Abstract
Purpose
To determine the localization of JAM-C in human RPE and characterize its functions.
Methods
Immunofluorescence, Western blot, and PCR was used to identify the localization and expression of JAM-C, ZO-1, N-cadherin, and ezrin in cultures of human fetal RPE (hfRPE) with or without si-RNA mediated JAM-C knockdown and in adult native RPE wholemounts. A transepithelial migration assay was used to study the migration of leukocytes through the hfRPE monolayer.
Results
JAM-C localized at the tight junctions of cultured hfRPE cells and adult native RPE. During initial junction formation JAM-C was recruited to the primordial cell– cell contacts and after JAM-C knockdown, the organization of N-cadherin and ZO-1 at those contacts was disrupted. JAM-C knockdown caused a delay in the hfRPE cell polarization, as shown by reduced apical staining of ezrin. JAM-C inhibition significantly decreased the chemokine-induced transmigration of granulocytes but not monocytes through the hfRPE monolayer.
Conclusions
JAM-C localizes specifically in the tight junctions of hfRPE and adult native RPE. It is important for tight junction formation in hfRPE, possibly by regulating the recruitment of N-cadherin and ZO-1 at the cell– cell contacts, and has a role in the polarization of hfRPE cells. Finally, JAM-C promotes the basal-to-apical transmigration of granulocytes but not monocytes through the hfRPE monolayer.
The blood–retinal barrier (BRB) has two components. The inner BRB at the vitreous surface of the retina is formed by tightly opposed retinal endothelial cells and the pericytes surrounding them, whereas the outer BRB consists of a uniform monolayer of retinal pigment epithelial (RPE) cells. The outer component of the BRB is particularly important for maintaining the health and integrity of the retina–RPE complex. In the distal retina, the RPE helps maintain the volume and chemical composition of the extracellular spaces on both the retinal and choroidal sides of the tissue. The RPE apical processes are in close anatomic association with photoreceptor outer segments, and consequently this interface mediates a wide range of metabolic, electrical, and functional interactions.1,2 In particular, the RPE avidly participates in the phagocytosis of the photoreceptor outer segments and the recycling of visual pigments during the light– dark cycle,3 whereas pathophysiological processes in the RPE–photoreceptor complex can lead to widespread photoreceptor degeneration and vision loss.4,5
The integrity of the RPE monolayer depends on the interepithelial junctions that are subdivided to include the tight and adherens junctions and the desmosomes. Adherens junctions are formed by cadherins linked to the actin cytoskeleton via intracellular catenins and the main constituents of tight junctions are three families of transmembrane proteins: occludins, claudins, and junctional adhesion molecules (JAMs).6 JAMs are part of the immunoglobulin superfamily and have two extra-cellular Ig domains, a single transmembrane region and one short cytoplasmic tail. The PDZ domain binding motif located in the cytoplasmic tail of JAMs mediates interactions with intracellular scaffolding proteins such as ZO-1, thereby providing a link to the cytoskeleton.7 JAM-A is found in several types of cells, including endothelial and epithelial cells, and it functions as a gatekeeper by regulating permeability through both endothelial and epithelial monolayers and leukocyte transmigration through endothelial cells.8–10 Furthermore, JAM-A promotes the apicobasal polarization of epithelial cells, including RPE cells.11–13 In contrast, JAM-B is principally found in endothelial cells and also has a role in endothelial cell permeability and leukocyte transmigration through these cells.14
The third member of the JAM family, JAM-C15 has been identified in various cell types including endothelial and gut epithelial cells, platelets, smooth muscle cells and B cells and recently also RPE.8,16 Its localization within intercellular contacts may be divergent among different cell types, as both association with tight junctions and desmosomes have been reported.17,18 Previous studies have shown that JAM-C may interact with factors that regulate cell polarization, tight junction assembly, and paracellular permeability in different cell types.17,19,20 In addition, JAM-C has been implicated in inflammatory processes and shown to participate in the transmigration of leukocytes through endothelial and gut epithelial cells, which may be attributed to the propensity of JAM-C to interact with JAM-B21 as well as with the leukocyte β2-integrin Mac-1.18,22,23
There is growing recognition of the RPE’s involvement in immune mediated processes and diseases24–28 like age-related macular degeneration (AMD) and other inflammatory processes in the posterior pole of the eye. Given that the integrity of the RPE junctions is essential to its function as a barrier between the blood and the retina, that JAM-A and JAM-C have been identified in the RPE junctions, and that JAM-C is known to mediate leukocyte transepithelial migration in other systems, we sought to examine the expression, localization, and function of JAM-C in human RPE. We found that JAM-C is localized at the tight junctions of intact monolayers of human RPE, that it is found at the initial cell– cell contacts of newly forming junctions, and that it helps initiate hfRPE junction formation and polarization. JAM-C also promotes the transepithelial migration of granulocytes through intact monolayers of cultured hfRPE. Thus, in the intact eye, JAM-C may be an important determinant of RPE initial junction formation, cell polarization, and immune system–mediated pathophysiology at the retina-RPE interface.
Materials and Methods
hfRPE Cell Culture and Adult Human RPE
Fetal eyes were obtained from Advanced Bioscience Resources (Alameda, CA) and adult eyes were provided by Analytical Biological Services (Wilmington, DE). All tissues were processed less than 26 hours after enucleation. Primary (P0) RPE cells were isolated from human fetal eyes, grown to confluence with significant pigmentation, and passaged (passage [P]1) on microporous cell culture inserts (Transwells; Corning Costar, Corning, NY) as previously described.29 In the case of adult eyes, after the anterior chamber was opened and the lens and hyaloid body removed, the retina was carefully peeled off to expose the RPE monolayer. Pieces of RPE still attached to the choroid and sclera were cut out, washed, fixed, and used for immunofluorescence. The research adhered to the tenets of the Declaration of Helsinki and was approved by the NIH institutional review board.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Real time PCR was used to quantify the amount of mRNA in each sample. Total mRNA was extracted from the cells in each well (miR-Vana kit; Ambion, Austin, TX) and cleaned (RNeasy mini cleanup Kit; Qiagen, Valencia, CA). Total RNA (1 μg) was mixed with 1 μM Oligo(dT)12–18 (Invitrogen, Carlsbad, CA) in 14-μL volume, incubated at 65°C for 5 minutes, then quickly chilled on ice. RNA and primer were mixed with 0.5 mM dNTP, 1 U/μL RNaseOUT, 2.5 mM DTT, and 0.02 U/μL reverse transcriptase (Omniscript; Qiagen) in 20 μL 1× first-strand buffer. After incubation at 37°C for 60 minutes, the cDNA was diluted 25-fold and 5 μL was used for each PCR reaction. Real-time PCR was performed (TaqMan Assays; Applied Biosystems, Inc. [ABI], Foster City, CA) for YWHAZ (tyrosine 3-monooxygenase/tryptophan 5-mono-oxygenase activation protein, and zeta polypeptide), JAM1(A), Jam2(B) and JAM3(C) with a sequence-detection system (7900; ABI) after standard 40-cycle reactions. PCR for each gene was performed in duplicates and repeated for three separate tissues. The mRNA concentration of each gene is normalized against YWHAZ. Relative expression data was averaged from three biological repeats.
