Abstract
Purpose
To compare subbasal nerve densities estimated from images recorded by the Tandem Scanning and the ConfoScan 4 confocal microscopes.
Methods
Confocal microscopy was used to estimate subbasal nerve density in 62 corneas of 40 subjects (18 corneas of 18 normal subjects and 44 corneas of 22 patients between 1 and 12 months after LASIK) At each examination, corneas were scanned first by using Tandem Scanning and then by using a ConfoScan 4 confocal microscope. Subbasal nerves from 2 to 4 scans per cornea were traced by using a semi-automated nerve analysis program. Nerve density was expressed as total nerve length divided by the sample area (µm/mm2). Differences in nerve density between instruments were examined by using paired tests.
Results
In normal corneas, subbasal nerve density was 10,658 ± 5,581 µm/mm2 (mean ± SD) with the ConfoScan 4 and 5,534 ± 1,850 µm/mm2 with the Tandem Scanning microscope (P<0.0001). One to 12 months after LASIK, mean subbasal nerve density was 2,477 ± 3,514 µm/mm2 estimated with the ConfoScan 4 and 844 ± 983 µm/mm2 estimated with the Tandem Scanning (P=0.0003). Estimates of nerve density were correlated between instruments (r=0.71, P<0.0001), although the mean difference between instruments was 2,308 ± 3,885 µm/mm2 (P<0.0001).
Conclusions
Mean subbasal nerve density estimated with the ConfoScan 4 was 2 to 3 times higher than density estimated with the Tandem Scanning confocal microscope. These differences must be considered when comparing subbasal nerve densities between studies that use different confocal microscopes.
INTRODUCTION
Confocal microscopy provides a noninvasive method of observing and estimating subbasal nerve density in living human corneas. Subbasal nerves are visible as bright, linear objects that are limited to a relatively thin region between Bowman’s layer and the basal cells of the epithelium.1–3
Various confocal microscope designs have been used to study the cornea. Two commonly used confocal microscopes are the Tandem Scanning (Tandem Scanning, Reston, VA) and the ConfoScan 4 (Nidek, Inc., Fremont, CA). Tandem Scanning uses a point scanning design. The focal plane is illuminated through an array of pinhole apertures and imaged through a conjugate set of apertures on the opposite side of the disk. The image is formed as the disk spins, scanning the apertures rapidly across the field. This design provides excellent transverse and axial resolution, and because of the small pinhole diameters (typically 30 µm), it has a narrow depth of field. Our measurements show a depth of field of approximately 11 µm.4 As a consequence of this resolution, images from this instrument have less field brightness and contrast than microscopes with larger apertures.
The ConfoScan 4 confocal microscope with a z-ring adapter uses a slit scanning design. The field is illuminated through a vertical slit aperture (180 µm wide) and the image is viewed through a conjugate slit of the same size. The wide slit aperture increases field brightness considerably compared to the field in the Tandem Scanning microscope, although it does so at the expense of a greater depth of field. We reported a depth of field of 26 µm for the ConfoScan 3, which has a similar optical design.4
Corneal nerves are visible in images from both the Tandem Scanning and ConfoScan 4 confocal microscopes and both microscopes have been used to estimate subbasal nerve density in humans in vivo.2,3.5,6 Subbasal nerve densities in normal corneas have ranged from 5,867 to 11,110 µm/mm2 when using these confocal microscopes, and it is not clear if differences in image brightness and contrast, or differences in depth of field affect instrument sensitivity for detecting subbasal nerves. 2,3,5,6 Some of the large variation of subbasal nerve densities reported by investigators may be attributed to differences in instrument design.
In this study, we measured and compared subbasal nerve densities in two groups, normal corneas with normal subbasal nerve densities and early post-LASIK corneas with diminished subbasal nerve densities. Subbasal nerve densities were estimated from images recorded by the Tandem Scanning and the ConfoScan 4 confocal microscopes and differences in densities were examined.
