I am pleased to provide an update on the policies and programs of the National Heart, Lung, and Blood Institute (NHLBI). This is an exciting time of many transitions for the federal government as a whole and the National Institutes of Health (NIH) in particular. As these changes take form, I want to assure you of our unwavering commitment to support our community of heart, lung and blood investigators and to the goals articulated in the NHLBI Strategic Plan. My goal in this editorial is to discuss the NHLBI budget for the 2009 fiscal year (October 1, 2008-September 30, 2009, FY 2009) and to describe new policies that we are implementing for early stage investigators and for revised grant applications.
FY 2009 Budget
The NIH is operating under a continuing resolution (CR)1, which means that Congress has not provided the NIH with an appropriation for FY 2009. This requires us to operate at the same level of funding as we had in FY 2008. Because we are obligated to provide FY 2009 cost of living increases, the actual purchasing power of the FY 2008 appropriation is reduced in FY 2009. The CR authorizes us to fund noncompeting research grants at 90% of the previously committed levels until a FY 2009 appropriation is made. The CR expires March 6, 2009, but we must be prepared for the possibility that the CR amount will become the FY 2009 appropriation. Accordingly, the NHLBI is currently operating with a CR budget of $2.922 billion. Similar to FY 2008, noncompeting awards will be provided a 1% inflation rate. Detailed information can be found in the NHLBI FY 2009 Funding and Operating Guidelines.2
New Investigators and Early Stage Investigators
We are strongly committed to helping early stage investigators launch their research careers. As you know, beginning in FY 2005, the NHLBI initiated a policy of increasing the payline for regular research grant (R01) applications of first time NIH applicants (termed New Investigators, NI) by 5 percentile points. NI applicants with scores 6 to 10 percentile points above the payline who successfully address the concerns of the initial review group in an expedited administrative review also have received funding.3
We were interested in knowing more about the NIs who have received funding since 2005. We analyzed the data based upon the following definitions. NIs who are within 10 years of completing their terminal research degree or their medical residency are designated Early Stage Investigators (ESIs), while NIs who are more than 10 years from their terminal degree or medical residency but have not previously applied for NIH support are termed non-ESIs. To our surprise, only 55% of NIs were ESIs. The remaining 45% were established investigators in other fields, often non-medical, applying to the NIH for the first time.
To help ESIs make a smooth transition to research independence, we and other Institutes have implemented an amended NI policy beginning in FY 2009.4 The career stage of NIs will be taken into account when their grant applications are reviewed. Review committees will be instructed to select the same proportion of applications from NIs, including ESIs, as from established investigators for discussion and scoring. Reviewers will score applications from NIs relative to other NI applications, rather than to the entire pool of applications. The NHLBI and other Institutes will make awards so that NIs have the same success rate as established investigators applying for new funding and ESIs constitute at least 60% of funded NIs in FY 2009, with the expectation that they will grow to at least 75% in the near future.
While these NIH-wide procedures are being put into place, the NHLBI payline for all NIs (ESIs and non-ESIs) will remain 5 percentile points above the regular R01 payline for FY 2009. This payline advantage for non-ESIs will be phased out in FY 2010. ESI applications that score 6 to 10 percentile points above the regular R01 payline will continue to be eligible for the expedited review procedure described above. All awards to ESI applicants will be funded for the full recommended project period.
Policies Regarding Amended Applications
Considerable attention has been focused on increasing the efficiency of the peer review system. The declining success rate for original grant applications, termed A0, and the increasing proportion of grants that are funded only after an application has been amended and resubmitted once or twice, termed A1 and A2, are particular concerns. Currently, fewer than 25% of funded applications are awarded after the A0 application.
