.9 |
| BA/MA/DR † | 28.9 | 30.6 | 30.1 | 29.4 | 29.4 | 30.1 |
| Current cigarette smoking | 9.9 | 9.7 | 10.2 | 9.4 | 10.0 | 9.5 |
| History of MI | 19.4 ‡ | 22.8 | 21.2 | 21.1 | 21.7 | 20.6 |
| History of Stroke | 9.0 | 8.3 | 9.7 § | 7.6 | 9.0 | 8.3 |
| History of Revascularization Surgery | 21.8 | 20.2 | 21.9 | 20.1 | 21.8 | 20.2 |
| History of Angina | 45.0 | 44.5 | 44.5 | 45.0 | 43.9 | 45.6 |
| History of Transient Ischemic Attack | 15.2 | 15.5 | 15.2 | 15.5 | 15.2 | 15.5 |
| History of Diabetes | 18.1 | 17.1 | 17.0 | 18.2 | 17.7 | 17.5 |
| History of Hypertension | 77.3 | 78.0 | 77.7 | 77.6 | 79.6 ║ | 75.7 |
| History of Hyperlipidemia | 72.9 | 76.7 | 74.2 | 75.3 | 72.8 ║ | 76.7 |
Characteristics as of randomization
LPVN: Licensed practical or vocational nurse; AD: Associate’s degree; RN: Registered nurse; BA: Bachelor’s degree; MA: Master’s degree; DR: Doctoral degree
p<0.05 for the difference between vitamin E active group and placebo
p<0.05 for the difference between vitamin C active group and placebo
p<0.05 for the difference between β-carotene active group and placebo
Mean score at each timepoint
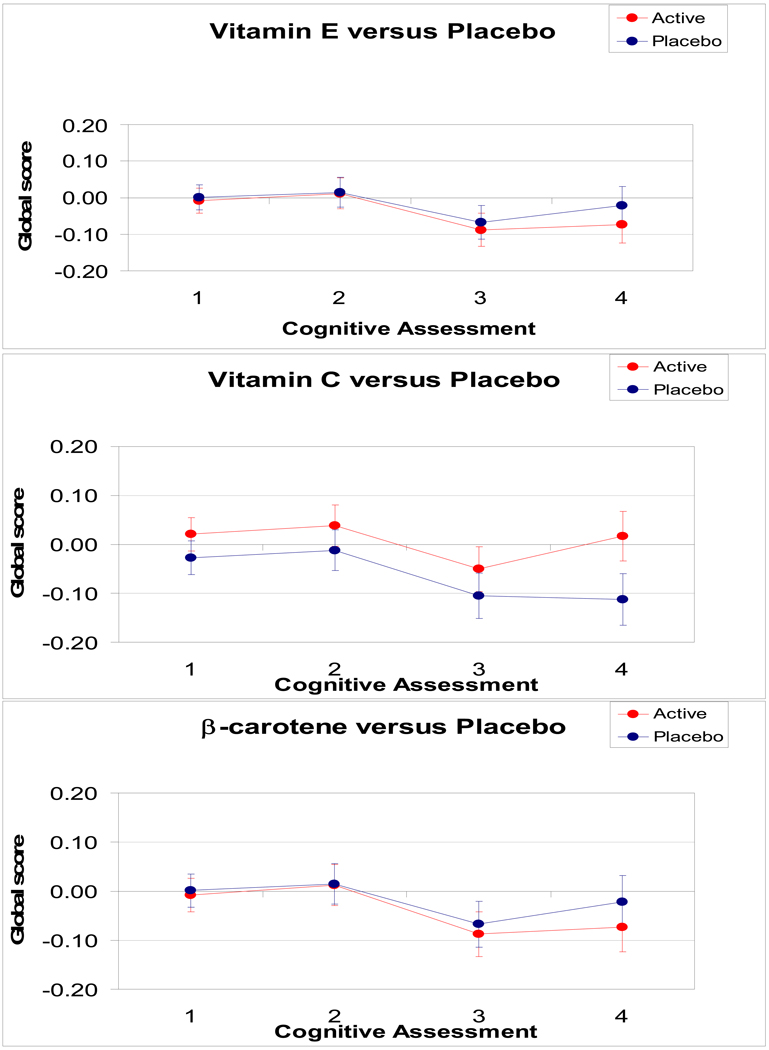
Mean scores over time by assignment group are shown in Figure 2. At the first cognitive assessment, after an average of 3.5 years of treatment, cognition did not differ significantly by treatment groups for vitamin E or β-carotene, but was borderline significant for vitamin C (Table 2) (mean difference in the global composite score between treatment and placebo groups for vitamin C was 0.05, 95% CI, 0.00, 0.10 with p=0.05). At the final assessment, for vitamin E and β-carotene, there was no difference by assignment; however, for vitamin C, the treatment group had higher scores than the placebo group for the global composite score (mean difference = 0.13 (95% CI, 0.06, 0.20; p=0.0005), verbal memory score (mean difference at last assessment = 0.14, 95% CI, 0.06, 0.21; p=0.0004) and TICS (mean difference at last assessment = 0.46, 95% CI, 0.14, 0.78; p=0.006). Overall, the vitamin C active group performed better than the vitamin C placebo group from the 1st through the last assessments as determined by the difference in pattern of performance in global score over time; however, this was of borderline significance (p = 0.06). The overall mean performance on global score over the four assessments did not differ between the active and placebo groups for vitamin E (p=0.54) and β-carotene (p=0.54).
FIGURE 2.
Mean global scores during follow-up (1998–2000 through 2004–2005) by active or placebo assignment for vitamin E, vitamin C and β-carotene
Table 2.