METHODS
Subjects and Examination
Eighteen normal corneas of 18 subjects and 44 corneas of 22 patients between 1 and 12 months after LASIK (total of 75 post-LASIK examinations) were examined by using confocal microscopy. The normal subjects (8 men and 10 women) had a mean age of 38 ± 10 years (± SD). The post-LASIK patients (5 men and 17 women) had a mean age of 39 ± 9 years. At each examination, corneas were examined first by using a Tandem Scanning confocal microscope and then by using a ConfoScan 4 confocal microscope with a z-ring adapter. Subjects with previous surgery or injury, glaucoma, diabetes, or who were using topical ophthalmic medications were excluded. Each subject gave informed consent to participate after the nature and possible consequences of the study had been explained. The study was approved by the Institutional Review Board of Mayo Clinic and followed the principles of the Declaration of Helsinki for research involving human subjects.
Confocal microscopy
Methods for using the Tandem Scanning and ConfoScan 4 confocal microscopes have been previously described.3,4 Briefly, an optical coupling solution, (GenTeal Gel, Novartis Ophthalmics, East Hanover, NJ) was placed on the tip of the Tandem Scanning objective lens, and the objective was advanced until the solution contacted the central cornea. The objective lens was aligned with the central cornea by centering light and dark rings in the epithelial image as the focal plane was positioned on the epithelial surface. The focal plane was scanned anterior to posterior along the z-axis through the cornea at approximately 72 µm/second while digital images were captured by a low-light camera (VE-1000 SIT, DAGE-MTI, Michigan City, IN) at 30 frames/second and stored on a computer workstation (Indy, Silicon Graphics Inc., Mountain View, CA). The camera automatically adjusted the video gain to optimize image brightness. Consecutive images were separated by approximately 2.4 µm.7 After each scan the objective lens was removed from the cornea. The objective lens was then repositioned on the central cornea for each subsequent scan. The cornea was scanned 2–4 times.
During examination with the ConfoScan 4 confocal microscope, GenTeal Gel was placed on the tip of the objective lens and on the z-ring adapter, and the operator advanced the objective lens and z-ring until the coupling solution contacted the central cornea. The operator adjusted the position of the objective lens until a bright image of the endothelium was visible in the center of the field. In most scans, the ConfoScan 4 completed the final alignment automatically by using the auto-adjust feature. In some corneas, the alignment was entirely manual. The operator then advanced the focal plane approximately 30 to 50 µm into the anterior chamber (i.e. deep to the endothelium) and initiated the exam. The instrument recorded video frames at 25 frames/second as the focal plane scanned from posterior to anterior through the cornea. The scan stopped when the focal plane moved anterior to the epithelium, as determined by the changing intensity in the image. The focal plane was then reset to the starting position and the scan was repeated. The step distance between frames was 5 µm, and scans were repeated until 350 frames were captured. The objective lens was removed and then repositioned for each subsequent scan. Each cornea was scanned 2–4 times.
Subbasal Nerve Density
Subbasal nerves were defined as all visible nerve fiber bundles anterior to Bowman’s layer. One investigator (EAE), who was masked to patient and postoperative time, measured the total length of all visible subbasal nerves (µm) and their branches longer than 50 µm in 2 to 4 scans of each cornea at each examination. Nerves were traced by using NeuronJ, a semi-automated nerve analysis plug-in program of ImageJ (Figure 1) 8 Each nerve was traced only once, but if the nerve length extended across several images, the total length was measured as if it were projected onto one image.
Figure 1.