Immunofluorescence
All primary antibodies were conjugated with red and green fluorophores using a commercial antibody labeling technology (Zenon; Invitrogen), according to the manufacturer’s instructions. hfRPE cells were either placed on ice and fixed for 10 minutes in ice-cold methanol or fixed in 4% formaldehyde for 30 minutes at room temperature and permeabilized with 0.1% Triton-X100. After treatment with a solution containing a signal enhancer (Image-IT FX; Invitrogen) for 30 minutes at room temperature, the cells were incubated with the following fluorophore labeled primary antibodies: mouse anti-JAM-C (BD Biosciences, San Jose, CA), rabbit anti-desmoplakin (AbD Serotec, Raleigh, NC), mouse anti-E-cadherin (EMD Biosciences-Calbiochem, San Diego, CA), mouse anti-ZO-1 (Zymed-Invitrogen), and mouse anti-occludin-FITC (Zymed-Invitrogen). After antibody incubation, the cells were treated with 4% paraformaldehyde-PBS (PFA) for an additional 15 minutes at room temperature, mounted on glass slides (Prolong Gold; Invitrogen), and imaged with a fluorescence microscope (Axioplan 2 with Axiovision 3.4 software using ApoTome; Carl Zeiss Meditec, Inc., Dublin, CA, or SP2 confocal microscope; Leica, Bannockburn, IL). To confirm the localization of JAM-C in the apical processes of human fetal RPE cells, we used the following three antibodies: (1) Gi11/human epitope (BD Pharmingen, San Diego, CA); (2) AF1213/mouse epitope (R&D Systems, Minneapolis, MN; and (3) PACA4 (generous gift of Tony Liang of Raven Biotechnologies, South San Francisco, CA). To maximize resolution in our localization studies of JAM-C, short-wave length–emitting fluorophores were used. The anti-JAM-C antibody was conjugated with a blue fluorochrome (350 nm), whereas the anti-ZO-1, anti-occludin, anti-E-cadherin, and anti-desmoplakin antibodies were conjugated with FITC (488 nm). The blue fluorophore data are presented in pseudocolor (red) instead of blue.
Adult RPE Wholemount
After the anterior chamber was opened and the lens and hyaloid body removed, the retina was carefully excised, to expose the RPE monolayer. Pieces of RPE still attached to choroid and sclera were cut out, washed, and fixed in ethanol for 30 minutes at 4°C. Explants were stained using the same method as for the hfRPE cells (see section on immunofluorescence). The following antibodies were used: mouse anti-JAM-C (BD Biosciences), mouse anti-E-cadherin (EMD Biosciences, Calbiochem), and mouse anti-ZO-1 (Zymed-Invitrogen). Stained tissues were fixed with paraformaldehyde (PFA), the RPE and choroid were detached from the sclera, and mounted on a glass slide for imaging.
JAM-C Knockdown by siRNA
For transfections, two chemically synthesized duplex siRNAs were used, one directed against human JAM-C, and the second, a non-targeting control siRNA (siGENOME-hJAM3 1 and siCONTROL nontargeting siRNA 1, respectively; Dharmacon, Chicago, IL). Single siRNAs were transfected at a concentration of 100 nM (Dharmafect 4 reagent; Dharmacon) in reduced-serum medium (OptiMEM; Invitrogen) according to the manufacturer’s protocol. Briefly, confluent cells were split by trypsinization, and when they reached approximately 90% confluence, the transfection reagents were added and left to incubate overnight. Cells were passaged 48–72 hours after transfection and either transferred to coated cell culture inserts (Transwell; Corning Costar) or seeded at a low density on coated glass coverslips.
Surface Protein Fraction Isolation
For the surface protein fraction isolation we used 90% confluent hfRPE cells that were transfected with JAM-C siRNA or control siRNA (siGENOME-hJAM3 1 and siCONTROL nontargeting siRNA 1, respectively; Dharmacon). For the protein extraction we used a biotinylation kit (Cell Surface Biotinylation kit; Pierce, Rockford, IL) and the cells were processed according to the manufacturer’s protocol. Briefly, the cell surface proteins were labeled with biotin, and the cells were scraped off the flasks and lysed. The cell lysates were run through Avidin columns, and the protein fraction that did not bind to the Avidin, was discarded. The rest of the proteins that were isolated represented the surface membrane-bound proteins. The product of the surface protein isolation was analyzed by Western blot.
Western Blot Analysis
hfRPE cultured cells or native adult human RPE cells were rinsed with cold PBS and lysed with RIPA buffer (Sigma-Aldrich, St. Louis, MO) supplemented with complete protease inhibitors (Roche, Basel, Switzerland) for 5 minutes on ice. The cell lysate was centrifuged at 13,000 rpm for 10 minutes and the supernatant was collected. The protein amount in the supernatant was quantified with a BCA protein assay (Pierce). Ten micrograms of total protein was separated on a 4% to 12% polyacrylamide gradient gel (NuPAGE gel; Invitrogen) under nonreducing conditions and transferred to nitrocellulose membranes. Membranes were probed with mouse anti-JAM-C (clone Gi11; BD Biosciences), mouse anti-JAM-C (clone PACA4, a generous gift from Raven Biotechnologies) mouse anti-α-tubulin (Abcam, Cambridge, MA), goat anti-JAM-A (R&D Biosystems), mouse anti-ezrin (Sigma-Aldrich), rabbit anti-ICAM-1 (Cell Signaling, Danvers, MA), and mouse anti-N-cadherin-1 (Zymed-Invitrogen) antibodies. An anti-mouse and anti-rabbit horse-radish peroxidase–conjugated antibody (Pierce) was used as secondary antibody. An enhanced chemiluminescence kit (Supersignal Pico ECL; Pierce) was used to detect the signal using a gel documentation system (Autochemie; UVP, Upland, CA). Densitometric analysis was performed with Image J software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Cytokine Stimulation
Passage 1 hfRPE cells grown for 4 months on microporous cell culture inserts (Transwells; Corning Costar) were stimulated for 24 hours with 10 ng/mL TNF-α, 10 ng/mL IL-1β, or 100 U/mL IFN-γ one by one, as pairs, or as a complete mixture. At the end of the experiment, the cells were either fixed with ice cold methanol and used for immunofluorescence or lysed and processed for Western blot analysis. In a different experiment, we exposed the cells to a 10-fold higher concentration of TNF-α (100 ng/mL) and we performed Western blot analysis to determine changes in JAM-C expression.
Cell Polarization Assessment
Primary hfRPE cells were transfected with JAM-C siRNA or nontargeting siRNA, trypsinized, and plated at high density (≈300,000 cells/cm2) on 12-well inserts and left to adhere and form cell–cell contacts. The cells were then fixed at 24, 48, and 72 hours and stained with a mouse anti-ezrin (Zymed-Invitrogen) and a mouse anti-ZO-1 (Zymed-Invitrogen) antibody. The antibodies were conjugated with red or green fluorophores by using a commercial antibody labeling technology (Zenon; Invitrogen) as described earlier (immunofluorescence). Cell polarization was assessed by the detection of ezrin which has been shown to be localized to the apical processes of human RPE.29
Transepithelial Migration of Leukocytes across RPE Monolayers
hfRPE cells in 100 μL media were plated, according to a published protocol,30 on the bottom side of 6.5-mm uncoated inverted cell culture wells (Transwells; Corning Costar) with an 8-μm pore size, at a density of 100,000 cells per membrane and left upside down for 24 hours at 37°C to attach.