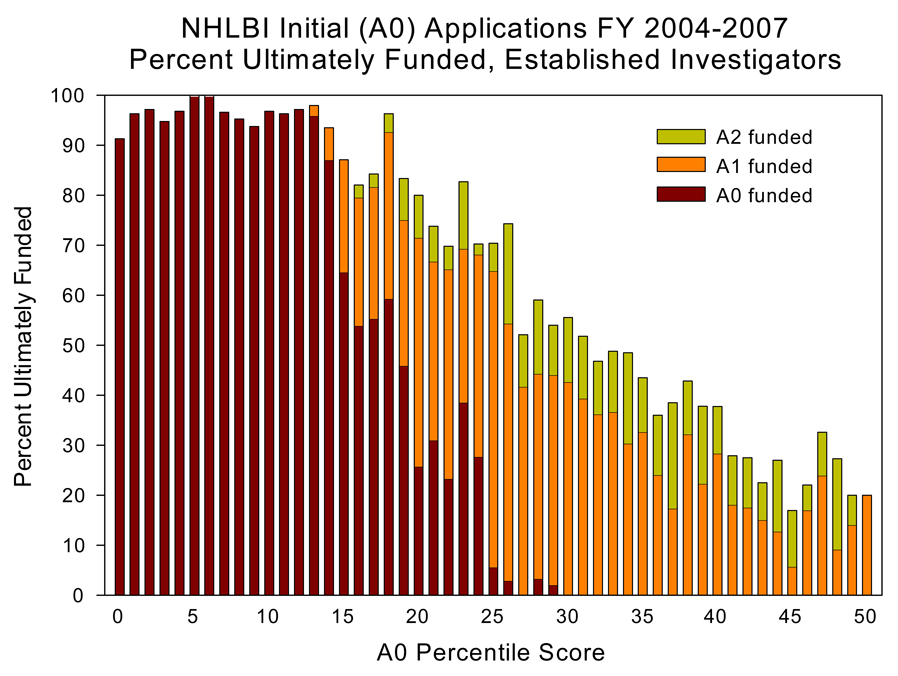
We analyzed the success rates of A0, A1 and A2 submissions received by the NHLBI in recent years and tracked the improvements in percentile scores that typically occurred with resubmissions. Data from the NHLBI is consistent with that of other Institutes. Over the past 5 years, the number of funded A0 applications has declined, while the number of funded A1 and A2 applications has increased (see Figure 1). Strikingly, >80% of A0 applications receiving an original priority score within the 20th percentile are eventually funded as A1 or A2 applications. Repeated submissions increase work for both applicants and reviewers, create unnecessary delays in funding high-quality science, and discourage investigators from pursuing scientific careers. To increase the likelihood that meritorious A0 applications are funded without multiple revisions, the NIH has changed its policy on resubmission (amended) applications.5 For any competing application submitted for the January 25, 2009, due date or thereafter, the NIH will accept only one subsequent amendment, A1. Our data support the elimination of second-amendment applications, A2, as a way to fund meritorious applications earlier and reduce the burden on investigators, reviewers, and the NIH.
Figure 1.
The percent of ultimately funded NHLBI initial applications (A0) from Established Investigators in the fiscal years 2004 to 2007. The y-axis indicated the percentile score of the A0 application. Note that >80% of A0 applications at the 20th percentile or better were funded during the A0, A1 or A2 submission.
Further, our analysis suggested the following strategy to enable the NHLBI to identify grants at the A0 review with a high probability of ultimately achieving a fundable score and fund them promptly. Beginning with awards made in FY 2010, the NHLBI will recalculate percentile rankings for grant applications according to their amendment status. A0 applications will be ranked together, and A1 applications will be ranked with other A1 applications. Awards will be made according to the new percentile rankings to achieve equivalent success rates for both A0 and A1 applications. This approach should enable us to make >60% of our grant awards after the A0 submission.
We are confident that these policy changes will enhance our ability to fund outstanding science efficiently on the first grant application and to bring a new generation of talented investigators into the research enterprise.
Other Updates on the Peer Review Process
I would like to bring you up to date on other changes that are being made in the NIH peer review process. In June of 2007, the NIH formed internal and external working groups to conduct an in-depth evaluation of the peer review system and develop recommendations for its enhancement. Based on their findings,6 an implementation plan was established for several high-priority areas. As discussed above, we have instituted several new policies to reduce the burden of repeated grant submission and review, and to ensure balanced and fair reviews for ESIs. In the upcoming months, the NIH will evaluate clustering clinical research applications as well.
To engage and retain the best reviewers, the NIH will develop more flexible tours of duty on study sections, explore alternatives to in-person meetings, identify best practices for recruiting reviewers, and enhance reviewer training. To improve the quality and transparency of review, we will soon be implementing review-criteria-based scoring7 and restructuring summary statements to provide clearer feedback to applicants. Applications that are not selected for detailed discussion will nonetheless be scored to help applicants assess the advisability of resubmission. A shortened and restructured application form will be introduced for receipt dates beginning in January 2010.
The implementation plan also calls for development of a permanent process for continuous reevaluation of the peer review system in light of evolving science. From time to time I will report on our progress in addressing the peer review recommendations. In the meantime, I encourage you to access the latest information via the NIH Enhancing Peer Review Web site for periodic updates.8
As always, I welcome your comments and suggestions as we continually try to improve our support of biomedical research.
Footnotes
Conflict of Interest Disclosures: none
References
- 1. http://thomas.loc.gov/cgi-bin/query/z?c110:H.R.2638.enr:
- 2. http://www.nhlbi.nih.gov/funding/policies/operguid.htm.
- 3. http://www.nhlbi.nih.gov/funding/training/redbook/newinvest.htm.
- 4. http://grants1.nih.gov/grants/guide/notice-files/not-od-09-013.html.
- 5. http://grants.nih.gov/grants/guide/notice-files/NOT-OD-09-003.html.
- 6. http://enhancing-peer-review.nih.gov/meetings/NIHPeerReviewReportFINALDRAFT.pdf.
- 7. http://grants.nih.gov/grants/guide/notice-files/NOT-OD-09-025.html; http://grants.nih.gov/grants/peer/peer.htm.
- 8. http://enhancing-peer-review.nih.gov/