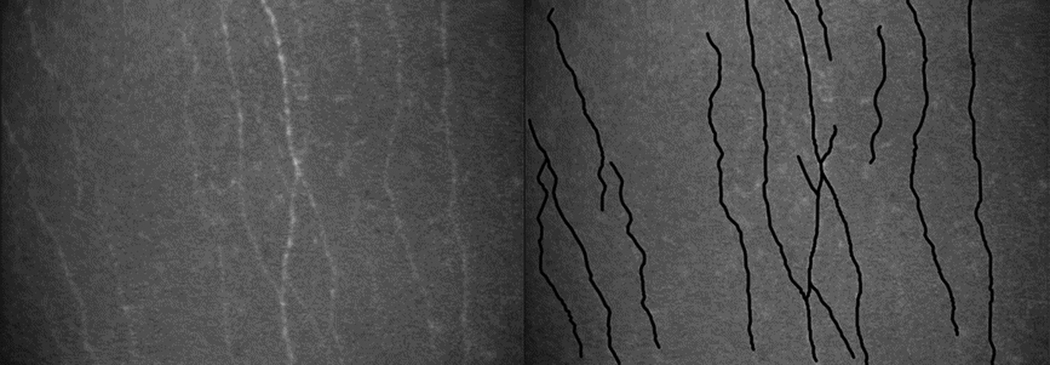
Subbasal nerve density measurement. (Left) Subbasal nerves in a normal cornea as recorded by using Tandem Scanning confocal microscope and (right) the same image after tracing nerves by using a semi-automated nerve analysis program. 8 Subbasal nerve density (µm/mm2) was the total length of nerve (µm) measured per image sample area (0.058 mm2 for the ConfoScan 4 and 0.166 mm2 for Tandem Scanning).
Nerve density was expressed as the total length of nerve fibers traced per image area (µm/mm2). Spatial dimensions in both instruments were determined by measuring the number of pixels that corresponded to 100 µm in images of a calibrated scale. All nerve lengths were calculated from a calibration factor of 0.56 µm/pixel in scans by the ConfoScan 4 and 0.78 µm/pixel in images from the Tandem Scanning.
The total nerve fiber length (µm) was determined from the coordinates of the tracings recorded by NeuronJ. Confocal image brightness diminished from the center to the edges of the full image field. 7 Image brightness may affect nerve visibility. In order to maintain uniform image brightness in both confocal microscopes, we retricted the image sample area to ≥ 60% maximum central brightness. As a result, the image sample area for the ConfoScan 4 was a central rectangle with an area of 0.081 mm2 or 59% of the full visible field area. The sample area for the Tandem Scanning confocal microscope was 0.166 mm2 or 99% of the full visible field area, and we did not reduce the size of the full field. The nerve density recorded for each cornea was the mean nerve density of all scans for that examination.
Statistical Analysis
Differences in subbasal nerve density between instruments were assessed by using paired tests. Generalized estimating equation models were used to adjust for possible correlation between eyes from the same subject.9 Linear correlation between microscopes was examined by calculating Spearman correlation coefficients. Limits of agreement between microscopes was the mean difference ± two standard deviations of the difference between microscopes, as described by Bland and Altman.10 All statistics were calculated by using Statistical Analysis Software (Version 9.1.3, SAS Institute Inc., Cary, NC).
RESULTS
Mean subbasal nerve densities estimated by using the ConfoScan 4 were significantly greater than those estimated by using the Tandem Scanning confocal microscope in normal corneas and in post-LASIK corneas (P≤0.0003; Table 1). Despite these differences, central subbasal nerve densities measured by using one instrument were correlated with those measured by using the other instrument in the same corneas (r= 0.71, P<0.0001; Figure 2). The mean difference in nerve density between the two instruments was 2,308 ± 3,885 µm/mm2 (P<0.0001; Figure 2), and nerve density was greater when measured with the ConfoScan 4.. The difference in estimated nerve density between the two instruments increased with increasing mean nerve densities (r = 0.78, P<0.0001; Figure 2).
Table 1.
Subbasal Nerve Density Estimates in Normal and Post-LASIK Corneas by using Two Confocal Microscopes
| Subbasal nerve density, µm/mm2(means ± SD) |
|||
|---|---|---|---|
| Instrument | Normal cornea (n = 18) |
Post-LASIK cornea (n = 75)a |
All Corneas (n = 93) |
| Confoscane 4 | 10,658 ± 5,581b | 2,477 ± 3,514c | 4,060± 5,124b |
| Tandem scanning | 5,534 ± 1,850b | 844 ± 983c | 1,752 ± 2,209b |
n, number of confocal microscopy examinations.