These inserts were returned to their right-side-up position, with the hfRPE apical membrane now facing the lower chamber. In this configuration, the cells were cultured for 3 weeks. For the transepithelial migration experiments, 600 μL of migration assay medium (serum-free RPMI in the absence or presence of 50ng/mL IL-8 (R&D Systems) or 50 ng/mL MCP-1 (R&D Systems) was added to the lower compartment of the cell culture system. Human granulocytes and elutriated monocytes from healthy donors were provided by the department of transfusion medicine of the NIH Blood Bank (Bethesda, MD). The elutriation procedure was performed with a semiautomated and closed cell-separation system (Elutra; Gambro, Lakewood, CO). The system is a semiautomated, centrifuge-based laboratory device that uses continuous counterflow centrifugation elutriation (CCE) to separate cells based on their size, shape, and density. The system is used to separate a mononuclear cell (MNC)–rich leukapheresis product into enriched fractions of lymphocytes and monocytes. An aliquot of 100 μL of medium containing 6 × 105 granulocytes or monocytes respectively was added to the upper compartment, in the presence or absence of Fc-JAM-C and Fc-JAM-A, bathing the basolateral side of the epithelial monolayer. After incubation for 3 hours at 37°C, the number of transmigrated cells in the lower (apical membrane facing) compartment was estimated by manual counting in a Neubauer chamber.23
Statistical Analysis
Results are expressed as mean ± SD. Comparisons between more than two groups were analyzed by analysis of variance with Bonferroni adjustment (SPSS 11.5 software; SPSS, Chicago, IL) unless otherwise stated. An unpaired and two tailed Student’s t-test was used (Excel software; Microsoft, Redmond, WA) for the comparison of two groups. P < 0.05 was considered statistically significant.
Results
Expression and Localization of JAM-C in Human RPE
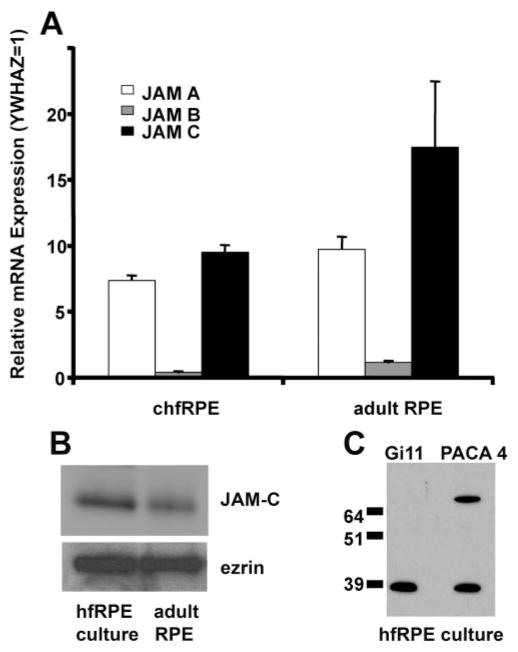
Recently, two members of the junctional adhesion molecule (JAM) family, JAM-A and JAM-C, were identified in mouse and human RPE cells.8,16 How these junctional proteins might help regulate inflammation in the eye is not yet clear.31 We first determined the expression of JAMs in human fetal RPE and adult native RPE by using quantitative PCR in cultured hfRPE cells and adult native tissue for all three members of the JAM family and found that JAM-A and -C are the two most prominently expressed members in human RPE (Fig. 1A). The medium abundance of YWHAZ in RPE also suggests a significant expression level for JAM-B. We focused on JAM-C, since to our knowledge its function in RPE is not yet known. In both cultured hfRPE and adult human native RPE (Fig. 1B) Western blot analysis was performed showing JAM-C expression. In addition, two distinct monoclonal antibodies to human JAM-C, clones Gi11 and PACA4, were used in hfRPE; both antibodies show the presence of a 39-kDa band, indicative of specific JAM-C expression (Fig. 1C).
Figure 1.
JAM-C expression in the RPE. (A) Quantitative PCR for JAM-A, -B, and -C was performed on RNA extracted from hfRPE cells and RPE cells from adult native tissue. JAM-C mRNA is the most abundant of the three family members in both adult native RPE and cultured hfRPE cells. The data are presented as the mean ± SD of resuslts in three experiments from three donors. (B) Western blot analysis of cultured hfRPE and adult human native RPE with mAb Gi11 specific for human JAM-C. Ezrin was used as a loading control. Similar results were obtained in three experiments. (C) Western blot analysis of cultured hfRPE with two mAbs specific for human JAM-C, mAb Gi11 and mAb PACA-4.
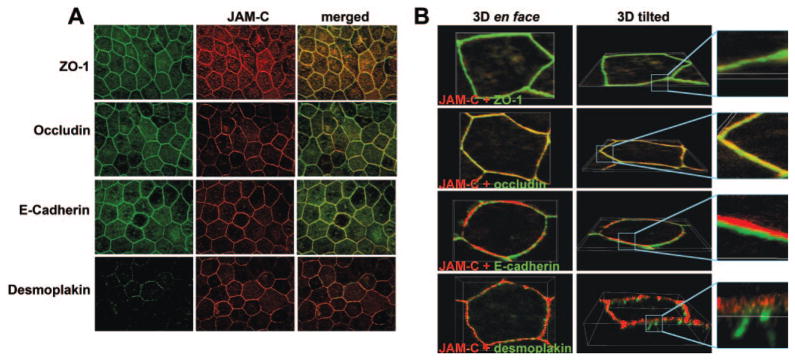
The immunofluorescence staining experiments in Figure 2A showed that JAM-C is localized within the junctional complex that surrounds hfRPE cells (middle column). To more closely determine the localization of JAM-C within the hfRPE junctional complex, we performed coimmunostaining of JAM-C with markers of the tight junctions (ZO-1, occludin), the adherens junctions (E-cadherin), and the desmosomes (desmoplakin). The merged images in Figure 2A (right) suggest that JAM-C colocalized with ZO-1 (yellow), occludin (yellow-green), and possibly E-cadherin (yellow-green). In contrast, colocalization between JAM-C and desmoplakin was not observed. Confocal microscopy analysis with 3-D reconstruction of single cells was then used to obtain a more exact localization of JAM-C (Fig. 2B). The tilted view of the 3-D reconstructed cells shows a striking colocalization of JAM-C with occludin and ZO-1 (top two rows), indicating that JAM-C was localized at the tight junctions. In the tilted view, the bottom two rows show that JAM-C staining was situation above that of E-cadherin and desmoplakin, indicating that JAM-C localizes apical to the adherens junctions and the desmosomes. The results shown are representative of those in three separate experiments. Similar data regarding the localization of JAM-C were obtained with another monoclonal antibody against JAM-C, PACA4 (data not shown).
Figure 2.
Localization of JAM-C in the cultured hfRPE cells. hfRPE monolayers were double stained with anti-JAM-C (red) and with anti-ZO-1, anti-occludin, anti-E-cadherin, and anti-desmoplakin (green). (A) Junctional localization of JAM-C. (B) En face and tilted views of 3-D reconstruction of single cells, showing that JAM-C colocalized with ZO-1 and occludin and was situated apically to E-cadherin and desmoplakin. Similar results were obtained in three experiments.