Mean difference in cognitive function at each cognitive assessment for each antioxidant
| VITAMIN E | VITAMIN C | β-CAROTENE | |||||
|---|---|---|---|---|---|---|---|
| Mean difference in score | Mean difference in score | Mean difference in score | |||||
| Cognitive Assessment |
N | [Active Group – Placebo Group] |
p-value | [Active Group – Placebo Group] |
p-value | [Active Group – Placebo Group] |
p-value |
| (95% CI) * | (95% CI) | (95% CI) | |||||
| PRIMARY ENDPOINT: Global score† (difference in score associated with being 1 year older = −0.03) | |||||||
| 1 | 2824 | −0.01 (−0.06, 0.04) | 0.71 | 0.05 (0.00, 0.10) | 0.05 | −0.03 (−0.08, 0.02) | 0.19 |
| 2 | 2511 | 0.00 (−0.06, 0.06) | 0.94 | 0.05 (−0.01, 0.11) | 0.09 | 0.00 (−0.06, 0.05) | 0.89 |
| 3 | 2271 | −0.02 (−0.09, 0.05) | 0.55 | 0.05 (−0.01, 0.12) | 0.10 | 0.01 (−0.06, 0.07) | 0.82 |
| 4 | 1586 | −0.05 (−0.13, 0.02) | 0.17 | 0.13 (0.06, 0.20) | 0.0005 | −0.01 (−0.09, 0.06) | 0.71 |
| KEY SECONDARY ENDPOINT: Verbal memory score† (difference in score associated with being 1 year older = −0.03) | |||||||
| 1 | 2824 | 0.02 (−0.03, 0.07) | 0.43 | 0.05 (0.00, 0.10) | 0.06 | −0.01 (−0.07, 0.04) | 0.62 |
| 2 | 2511 | 0.02 (−0.04, 0.09) | 0.45 | 0.05 (−0.01, 0.11) | 0.13 | 0.00 (−0.06, 0.06) | 0.99 |
| 3 | 2271 | −0.01 (−0.08, 0.06) | 0.75 | 0.07 (0.00, 0.13) | 0.05 | 0.02 (−0.04, 0.09) | 0.50 |
| 4 | 1586 | −0.06 (−0.13, 0.02) | 0.13 | 0.14 (0.06, 0.21) | 0.0004 | −0.02 (−0.09, 0.06) | 0.68 |
| TICS score† (difference in score associated with being 1 year older = −0.13) | |||||||
| 1 | 2824 | −0.01 (−0.25, 0.23) | 0.95 | 0.16 (−0.08, 0.39) | 0.20 | −0.18 (−0.42, 0.06) | 0.14 |
| 2 | 2511 | 0.03 (−0.23, 0.29) | 0.80 | 0.15 (−0.11, 0.41) | 0.24 | 0.06 (−0.20, 0.32) | 0.63 |
| 3 | 2270 | 0.08 (−0.37, 0.21) | 0.61 | 0.15 (−0.14, 0.44) | 0.31 | 0.14 (−0.15, 0.43) | 0.35 |
| 4 | 1586 | −0.16 (−0.49, 0.16) | 0.33 | 0.46 (0.14, 0.78) | 0.006 | −0.13 (−0.46, 0.19) | 0.42 |
| Category fluency score (difference in score associated with being 1 year older = −0.18) | |||||||
| 1 | 2819 | −0.42 (−0.78, −0.06) | 0.02 | 0.03 (−0.33, 0.39) | 0.87 | −0.22 (−0.58, 0.14) | 0.23 |
| 2 | 2504 | −0.45 (−0.83, −0.06) | 0.02 | 0.03 (−0.36, 0.41) | 0.90 | −0.05 (−0.44, 0.34) | 0.80 |
| 3 | 2261 | −0.25 (−0.66, 0.16) | 0.24 | 0.05 (−0.36, 0.46) | 0.80 | −0.05 (−0.46, 0.36) | 0.80 |
| 4 | 1583 | −0.35 (−0.80, 0.10) | 0.13 | 0.25 (−0.20, 0.70) | 0.27 | −0.01 (−0.46, 0.44) | 0.96 |
From longitudinal linear models of adjusted mean cognitive performance