Post-LASIK examination were at 1-month (n=10), 3 months(n=26), 6-months1-years (n=29).
P<0.0001, paired, test generalized estimating equal models, confoscan 4 vs. tandem scanning microscope.
P=0.0003,paired test, generalized estimating equation models confoscane 4 vs tandem scanning confocal microscope.
Figure 2.
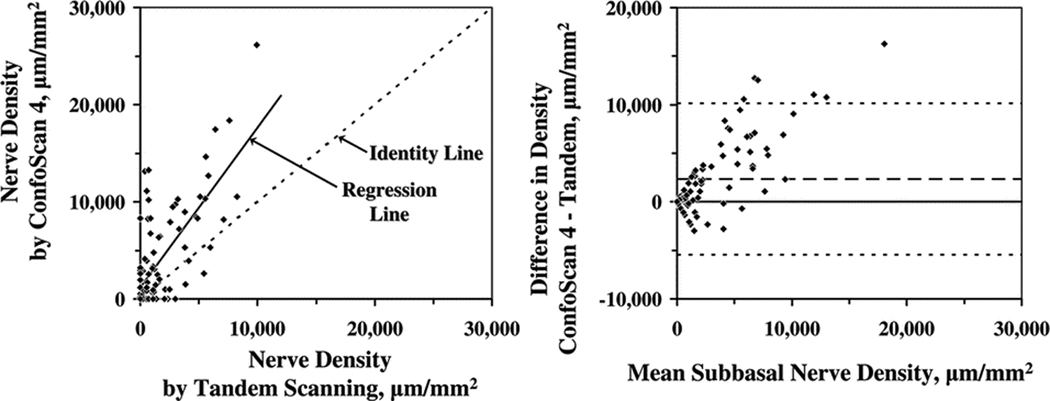
(Left) Subbasal nerve densities measured by using the ConfoScan 4 were greater than those measured by using Tandem Scanning confocal microscope, although the data were well correlated (r=0.71, P<0.0001, n=82). The dotted line is the identity line and the solid line is the linear regression. (Right) The mean difference in subbasal nerve density between microscopes (ConfoScan 4 – Tandem Scanning) was 2,308 ± 3,885 µm/mm2 (dashed line, P<0.0001), and the difference increased with increasing density. The limits of agreement are indicated by the fine dotted lines.
Subbasal nerve densities estimated from the ConfoScan 4 were normalized by using the regression equation y = mx + b and were compared with estimates from the Tandem Scanning confocal microscope, where y is normalized nerve density and x is density estimated by the ConfoScan 4. The coefficients m = 0.608 and b = -718 were determined by regression of the measurements from the ConfoScan 4 on those from the Tandem Scanning confocal microscope (Figure 3). There was no significant difference between the normalized density and density determined from the Tandem Scanning confocal microscope (0 ± 2,199 µm/mm2). The limits of agreement, the mean difference ± 2 standard deviations of the difference, extended from −4,398 µm/mm2 to 4,398 µm/mm2 (Figure 4). The mean difference between the normalized density from the ConfoScan 4 and density determined from the Tandem Scanning microscope images increased somewhat as mean density increased, although with a much weaker relationship than it did before normalization (r=0.43, p=0.04; Figure 3).
Figure 3.