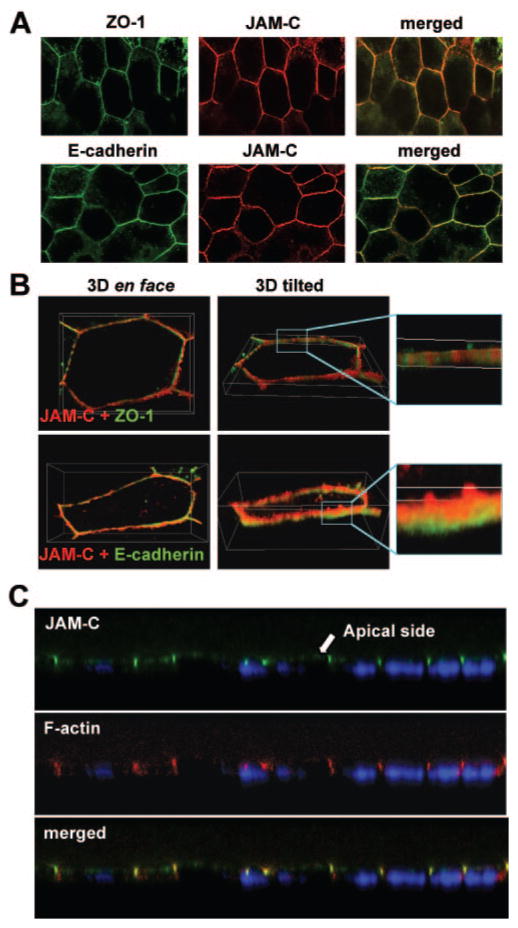
Similar results were obtained in adult human native RPE (Fig. 3A). The merged images indicated that JAM-C was localized within the junctional complex that surrounds each cell, as shown by its close localization with ZO-1 and E-cadherin. In Figure 3B, the 3-D reconstruction of single cells shows that JAM-C colocalized with ZO-1 at the tight junctions and was located apical to the adherens junctions, as marked by E-cadherin.
Figure 3.
Localization of JAM-C in fetal and adult human native RPE. Native RPE monolayers of adult donors were double stained with anti-JAM-C (red) and with anti-ZO-1 and anti-E-cadherin (green). (A) Junctional localization of JAM-C. (B) En face and tilted views of 3-D reconstruction of single cells, showing that JAM-C colocalized with ZO-1 and lies apically to E-cadherin. (C) Immunofluorescence staining for JAM-C (green) and F-actin (red) in human fetal RPE. The presence of JAM-C on the apical side of the RPE (microvilli) and in the tight junctions is shown in confocal cross-sectional images. Nuclei are stained with DAPI (blue).
In a previous report, JAM-C was shown to be present in the microvilli of murine RPE cells.16 To corroborate this result in human RPE, we performed immunofluorescence analysis for JAM-C in human fetal RPE cultures (Fig. 3C). JAM-C colocalized with F-actin, both in the microvilli and tight junctions. In the merged image the red (F-actin) and green (JAM-C) colors coincide (yellow), confirming the close proximity of these proteins in microvilli and tight junctions.
Proinflammatory Cytokines and Expression or Localization of JAM-C in hfRPE Cells
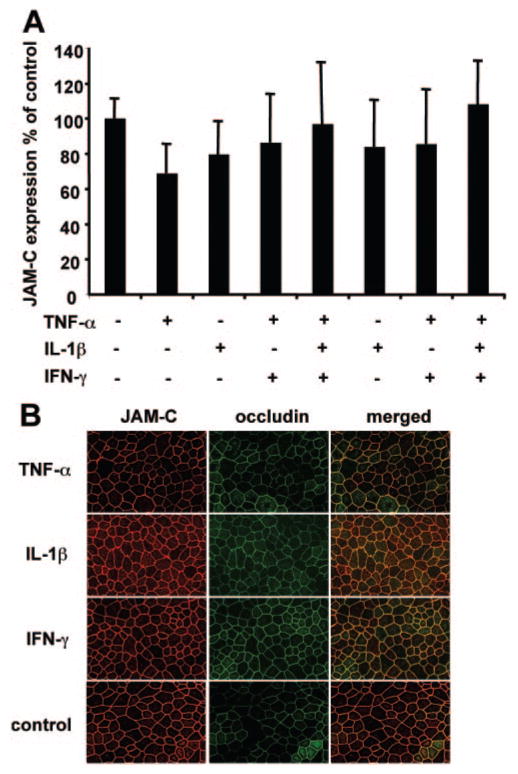
Confluent monolayers of hfRPE cells were grown for 6 weeks and stimulated by various combinations of cytokines for 24 hours to test whether JAM-C expression or localization is altered by proinflammatory cytokines.32,33 The expression of JAM-C was studied by Western blot and semiquantitative estimates of protein amount were obtained by a densitometric analysis of the blots. In Figure 4A, the 100% control represents the expression of JAM-C in hfRPE cells not stimulated with cytokines. Singly or in various combinations, the proinflammatory cytokines did not significantly alter the expression of JAM-C in hfRPE compared with the nonstimulated control. In addition, a 10-fold higher concentration of TNF-α had no effect on the expression of JAM-C (data not shown). Furthermore, immunofluorescence measurements show that the localization of JAM-C is not appreciably changed on stimulation of the hfRPE cells with TNF-α, IL-1β, or IFN-γ (Fig. 4B, merged image).
Figure 4.
JAM-C expression and localization is not affected by proinflammatory cytokines. (A) After stimulation of hfRPE monolayers with TNF-α, IL-1β, IFN-γ, all three together, TNF-α and IL-1β, TNF-α and IFN-γ, and IL-1β and IFN-γ, no significant change was found in the expression of JAM-C. The densitometry data are presented as a percentage of JAM-C expression of control. (B) Incubation of hfRPE cells with TNF-α, IL-1β, or IFN-γ caused no change in the localization of JAM-C when compared to the control.
A Role for JAM-C in Junction Formation and Polarization of hfRPE
Tight junction–associated proteins, such as ZO-1, are involved in the formation of primordial epithelial junctions.11,34,35 Given our observation of a close association of ZO-1 and JAM-C in hfRPE tight junctions, we examined the role of JAM-C in de novo junction formation by determining its localization during initial junction formation. In these experiments, summarized in Figure 5, cells were sparsely seeded on coverslips for 24 hours and then stained for JAM-C and ZO-1. Figure 5A, top, demonstrates that JAM-C was present at the sites of the initial cell– cell contacts (arrowheads) and in filopodia-like structures (arrows) of nonconfluent hfRPE cells that formed primordial junctions. These filopodia-like structures are reminiscent of the transient contacts that precede junction formation between MDCK cells.36 In these initial contacts and filopodia-like structures, JAM-C colocalized with ZO-1, which has been implicated in the assembly of the primordial junctional complex.11 In contrast, we found a diffuse intracellular JAM-C and ZO-1 staining in hfRPE cells that were not engaged in contacts with other cells (bottom). The observations in Figure 5A duplicate what we observed in hfRPE cells from three different donors.
Figure 5.
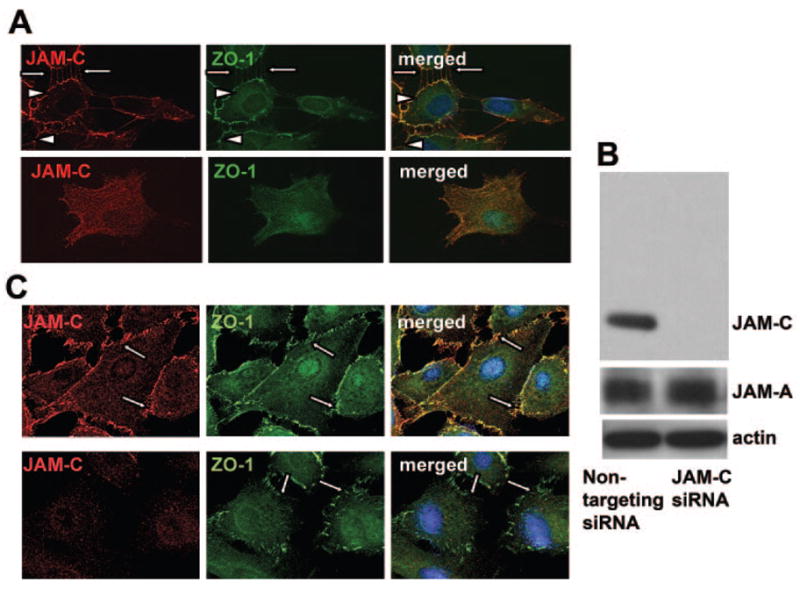
Role of JAM-C in the recruitment of ZO-1 to the initial hfRPE contacts. (A) Double staining for JAM-C (red) and ZO-1 (green) in contacting hfRPE cells show recruitment of JAM-C at the contacting surfaces of adjacent cells, similar to the distribution of ZO-1. In noncontacting hfRPE cells, immunofluorescence for both JAM-C and ZO-1 showed no specific staining. Similar results were obtained in three experiments. (B) Western blot analysis of hfRPE cells transfected with siRNA against JAM-C or with control siRNA as indicated, show the expression of JAM-C, JAM-A, and actin. JAM-A expression was not changed after JAM-C knockdown. (C) JAM-C knockdown caused a disruption in the ZO-1 localization at the initial contacts among hfRPE cells (bottom) compared with the control transfected cells (top). Similar results were obtained in three experiments.