TICS = Telephone Interview of Cognitive Status; Verbal score is a composite score of the z-scores of the immediate and delayed recalls of both the TICS 10-word and the East Boston Memory Test; Global score is a composite score of the z-scores of TICS, immediate and delayed recalls of the East Boston Memory Test, category fluency, delayed recall of the TICS 10-word list
Change from baseline during follow-up
The primary, pre-specified analyses were of change in cognitive function over time in the treated compared with placebo groups.
When we evaluated the differences in the mean rate of change from baseline in cognitive performance from the second through the fourth assessments, we did not observe differences by treatment assignment for any of the antioxidants across all cognitive outcomes (Table 3). On the global composite score, the mean difference in cognitive change from baseline between the vitamin E treatment and placebo groups was −0.01 standard units (95% CI, −0.05, 0.04; p=0.78); for vitamin C, the difference was 0.02 (95% CI, −0.03, 0.07; p=0.39) and for β-carotene, the difference was 0.03 (95% CI, −0.02, 0.07; p=0.28).
Table 3.
Mean differences in cognitive change over follow–up for each antioxidant *
| VITAMIN E | VITAMIN C | β–CAROTENE | |||||
|---|---|---|---|---|---|---|---|
| Mean difference in change in score | Mean difference in change in score | Mean difference in change in score | |||||
| [Active Group – Placebo Group] | p-value | [Active Group – Placebo Group] | p-value | [Active Group – Placebo Group] | p-value | ||
| (95% CI) | (95% CI) | (95% CI) | |||||
| PRIMARY ENDPOINT: Global score (difference in change associated with being 1 year older = −0.03) | |||||||
| −0.01 (−0.05, 0.04) | 0.78 | 0.02 (−0.03, 0.07) | 0.39 | 0.03 (−0.02, 0.07) | 0.28 | ||
| KEY SECONDARY ENDPOINT: Verbal memory score† (difference in change associated with being 1 year older = −0.03) | |||||||
| −0.03 (−0.08, 0.03) | 0.33 | 0.03 (−0.03, 0.08) | 0.32 | 0.01 (−0.04, 0.07) | 0.61 | ||
| TICS score† (difference in change associated with being 1 year older = −0.03) | |||||||
| −0.01 (−0.24, 0.23) | 0.96 | 0.08 (−0.15, 0.32) | 0.49 | 0.20 (−0.03, 0.44) | 0.09 | ||
| Category fluency score (difference in change associated with being 1 year older = −0.03) | |||||||
| 0.11 (−0.22, 0.44) | 0.52 | 0.06 (−0.27, 0.39) | 0.70 | 0.16 (−0.17, 0.49) | 0.36 | ||
From longitudinal linear models of adjusted mean cognitive change. “Cognitive change” is defined as the follow-up score minus the baseline score, with negative values indicating worsened scores. “Mean difference in change in score” is defined as the cognitive change in the active group minus the cognitive change in the placebo group, with negative values indicating an adverse effect of the active agent.