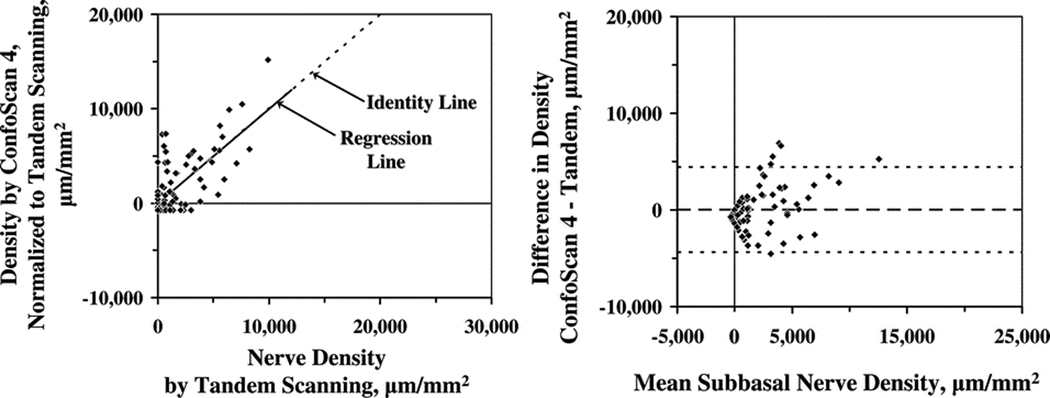
(Left) Relationship between subbasal nerve densities estimated by using the ConfoScan 4 and by using the Tandem Scanning confocal microscope after densities were normalized by using the regression formula: y = 0.608x - 718, where y is normalized nerve density and x is density estimated by the ConfoScan 4. (Right) The adjusted mean difference between microscopes (ConfoScan 4 – Tandem Scanning) was 0 ± 2,199 µm/mm2. The limits of agreement are indicated by the fine dotted lines.
DISCUSSION
Subbasal nerve densities estimated by using the ConfoScan 4 confocal microscope were approximately 2–3 times greater than those estimated by using the Tandem Scanning confocal microscope. The difference in estimates between the instruments increased as density increased. Why should the ConfoScan 4 estimate a higher nerve density than the Tandem Scanning when examining the same corneas? The brighter, higher contrast images recorded from the ConfoScan 4 likely provide visualization of nerves that are too narrow or not bright enough to be detected in images from the Tandem Scanning confocal microscope. Despite these absolute differences, estimates of subbasal nerve density between instruments were well correlated and can be compared if they are normalized by using regression analysis. However, normalization does not consider differences in the ratio of small to large nerves, if these differences exist between the treatments that are being compared.
The ConfoScan 4 was able to detect significantly more nerves in both normal corneas and in post-LASIK corneas. After LASIK, regenerating subbasal nerves are initially smaller in caliber than subbasal nerves in normal, unoperated corneas.3, 5 There may be multiple reasons for the greater visibility of smaller nerves in images recorded by the ConfoScan 4. First, nerve visibility may be related to differences in confocal design, particularly differences in the size and shape of the confocal apertures between the two microscopes. The small pinhole apertures of the Tandem Scanning microscope limit field brightness and image contrast. The relatively wide linear slit aperture of the ConfoScan 4 provides brighter illumination of objects in the field, including nerves, and greater contrast between the objects and their background. Consequently, nerves appear brighter, sharper, and more textured than they do in images from the Tandem Scanning confocal microscope. Second, the ConfoScan 4 has slightly higher magnification than the Tandem Scanning which could contribute to greater visibility of nerves. Third, the ConfoScan 4 may have estimated a higher subbasal nerve density if the ConfoScan 4’s thicker optical section (26 µm) included sub-Bowman’s stromal nerves, assuming they were present in the central stroma. 12 We recently measured the optical section thickness of our ConfoScan 4, and it was similar to that of our ConfoScan 3, approximately 26 µm
In contrast, the Tandem Scanning and ConfoScan 4 confocal microscopes do not estimated corneal keratocyte density differently when differences in focal depth are considered.13 This may be because keratocyte nuclei are relatively large (12 µm in diameter) and are visible with both microscopes, whereas the population of smallest subbasal nerves and nerve fiber bundles (< 3 µm in diameter) are detectable with the Confoscan 4, but are below the threshold of detection with the Tandem Scanning microscope. Other confocal microscopes, such as the HRT II laser scanning confocal microscope (Heidelberg Engineering, Heidelberg, Germany) may be able to detect even smaller-caliber nerves than were detected by the ConfoScan 4. Patel and McGhee estimated 25% greater subbasal nerve densities in normal corneas with a laser scanning confocal microscope than our estimates using the ConfoScan 4.12 Therefore, it is important to consider the optical properties of the instrument used to record images of the subbasal nerves when comparing densities of nerves across studies.