To analyze the functional importance of the appearance of JAM-C in primordial junctions, we performed siRNA-mediated knockdown of JAM-C in primary cultures of hfRPE cells. As assessed by Western blot analysis, siRNA targeting JAM-C clearly downregulated JAM-C expression, whereas control, nontargeting siRNA did not (Fig. 5B). In contrast, the expression of a related protein, JAM-A was not affected by the siRNA-mediated knockdown of JAM-C, suggesting that compensatory changes in the expression of other JAM family members did not take place after JAM-C knockdown. In addition, knockdown of JAM-C in hfRPE cells disrupted the localization of ZO-1 at the primordial cell junctions (Fig. 5C, bottom, arrows). Similar results were obtained from three different experiments.
N-cadherin is a prominent marker of primordial junctions in RPE and participates in the organization of the adherens junctions.11,37 As assessed by immunofluorescence, the organization of N-cadherin in the primordial junctions of hfRPE was apparently altered after siRNA-mediated knockdown of JAM-C (Fig. 6A, bottom), Immunofluorescence staining, however, does not allow for quantitative analysis of changes in N-cadherin organization. Therefore, Western blots were used to compare the expression of surface-associated N-cadherin in control (nontargeting siRNA) and JAM-C siRNA-transfected hfRPE cells. An overall decrease of the surface-associated N-cadherin protein fraction was observed after JAM-C knockdown (Fig. 6B, top), whereas the total cell N-cadherin was not altered (Fig. 6B, middle). In contrast, the surface expression of another plasma membrane–associated protein, ICAM-1, which is not involved in primordial junction formation, was not affected after JAM-C knockdown (Fig. 6C). This latter experiment provides an additional internal loading control for the surface biotinylation experiments. The densitometric analysis of the ratio of surface N-cadherin to surface ICAM-1 from three different experiments (Fig. 6D) showed a significant decrease in the surface membrane N-cadherin after JAM-C knockdown, compared with the nontargeting siRNA control (P = 0.01; n = 3). These data indicate an early role for JAM-C in the formation of RPE adherens junctions.
Figure 6.
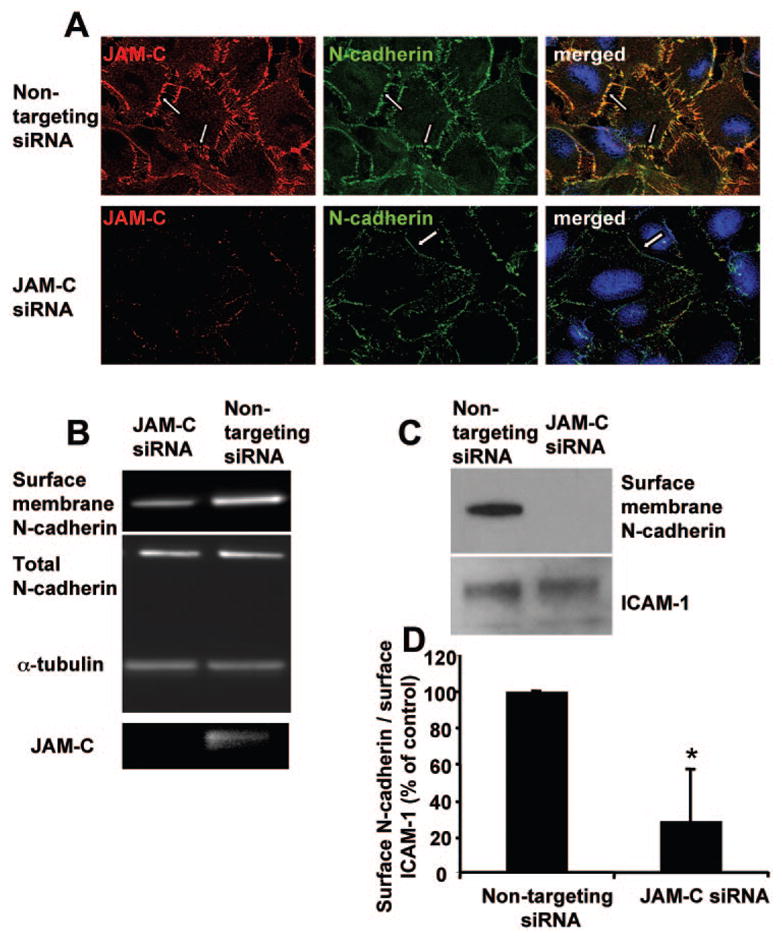
Effect of JAM-C in the recruitment of N-cadherin at the initial hfRPE contacts. (A) JAM-C and N-cadherin staining of control siRNA transfected and JAM-C siRNA transfected hfRPE cells. (B) Western blot analysis for the surface membrane fraction of N-cadherin, total N-cadherin, α -tubulin, and JAM-C in hfRPE cells transfected with control nontargeting siRNA or specific JAM-C targeting siRNA. A reduction in surface-associated N-cadherin but no change in total N-cadherin was observed on JAM-C knockdown. (C) Western blot analysis for the surface membrane fraction of N-cadherin and the surface membrane fraction of ICAM-1 in hfRPE cells transfected with control nontargeting siRNA or specific JAM-C targeting siRNA. (D) Densitometric analysis of the surface membrane fraction of N-cadherin and the surface membrane fraction of ICAM-1 in hfRPE cells transfected with control nontargeting siRNA or specific JAM-C targeting siRNA. Data are shown as a percentage of control and represent the ratio of surface membrane fraction of N-cadherin to the surface membrane fraction of ICAM-1 in cells transfected with nontargeting siRNA (mean ± SD; n = 3; *P < 0.05).
Primordial Junctional Complex Assembly and Epithelial Cell Polarity
The role of JAM-C in the polarization of hfRPE cells38 was investigated in immunofluorescence experiments using ezrin, which is localized strictly on the apical side of well-polarized hfRPE cells29,39 and is one of the earliest proteins to be polarized. Figures 7A and 7B (representative of three experiments) show that after JAM-C knockdown, ezrin immunolocalized on both the apical and basolateral sides of the hfRPE cells, indicating incomplete polarity (48 hours). As expected, the cells treated with a nontargeting siRNA showed a strictly apical localization of ezrin 48 and 72 hours after seeding. The images shown in Figure 7A are reconstructed cut views of z-stacks. The Western blot in Figure 7B shows that the JAM-C knockdown did not affect the expression of ezrin in the hfRPE cells. These data are consistent with an early role for JAM-C in the formation of the junctional complex.
Figure 7.