Same footnotes as Table 2
In secondary analyses, those on at least one of the three antioxidant supplements (n=2471) did not differ in their cognitive change from baseline compared with those assigned to all placebos (n=353): mean difference in cognitive change over time was 0.02 standard units (95% CI, −0.04, 0.09; p=0.64). We also examined those taking various combinations of 2 of the 3, compared with those on the corresponding placebos and did not observe any associations; for example, the mean difference in cognitive change over time for those on both active vitamin C and active vitamin E was 0.01 (95% CI, −0.05, 0.08; p=0.67). When we compared those assigned to all three active antioxidant agents (n=349) compared with those assigned to all placebos (n=353), those on all three antioxidants showed a suggestion of cognitive benefits; however, the difference in cognitive change was not significant: 0.08 (95% CI, −0.01, 0.17; p=0.08).
We investigated the risk of substantial cognitive change, defined as those in the worst 10% of the distribution of change from the first to the final assessment. Compared with placebo, the relative risk (RR) was 1.20 (95% CI, 0.86, 1.66) for the vitamin E group, and 1.04 (95% CI, 0.75, 1.44) for the β-carotene group. The RR for the vitamin C group was 0.73 (95% CI, 0.52, 1.01).
In further secondary analyses, we investigated whether the influence of antioxidants differed by various participant characteristics at randomization (Table 4). We observed effect modification by new cardiovascular events occurring after randomization for vitamin C (p for interaction was 0.009). Vitamin C supplementation was associated with better change from baseline (difference in change from baseline in global score for active versus placebo = 0.15 (95% 0.04, 0.26)) among those who developed cardiovascular events during follow-up, while it was not associated among those who had not developed incident events (difference in change = 0.00 (95% CI, −0.05, 0.05). We also found a benefit of β-carotene supplements among those with “low” dietary intakes of total carotenoids but not among those with higher intakes (p-interaction = 0.02). As there is no recommended dietary allowance (RDA) values for total carotenoids, we defined “low” total carotenoids intake as consuming at the lowest 20th percentile (<3.09 mg). There were no such significant interactions by dietary intake with vitamin C and vitamin E.
Table 4.
Mean difference in cognitive change between antioxidant and placebo groups by subgroups *
| Characteristics | Mean difference in cognitive change [Active Group – Placebo Group] (95% CI) |
||
|---|---|---|---|
| Vitamin E | Vitamin C | β−carotene | |
| Age at 1st assessment | |||
| ≤ 72 years (n=1440) | −0.03 (−0.09, 0.04) | 0.05 (−0.01, 0.12) | 0.03 (−0.03, 0.10) |
| > 72 years (n=1384) | 0.02 (−0.05, 0.08) | −0.01 (−0.08, 0.05) | 0.02 (−0.05, 0.09) |
| P†=0.34 | P=0.16 | P=0.80 | |
| 1st cognitive assessment score | |||
| Below median (n=1412) | −0.01 (−0.08, 0.06) | 0.05 (−0.02, 0.12) | 0.05 (−0.02, 0.12) |
| Above median (n=1412) | −0.01 (−0.07, 0.05) | 0.01 (−0.05, 0.07) | 0.00 (−0.06, 0.05) |
| P=1.00 | P=0.37 | P=0.21 | |
| Highest attained education | |||
| LPVN/AD/RN ‡ (n=1861) | −0.04 (−0.10, 0.01) | 0.03 (−0.03, 0.08) | 0.01 (−0.05, 0.06) |
| BA, MA, DR (n=788) | 0.06 (−0.03, 0.15) | −0.01 (−0.10, 0.08) | 0.04 (−0.05, 0.13) |
| P=0.05 | P=0.53 | P=0.50 | |
| Prevalent CVD event/Risk factors § | |||
| CVD event (n=2120) | 0.00 (−0.06, 0.05) | 0.04 (−0.01, 0.10) | 0.03 (−0.02, 0.09) |