The apparent density of subbasal nerves in images recorded with the ConfoScan 4 was greatest in the center of the field and diminished laterally in the peripheral field. This pattern is similar to the change in brightness across the field and represents a variation in sensitivity that limits this method to detect nerve fibers. For this reason, we used a sample area in the center of the field (59% of the full field) where the apparent nerve density was uniform to within 60% of the maximum. Nevertheless, within the bounding area, the sensitivity was still non-uniform and may cause an underestimate when using the ConfoScan 4 to determine subbasal nerve density. In addition, the use of different bounding areas in other studies might affect comparisons of ConfoScan 4 data.
After transformation, the mean difference between the transformed density by the ConfoScan 4 and the density by the Tandem Scanning microscope still increased somewhat as mean nerve density increased (Figure 3). This increase was considerably less than it was before transformation. Nevertheless, the weaker correlation suggests that one must use care in comparing nerve densities measured by using one instrument with densities measured by using the other, even after transformation.
In summary, subbasal nerve densities estimated by using the ConfoScan 4 confocal microscope are significantly greater than those estimated by using the Tandem Scanning confocal microscope. Because of this large difference, these confocal microscopes should not be used interchangeably within the same study. If it is necessary to compare nerve densities between these two microscopes, then densities estimated from the ConfoScan 4 must first be normalized to the Tandem Scanning confocal microscope.
Acknowledgments
Supported by NIH Grant EY02037, Research to Prevent Blindness, Inc., New York, New York (SVP as Olga Keith Wiess Special Scholar and an unrestricted departmental grant), and Mayo Foundation, Rochester, Minnesota.
Footnotes
Presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, April 30, 2008.
REFERENCES
- 1.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents, and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–384. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Erie JC. Corneal wound healing after photorefractive keratectomy: a 3-year 2 confocal microscopy study. Trans Am Ophthalmol Soc. 2003;101:293–333. [PMC free article] [PubMed] [Google Scholar]
- 4.McLaren JW, Nau CB, Patel SV, Bourne WM. Measuring corneal thickness with 4 the ConfoScan 4 and Z-ring adapter. Eye Contact Lens. 2007;33:185–190. doi: 10.1097/ICL.0b013e31802b3114. [DOI] [PubMed] [Google Scholar]
- 5.Calvillo MP, McLaren JW, Hodge DO, Bourne WM. Corneal reinnervation after LASIK: Prospective 3-year longitudinal study. Invest Ophthal Vis Sci. 2004;45:3991–3996. doi: 10.1167/iovs.04-0561. [DOI] [PubMed] [Google Scholar]
- 6.Erie JC, McLaren JW, Hodge DO, Bourne WM. The affect of age on the corneal subbasal nerve plexus. Cornea. 2005;24:705–709. doi: 10.1097/01.ico.0000154387.51355.39. [DOI] [PubMed] [Google Scholar]
- 7.Mclaren JW, Nau CB, Erie JC, et al. Corneal thickness measurement by confocal microscopy, ultrasound, and scanning slit methods. Am J Ophthalmol. 2004;137:1011–1020. doi: 10.1016/j.ajo.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 8.Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry. 2004;58:167–176. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- 9.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurements. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 11.Darwish T, Brahma A, O’Donnell C, Efron N. Subbasal nerve fiber regeneration after LASIK and LASEK assessed by noncontact esthesiometry and in vivo confocal microscopy: Prospective study. J Cataract Refract Surg. 2007;33:1515–1521. doi: 10.1016/j.jcrs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Patel DV, McGhee CN. Mapping of the human corneal subbasal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthal Vis Sci. 2005;46:4485–4488. doi: 10.1167/iovs.05-0794. [DOI] [PubMed] [Google Scholar]
- 13.McLaren JW, Nau CB, Kitzmann AS, Bourne WM. Keratocyte density. Comparison of two confocal microscopes. Eye Contact Lens. 2005;31:28–33. doi: 10.1097/01.icl.0000151948.92593.c3. [DOI] [PubMed] [Google Scholar]