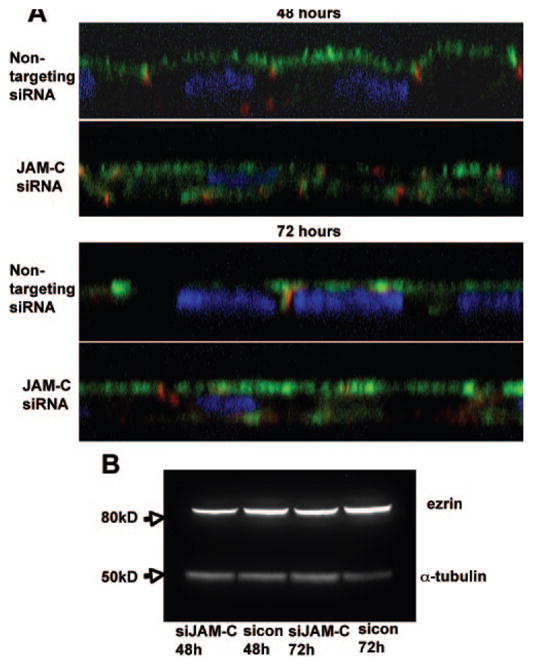
Role of JAM-C in the polarization of hfRPE cells. (A) Side views of immunofluorescent staining for ZO-1(red), ezrin (green), and DAPI (blue) in nontargeting siRNA and JAM-C siRNA-transfected hfRPE cells, 48 and 72 hours after seeding, show an apical and basolateral distribution of ezrin when JAM-C was downregulated compared with the control hfRPE cells, where ezrin staining is found strictly on the apical side. (B) Western blot of hfRPE cells transfected with JAM-C siRNA or nontargeting siRNA 48 and 72 hours after seeding, showing that there is no effect of JAM-C knockdown on the expression of ezrin.
Mediation of the Transepithelial Migration of Granulocytes but not Monocytes across Confluent hfRPE Monolayers
JAM-C has been shown to participate in the transmigration of inflammatory cells through endothelial and gut epithelial cells.18,22,23,40 Given the immunologic environment of the RPE and the variety of RPE–immune system interactions,41–44 we examined the possible role of JAM-C in mediating the basolateral-to-apical transmigration of granulocytes toward a chemotactic gradient of interleukin-8 and of monocytes toward MCP-1. The absolute number of transmigrated leukocytes was 6.22 ± 1.04 × 104 in the case of granulocytes and 11.13 ± 7.55 × 104 in the case of monocytes. The data summarized in Figure 8 show that inhibition of JAM-C by Fc-JAM-C significantly decreased the basolateral-to-apical transmigration of granulocytes through the hfRPE monolayer (Fig. 8A; P = 0.02; n = 3), whereas soluble JAM-A expressed as an Fc-fusion protein (Fc-JAM-A) did not affect the transepithelial migration of granulocytes through the hfRPE monolayer. On the other hand, Fc-JAM-C had no effect on the monocyte transmigration through the hfRPE (n = 3). These data suggest that JAM-C plays a role in the granulocyte basal-to-apical transepithelial migration through the hfRPE but may not participate in monocyte transmigration through the hfRPE.
Figure 8.
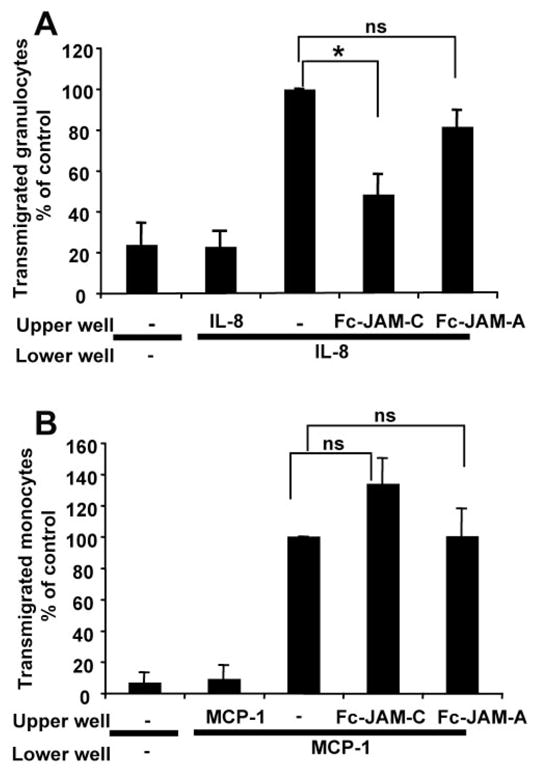
Effect of JAM-C on neutrophil transepithelial migration through the hfRPE monolayer. (A) Basolateral-to apical-transepithelial migration of granulocytes toward IL-8. In the absence of IL-8 or IL-8 gradient, almost no cells migrated through the hfRPE monolayer. A significant decrease in transmigrating granulocytes was observed when the JAM-C inhibitor Fc-JAM-C (P = 0.03) was added to the upper well. The inhibition of JAM-A had no significant effect on the number of transmigrating granulocytes. The number of transmigrating granulocytes is shown as a percentage of the control. The transmigration of granulocytes toward IL-8 in the lower well represents the 100% control. Data were pooled from three experiments. (B) In contrast, Fc-JAM-C had no significant effect on the basolateral-to-apical transepithelial migration of monocytes toward MCP-1. The number of transmigrating monocytes is shown as a percentage of the control. The transmigration of monocytes toward MCP-1 in the lower well represents the 100% control. Data were pooled from three experiments (*P < 0.05; ns, not significant).
Discussion
The RPE forms the outer BRB and, among epithelia, has the unusual property that its apical surface is in close contact with a cellular layer of photoreceptors, whereas the apical sides of most other epithelia face a lumen devoid of neuronal cells. As in other epithelia, RPE polarity is determined by a large variety of proteins that are localized at an apical junction complex that includes tight junctions and adherens junctions and help regulate interepithelial junctional integrity and permeability.29,45,46 The RPE maintains a constant active-solute–linked fluid transport from the subretinal space (apical side) to the choroid (basolateral side) and helps provide the close apposition of the retina and RPE.1,4 This vectorial function of the RPE is also determined by the proper formation of tight junctions and the regulated trafficking of different proteins to the apical and basolateral membranes. In situ, the correct polarization of the RPE is crucial for its ability to phagocytose photoreceptor outer segments, support the visual cycle, and maintain a constant chemical environment in the subretinal space. All these interactions at the RPE/distal retina interface are fundamentally important for maintaining the health and integrity of the neural retina.1–5
In the present study, we identified JAM-C as a tight junction component of both cultured hfRPE and adult human native RPE, colocalizing with the tight junction markers occludin and ZO-1 and lying apical to the adherens junctions and desmosomes. Previously, JAM-C was found in tight junctions or desmosomes of kidney and gut epithelial cells, respectively.15,18 More recently JAM-C has been localized to the interepithelial junctions of ARPE-19 cells and to the apical processes and junctional complex of mouse RPE, but the functional implications of this distribution remain to be determined.8,16
In chick RPE, it has been shown that junctional complex proteins like ZO-1, ZO-2, JAM-A, N-cadherin, AF-6, and PAR-3 participate in the development of the tight junctions.11,13 Previously, it was shown that JAM-A is localized at the initial junctions of Madin-Darby Canine Kidney (MDCK) epithelial cells.48 By contrast, in human RPE it seems clear (Figs. 5, 6) that JAM-C is intimately involved in the formation of initial cell– cell contacts. N-cadherin and ZO-1 are two of the proteins found at primordial adherens junctions and play a significant role in the assembly of the intercellular junctions11,34,49 Of note, JAM-C colocalized with ZO-1 and was found at the initial cell– cell contacts and around the filopodia-like structures of nonconfluent hfRPE cells that were forming primordial junctions. In MDCK cells, similar filopodia-like structures formed and disengaged until stable contacts formed and expanded into a continuous apical junctional complex. The accumulation of JAM-C at these sites might presage de novo junction assembly. The siRNA-mediated knockdown of JAM-C resulted in the disruption of ZO-1 distribution at the primordial cell– cell contacts during initial junction formation. More strikingly, downregulation of JAM-C expression resulted in an overall decrease in the surface expression of N-cadherin after JAM-C knockdown. Therefore, JAM-C aids in the initial junction formation of hfRPE cells presumably by affecting the organization of ZO-1 and N-cadherin at the primordial adherens junctions.