| Risk factors (n= 704) | −0.01 (−0.10, 0.08) | −0.04 (−0.13, 0.05) | 0.00 (−0.09, 0.09) |
| P=0.91 | P=0.12 | P=0.51 | |
| Incident Cardiovascular disease ║ | |||
| Present (n= 501) | − 0.06 (−0.05, 0.17) | 0.15 (0.04, 0.26) | 0.00 (−0.11, 0.11) |
| Absent (n=2323) | −0.02 (−0.07, 0.03) | 0.00 (−0.05, 0.05) | 0.03 (−0.02, 0.08) |
| P=0.17 | P=0.009 | P=0.64 | |
| Dietary intake of specific antioxidant | |||
| “Low” ¶| | 0.01 (−0.05, 0.08) | −0.06 (−0.27, 0.14) | 0.14 (0.04, 0.24) |
| “Adequate” | −0.01 (−0.08, 0.05) | 0.03 (−0.02, 0.07) | 0.00 (−0.05, 0.06) |
| P=0.62 | P=0.40 | P=0.02 | |
| Cigarette smoking | |||
| Never smoker (n=1314) | −0.03 (−0.10, 0.04) | 0.00 (−0.07, 0.06) | 0.03 (−0.04, 0.10) |
| Ever smoker (n=1510) | 0.01 (−0.05, 0.07) | 0.04 (−0.02, 0.10) | 0.02 (−0.04, 0.09) |
| P=0.40 | P=0.34 | P=0.96 | |
| Alcohol drinking | |||
| Non-drinker (n=1416) | −0.01 (−0.08, 0.06) | 0.02(−0.04, 0.09) | 0.04 (−0.03, 0.11) |
| Drinker (n=1280) | 0.01 (−0.06, 0.08) | 0.02 (−0.05, 0.08) | 0.02 (−0.05, 0.08) |
| P=0.64 | P=0.85 | P=0.64 | |
| Multivitamin | |||
| Non-user (n=1970) | −0.01 (−0.06, 0.05) | 0.01 (−0.05, 0.06) | 0.02 (−0.04 0.07) |
| User (n= 833) | 0.00 (−0.09, 0.09) | 0.05 (−0.04, 0.13) | 0.03 (−0.05, 0.12) |
| P=0.91 | P=0.46 | P=0.79 | |
Characteristics as of randomization, except for cumulative cardiovascular disease which occurred during follow-up and compliance. “Mean difference in change in score” is defined as the change in the global composite score from baseline in the active group minus the change in the global composite score from baseline in the placebo group, with negative values indicating an adverse effect of the active agent.
P =p value for interaction for testing effect modification
LPVN: Licensed practical or vocational nurse; AD: Associate’s degree; RN: Registered nurse; BA: Bachelor’s degree; MA: Master’s degree; DR: Doctoral degree
Non-fatal MI, non-fatal stroke, revascularization surgery, or cardiovascular death as of randomization
“Incident cardiovascular disease” refers to an updated history of cardiovascular disease as of each follow-up assessment
“Low” dietary intake refers to intake from diet and supplements and is defined for vitamin C, as <75 mg/day (RDA; n=164), for vitamin E, as <15 mg/d (RDA; n=1315), for total carotenoids, as <3.09 mg/d (lowest quintile cutpoint; n= 539); “Adequate” refers to intakes greater than the cutpoints for “low”
We did not observe major differences in the effect of supplementation for any of the antioxidants when we excluded women with poor compliance. Among those who reported good compliance, the mean difference in cognitive change from baseline was −0.02 standard units (95% CI, −0.07, 0.03; p=0.49) for vitamin E; 0.02 (95% CI, −0.03, 0.07; p=0.49) for vitamin C and 0.03 (95% CI, −0.02, 0.08; p=0.23) for β-carotene.
DISCUSSION
In this randomized placebo-controlled trial of 2824 older women at high risk of cognitive decline due to existing cardiovascular disease or cardiovascular risk factors, use of antioxidant supplements was not clearly associated with slowing of cognitive decline.
With our a priori determined outcome of differences in cognitive change over the entire follow-up period, we did not observe effects with any of the individual antioxidants. However, in secondary analyses, a suggestive late effect of vitamin C was observed where women assigned to active vitamin C performed better than women assigned to vitamin C placebo across several cognitive measures at the last assessment,.