JAM-C could directly interact with ZO-1 via its C-terminal PDZ-domain binding motif.17 Such interactions may provide the molecular platform by which JAM-C regulates assembly of the apical junction complex. In contrast to a possible direct interaction between JAM-C and ZO-1, or any PDZ protein, it is as yet unclear how JAM-C affects the recruitment of N-cadherin to the primordial junctions of the RPE. It is possible that the reduction in JAM-C expression (knockdown experiments) causes structural disorganization of the primordial cell– cell contacts and indirectly affects the recruitment of N-cadherin. In any case, our findings indicate that JAM-C is a critical participant in the formation of RPE tight junctions. Alterations in its expression or function may affect RPE barrier function and could be implicated in the pathogenesis of diseases such as central serous chorioretinopathy and diabetic retinal edema.49–51
Of note, knockdown experiments showed that JAM-C can participate in the establishment of RPE cell polarity, as indicated by the apical localization of ezrin (Fig. 7A). The effect of JAM-C on cell polarity could be related to its propensity to recruit ZO-1 and N-cadherin to the initial contacts since the proximity of both of these proteins in the region of contact is important for the development of cell polarization.11,34,38,48 Moreover, JAM-C is known to associate with the polarity complex comprising the factors PAR-3, PAR-6, and the atypical protein kinase C.7,17 Thus, the presence of JAM-C at the initial contacts of the RPE may serve to recruit the PAR-3/aPKC/PAR-6 complex. In addition, JAM-C regulates the activity of small GTPases, such as cdc42 and Rap1.20,52 Whether the PAR-3/aPKC/PAR-6 complex or small GTPases act to mediate the effect of JAM-C in RPE polarization remains to be determined.
It is tempting to speculate that JAM-C can act as a developmental switch since hfRPE cultures strongly express JAM-C in the tight junctions, are fully polarized, and have a high trans-epithelial resistance compared with native tissue.29 In support of this notion is the recent experiments with the tumor cell line KLN205 which show that JAM-C expression can reversibly restore tight junction formation, significantly increase epithelial resistance, and stimulate integrin-mediated cell migration and adhesion.46
Recent studies in different organ systems have closely linked the pathophysiology of AMD, Alzheimer’s disease, and glomerular basement membrane disease.53–57 An element common to all three diseases is the accumulation of extracellular deposits that generate local inflammatory responses by activating the complement cascade and immune system.40,42 Several recent animal models have implicated chemokine ligand 2 (Ccl2/MCP-1) and fractalkine receptors in retinal microglia as critical regulators of drusen accumulation, decreased local inflammation, and the development of AMD.58–60 We and others61 have observed that significant amounts of MCP-1 and IL-8 are consecutively secreted at the apical side of RPE cells in a polarized manner.62 Both of these chemokines could form gradients across the RPE that coordinate monocyte and neutrophil presence at the RPE basement membrane. This could provide local surveillance/protection against the accumulation of drusen, which decreases over time due to RPE aging and cellular stress (e.g., phagocytosis), leading eventually to AMD.
All three JAM family members have been associated with leukocyte transmigration and are linked to inflammatory processes throughout the body.14 JAM-C is an established ligand of the leukocyte β2-integrin Mac-1, and this interaction has been implicated in leukocyte transmigration through gut epithelial cells.18 Our finding that Fc-JAM-C, but not soluble JAM-A, can reduce the transepithelial migration of granulocytes (Fig. 8A) to the apical side of the hfRPE suggests that JAM-C specifically helps mediate the transepithelial migration of granulocytes through the hfRPE monolayer. In contrast, Fc-JAM-C had no effect on monocyte transmigration driven by MCP-1. From other systems, it has been found that different molecules appear to mediate leukocyte transmigration in a leukocyte-specific manner. For example, the endothelial cell-selective adhesion molecule (ESAM) appears to mediate neutrophil rather than T-cell transmigration through endothelial cells and a similar situation may apply in the case of JAM-C and neutrophil or monocyte transmigration through the RPE.63 The RPE holds a strategic position in inflammatory processes within the retina, as it forms the outer BRB between the choroid and the sub-retinal space and selectively allows leukocytes from the circulation to the distal retina. Therefore, JAM-C may play a critical role in the initiation of inflammatory pathologies of the retina such as uveitis, chorioretinitis, and AMD.28,52,54,55,64
Acknowledgments
The authors thank Larry Rizzolo for critical reading of the manuscript, Triantafyllos Chavakis for helpful discussions, and Connie Zhi for expert technical assistance.
Supported by the Intramural Research Program of the National Eye Institute.
Footnotes
Disclosure: M. Economopoulou, None; J. Hammer, None; F. Wang, None; R. Fariss, None; A. Maminishkis, None; S.S. Miller, None
References
- 1.Marmor MF, Wolfensberger TJ. The Retinal Pigment Epithelium. Oxford: Oxford University Press; 1998. [Google Scholar]
- 2.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 3.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Ann Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher SK, Lewis GP, Linberg KA, Verardo MR. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment. Prog Retin Eye Res. 2005;24:395–431. doi: 10.1016/j.preteyeres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci. 1993;17(suppl):189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- 6.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 7.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 8.Mandell KJ, Berglin L, Severson EA, Edelhauser HF, Parkos CA. Expression of JAM-A in the human corneal endothelium and retinal pigment epithelium: localization and evidence for role in barrier function. Invest Ophthalmol Vis Sci. 2007;48:3928–3936. doi: 10.1167/iovs.06-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandell KJ, Holley GP, Parkos CA, Edelhauser HF. Antibody blockade of junctional adhesion molecule-A in rabbit corneal endothelial tight junctions produces corneal swelling. Invest Ophthalmol Vis Sci. 2006;47:2408–2416. doi: 10.1167/iovs.05-0745. [DOI] [PubMed] [Google Scholar]
- 10.Dejana E, Spagnuolo R, Bazzoni G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb Haemost. 2001;86:308–315. [PubMed] [Google Scholar]
- 11.Rizzolo LJ. Development and role of tight junctions in the retinal pigment epithelium. Int Rev Cytol. 2007;258:195–234. doi: 10.1016/S0074-7696(07)58004-6. [DOI] [PubMed] [Google Scholar]
- 12.Rehder D, Iden S, Nasdala I, et al. Junctional adhesion molecule-a participates in the formation of apico-basal polarity through different domains. Exp Cell Res. 2006;312:3389–3403. doi: 10.1016/j.yexcr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Luo Y, Fukuhara M, Weitzman M, Rizzolo LJ. Expression of JAM-A, AF-6, PAR-3 and PAR-6 during the assembly and remodeling of RPE tight junctions. Brain Res. 2006;1110:55–63. doi: 10.1016/j.brainres.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 14.Aurrand-Lions M, Johnson-Leger C, Lamagna C, Ozaki H, Kita T, Imhof BA. Junctional adhesion molecules and interendothelial junctions. Cells Tissues Organs. 2002;172:152–160. doi: 10.1159/000066967. [DOI] [PubMed] [Google Scholar]
- 15.Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98:3699–3707. doi: 10.1182/blood.v98.13.3699. [DOI] [PubMed] [Google Scholar]