The late protective effect of vitamin C particularly among those who developed cardiovascular disease during follow-up should be interpreted with caution, as this result could be due to chance. Furthermore, it is not clear that the water-soluble vitamin C may have stronger neuroprotective actions over the lipid-soluble vitamin E or β-carotene. Biologically, the brain has a high concentration of vitamin C,14 and within the brain, the highest levels are in the cerebral cortex and hippocampus (important in memory).15 In the brain extracellular fluid, vitamin C is involved in broad spectrum radical scavenging, and acts with vitamin E to inhibit peroxidation of membrane phospholipids, particularly in cerebral ischemia.16 However, supplementation with ascorbic acid is unlikely to greatly increase brain levels of vitamin C because ascorbic acid itself does not readily penetrate the blood brain barrier (only the oxidized form of ascorbic acid does).17 In human studies, the evidence for vitamin C protecting against cognitive impairment or dementia is inconsistent, with studies finding both protective associations18, 19 and null associations.20–23 If there was a putative vitamin C specific neuroprotective effect, particularly among those with recent development of cardiovascular events, these data suggest that long-term treatment might be necessary for any effects; this is consistent with a recent antioxidant trial showing protective associations only with long-durations. 6 Clearly, further research on specific effects of vitamin C is needed.
Neurons contain oxidizable lipids that need protection by lipophilic antioxidants such as β-carotene and vitamin E.24 Vitamin E has been extensively studied in relation to cognitive function, including several randomized trials in different populations with different durations (2–10 years) and dosages (134–1340 mg or 200–2000IU). 4, 5, 7, 25, 26 Consistent with the results of these trials, our study among women with cardiovascular conditions showed no cognitive benefits with vitamin E when used for ~9 years. β-carotene has been less studied; however, a recent trial by Grodstein et al.6 among 4052 healthy male physicians showed that men treated with 50 mg on alternate days (same dose as this study) for 18 years had significantly better performance compared with men on placebo, while no association was observed among 1904 men treated for a short duration (1 year). This raises the possibility that either the duration of this study was too short (8.9 years) or the effect of β-carotene on cognition is different among those with cardiovascular disease. There was some evidence that β-carotene supplementation may be beneficial among those with the lowest dietary intake of carotenoids; however, this finding needs replication.
This present study has important strengths. The WACS trial is unique in that it provides cognitive data from a large study sample (n=2824) of older women at elevated risk of cognitive decline, due to vascular disease or vascular risk factors. There was a long duration of treatment (8.9 years treatment and 5.4 years of follow-up), and follow-up and compliance were high. This study provides unique data in that other similar trials of antioxidants have not specifically tested the effect of vitamin C. Finally, cognitive assessments included tests measuring a variety of cognitive domains.
A primary limitation of this study was initiating cognitive testing 3.5 years after randomization, which did not allow for evaluating cognitive change from randomization. However, at randomization, the distribution of various risk factors for cognitive decline was comparable across treatment groups. Thus, it is highly likely that cognitive function at randomization was also similar across treatment groups. Finally, because this study was limited to women with cardiovascular conditions, the results may not be generalizable to men or healthy women.
In conclusion, supplementation with vitamin E, vitamin C or β-carotene did not slow cognitive decline among women with preexisting CVD or risk factors. A late effect of vitamin C or an effect of β-carotene supplements among those with low dietary intake may have been due to chance, but they warrant further study with trials of long treatment durations (>10 years). The clinical interpretation and implications of this study are that supplementation with vitamin E, vitamin C or β-carotene for older women with cardiovascular conditions is unlikely to reduce their risk of cognitive decline.
Acknowledgements
Vitamin E and its placebo were provided by Cognis Corporation (LaGrange, IL); all other agents and their placebos were provided by BASF Corporation (Mount Olive, NJ).
We are indebted to the 2824 participants and staff of the Women’s Antioxidant Cardiovascular Study.
Funding Source
This work is supported by grants AG15933, HL046959 from the National Institutes of Health.
Footnotes
Disclosures
None.