- 16.Daniele LL, Adams RH, Durante DE, Pugh EN, Jr, Philp NJ. Novel distribution of junctional adhesion molecule-C in the neural retina and retinal pigment epithelium. J Comp Neurol. 2007;505:166–176. doi: 10.1002/cne.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebnet K, Aurrand-Lions M, Kuhn A, et al. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J Cell Sci. 2003;116:3879–3891. doi: 10.1242/jcs.00704. [DOI] [PubMed] [Google Scholar]
- 18.Zen K, Babbin BA, Liu Y, Whelan JB, Nusrat A, Parkos CA. JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol Biol Cell. 2004;15:3926–3937. doi: 10.1091/mbc.E04-04-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satohisa S, Chiba H, Osanai M, et al. Behavior of tight-junction, adherens-junction and cell polarity proteins during HNF-4alpha-induced epithelial polarization. Exp Cell Res. 2005;310:66–78. doi: 10.1016/j.yexcr.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Orlova VV, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell–cell contacts. J Exp Med. 2006;203:2703–2714. doi: 10.1084/jem.20051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamagna C, Meda P, Mandicourt G, et al. Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion. Mol Biol Cell. 2005;16:4992–5003. doi: 10.1091/mbc.E05-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santoso S, Sachs UJ, Kroll H, et al. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chavakis T, Keiper T, Matz-Westphal R, et al. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J Biol Chem. 2004;279:55602–55608. doi: 10.1074/jbc.M404676200. [DOI] [PubMed] [Google Scholar]
- 24.Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 26.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 27.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nussenblatt RB, Ferris F., 3rd Age-related macular degeneration and the immune response: implications for therapy. Am J Ophthalmol. 2007;144:618–626. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geisen P, McColm JR, Hartnett ME. Choroidal endothelial cells transmigrate across the retinal pigment epithelium but do not proliferate in response to soluble vascular endothelial growth factor. Exp Eye Res. 2006;82:608–619. doi: 10.1016/j.exer.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 32.Li R, Maminishkis A, Wang FE, Miller SS. PDGF-C and -D induced proliferation/migration of human RPE is abolished by inflammatory cytokines. Invest Ophthalmol Vis Sci. 2007;48:5722–5732. doi: 10.1167/iovs.07-0327. [DOI] [PubMed] [Google Scholar]
- 33.McKenzie JA, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–228. doi: 10.1002/jcp.21114. [DOI] [PubMed] [Google Scholar]
- 34.Ikenouchi J, Umeda K, Tsukita S, Furuse M, Tsukita S. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol. 2007;176:779–786. doi: 10.1083/jcb.200612080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 36.McNeill H, Ryan TA, Smith SJ, Nelson WJ. Spatial and temporal dissection of immediate and early events following cadherin-mediated epithelial cell adhesion. J Cell Biol. 1993;120:1217–1226. doi: 10.1083/jcb.120.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams CL, Nelson WJ, Smith SJ. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell–cell adhesion. J Cell Biol. 1996;135:1899–1911. doi: 10.1083/jcb.135.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzolo LJ. Polarity and the development of the outer blood-retinal barrier. Histol Histopathol. 1997;12:1057–1067. [PubMed] [Google Scholar]
- 39.Kivela T, Jaaskelainen J, Vaheri A, Carpen O. Ezrin, a membrane-organizing protein, as a polarization marker of the retinal pigment epithelium in vertebrates. Cell Tissue Res. 2000;301:217–223. doi: 10.1007/s004410000225. [DOI] [PubMed] [Google Scholar]
- 40.Aurrand-Lions M, Lamagna C, Dangerfield JP, et al. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J Immunol. 2005;174:6406–6415. doi: 10.4049/jimmunol.174.10.6406. [DOI] [PubMed] [Google Scholar]
- 41.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 42.Mandal MN, Ayyagari R. Complement factor H: spatial and temporal expression and localization in the eye. Invest Ophthalmol Vis Sci. 2006;47:4091–4097. doi: 10.1167/iovs.05-1655. [DOI] [PubMed] [Google Scholar]
- 43.Hageman GS, Anderson DH, Johnson LV, et al. A common haplo-type in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bok D. Evidence for an inflammatory process in age-related macular degeneration gains new support. Proc Natil Acad Sci U S A. 2005;102:7053–7054. doi: 10.1073/pnas.0502819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin M, Barron E, He S, Ryan SJ, Hinton DR. Regulation of RPE intercellular junction integrity and function by hepatocyte growth factor. Invest Ophthalmol Vis Sci. 2002;43:2782–2790. [PubMed] [Google Scholar]
- 46.Mandicourt G, Iden S, Ebnet K, Aurrand-Lions M, Imhof BA. JAM-C regulates tight junctions and integrin-mediated cell adhesion and migration. J Biol Chem. 2007;282:1830–1837. doi: 10.1074/jbc.M605666200. [DOI] [PubMed] [Google Scholar]
- 47.Ebnet K, Suzuki A, Horikoshi Y, et al. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) EMBO J. 2001;20:3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tepass U, Truong K, Godt D, Ikura M, Peifer M. Cadherins in embryonic and neural morphogenesis. Nat Rev. 2000;1:91–100. doi: 10.1038/35040042. [DOI] [PubMed] [Google Scholar]
- 49.Eandi CM, Ober M, Iranmanesh R, Peiretti E, Yannuzzi LA. Acute central serous chorioretinopathy and fundus autofluorescence. Retina. 2005;25:989–993. doi: 10.1097/00006982-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Nakazawa T, Hisatomi T, Nakazawa C, et al. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photo-receptor apoptosis. Proc Natl Acad Sci U S A. 2007;104:2425–2430. doi: 10.1073/pnas.0608167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyamoto N, de Kozak Y, Jeanny JC, et al. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia. 2007;50:461–470. doi: 10.1007/s00125-006-0539-2. [DOI] [PubMed] [Google Scholar]
- 52.Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 53.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration: emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker PD. Dense deposit disease: new insights. Curr Opin Nephrol Hypertens. 2007;16:204–212. doi: 10.1097/MNH.0b013e3280bdc0f4. [DOI] [PubMed] [Google Scholar]
- 55.Zipfel PF, Heinen S, Jozsi M, Skerka C. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol Immunol. 2006;43:97–106. doi: 10.1016/j.molimm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 56.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 57.Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J, Connor KM, Smith LE. Overstaying their welcome: defective CX3CR1 microglia eyed in macular degeneration. J Clin Invest. 2007;117:2758–2762. doi: 10.1172/JCI33513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 60.Combadiere C, Feumi C, Raoul W, et al. CX3CR1-dependent sub-retinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holtkamp GM, De Vos AF, Peek R, Kijlsta A. Analysis of the secretion pattern of monocyte chemotactic protein-1 (MCP-1) and transforming growth factor-beta 2 (TGF-beta2) by human retinal pigment epithelial cells. Clin Exp Immunol. 1999;118:35–40. doi: 10.1046/j.1365-2249.1999.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi G, Maminishkis A, Banzon T, et al. Control of chemokine gradients by the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2008;49(10):4620–4630. doi: 10.1167/iovs.08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 64.de Boer JH, Hack CE, Verhoeven AJ, et al. Chemoattractant and neutrophil degranulation activities related to interleukin-8 in vitreous fluid in uveitis and vitreoretinal disorders. Invest Ophthalmol Vis Sci. 1993;34:3376–3385. [PubMed] [Google Scholar]