REFERENCES
- 1.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002 Feb;1(1):61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 2.Pratico D, Clark CM, Liun F, et al. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 3.Nunomura A, Perry G, Aliev G, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001 Aug;60(8):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005 Jun 9;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe K, Clemons TE, McBee WL, et al. Impact of antioxidants, zinc, and copper on cognition in the elderly: a randomized, controlled trial. Neurology. 2004 Nov 9;63(9):1705–1707. doi: 10.1212/01.wnl.0000142969.19465.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grodstein F, Kang JH, Glynn RJ, et al. A Randomized Trial of Beta-carotene Supplementation and Cognitive Function in Men: The Physicians' Health Study II. Arch Intern Med. 2007;167:2184–2190. doi: 10.1001/archinte.167.20.2184. [DOI] [PubMed] [Google Scholar]
- 7.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 8.Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med. 2007 Aug 13–27;167(15):1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt J, Folstein MF. Telephone Interview for Cognitive Status: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 10.Scherr PA, Albert MS, Funkenstein HH, et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol. 1988;128:1084–1101. doi: 10.1093/oxfordjournals.aje.a115051. [DOI] [PubMed] [Google Scholar]
- 11.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 12.Small BJ, Fratiglioni L, Viitanen M, et al. The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population based sample. Arch Neurol. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 13.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons, Inc; 2004. Chapter 5. Modelling the Mean: Analyzing Response Profiles; pp. 103–139. [Google Scholar]
- 14.Hornig D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann N Y Acad Sci. 1975 Sep 30;258:103–118. doi: 10.1111/j.1749-6632.1975.tb29271.x. [DOI] [PubMed] [Google Scholar]
- 15.Rice ME, Lee EJ, Choy Y. High levels of ascorbic acid, not glutathione, in the CNS of anoxia-tolerant reptiles contrasted with levels in anoxia-intolerant species. J Neurochem. 1995 Apr;64(4):1790–1799. doi: 10.1046/j.1471-4159.1995.64041790.x. [DOI] [PubMed] [Google Scholar]
- 16.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000 May;23(5):209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 17.Agus DB, Gambhir SS, Pardridge WM, et al. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997 Dec 1;100(11):2842–2848. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paleologos M, Cumming RG, Lazarus R. Cohort study of vitamin C intake and cognitive impairment. Am J Epidemiol. 1998 Jul 1;148(1):45–50. doi: 10.1093/oxfordjournals.aje.a009559. [DOI] [PubMed] [Google Scholar]
- 19.Masaki KH, Losonczy KG, Izmirlian G, et al. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology. 2000;54:1265–1272. doi: 10.1212/wnl.54.6.1265. [DOI] [PubMed] [Google Scholar]
- 20.Perkins AJ, Hendrie HC, Callahan CM, et al. Association of antioxidants with memory in a multiethnic elderly sample using the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 1999;150:37–44. doi: 10.1093/oxfordjournals.aje.a009915. [DOI] [PubMed] [Google Scholar]
- 21.Grodstein F, Chen J, Willett WC. High-dose antioxidant supplements and cognitive function in community-dwelling elderly women. Am J Clin Nutr. 2003;77:975–984. doi: 10.1093/ajcn/77.4.975. [DOI] [PubMed] [Google Scholar]
- 22.Jama JW, Witteman JC, Den Breeijen JH, et al. Dietary antioxidants and cognitive function in a population-based sample of older persons: the Rotterdam study. Am J Epidemiol. 1996;144:275–280. doi: 10.1093/oxfordjournals.aje.a008922. [DOI] [PubMed] [Google Scholar]
- 23.Peacock JM, Folsom AR, Knopman DS, et al. The Atherosclerosis Risk in Communities (ARIC) Study investigators. Dietary antioxidant intake and cognitive performance in middle-aged adults. Public Health Nutr. 2000 Sep;3(3):337–343. doi: 10.1017/s1368980000000380. [DOI] [PubMed] [Google Scholar]
- 24.Kontush K, Schekatolina S. Vitamin E in neurodegenerative disorders: Alzheimer's disease. Ann N Y Acad Sci. 2004 Dec;1031:249–262. doi: 10.1196/annals.1331.025. [DOI] [PubMed] [Google Scholar]
- 25.Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease: the Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 26.Kang JH, Cook NR, Manson JE, et al. A randomized trial of vitamin E and cognitive function in women. Arch Intern Med. 2006;166:2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]