Abstract
Background
Targeted delivery of mesenchymal precursor cells (MPCs) can modify left ventricular (LV) cellular and extracellular remodeling following myocardial infarction (MI). However, whether and to what degree LV remodeling may be affected by MPC injection post-MI, and whether these effects are concentration dependent remain unknown.
Methods and Results
Allogeneic MPCs were expanded from sheep bone marrow, and direct intra-myocardial injection was performed within the borderzone region one hour following MI induction (coronary ligation) in sheep at the following concentrations: 25×106 (25 M, n=7), 75 ×106 (75 M, n=7), 225 ×106 (225 M, n=10), 450 ×106 (450 M, n=8), and MPC free media only (MI Only, n=14). LV end diastolic volume increased in all groups but was attenuated in the 25 and 75 M groups. Collagen content within the borderzone region was increased in the MI Only, 225, and 450 M groups. Whereas plasma ICTP, an index of collagen degradation, was highest in the 25 M group. Within the borderzone region matrix metalloproteinases (MMPs) and MMP tissue inhibitors (TIMPs) also changed in a MPC concentration dependent manner. For example, borderzone levels of MMP-9 were highest in the 25 M group when compared to the MI Only and other MPC treatment group values.
Conclusions
MPC injection altered collagen dynamics, MMP, and TIMP levels in a concentration dependent manner, and thereby influenced indices of post-MI LV remodeling. However, the greatest effects with respect to post-MI remodeling were identified at lower MPC concentrations, thus suggesting a therapeutic threshold exists for this particular cell therapy.
Keywords: Infarct Expansion, Stem Cells, Matrix Metalloproteinase, Tissue Inhibitor of Matrix Metalloproteinase, Fibrosis
INTRODUCTION
Despite advances in reperfusion therapy, acute coronary syndromes still result in myocardial injury and subsequent myocardial infarction (MI). Molecular, cellular, and interstitial events antecedent to the acute MI culminate in changes in the size, shape, and function of the left ventricle (LV), collectively termed LV remodeling. A pathophysiological underpinning of the LV remodeling process is that continuous changes occur in the structure and function of the fully perfused myocardium surrounding the infarct region, described as the borderzone myocardium. Extension of these changes from the borderzone to contiguous normal myocardium is a process defined as infarct expansion. Indeed, adverse LV remodeling and infarct expansion have been identified as independent predictors of mortality following MI.1 Accordingly, significant effort has been directed toward surgical and pharmacologic methods to interrupt post-MI LV remodeling and infarct expansion. While numerous pharmacological and surgical therapies have been shown to ameliorate post-MI LV remodeling, none have been able to halt the process completely. Of more recent import, the utilization of multiple cell types, i.e. stem cells, implanted into the post-MI myocardium has been evaluated.2-10 While pre-clinical studies demonstrated improved LV function following MI2-6 clinical trials have yielded disparate results thus far.7-10 The reasons for the difficulties in translating the basic studies regarding stem cells to clinical applications are likely multifactorial, and include the type of cell utilized, the concentration of cells administered, and the method of delivery. A direct relationship between stem cell concentration and the degree of post-MI remodeling, with respect to cellular and extracellular processes, has not been examined.
One specific stem cell line are the mesenchymal precursor cells (MPCs) which are defined as self-renewing, pluripotent cells contained within the adult bone marrow.2,3 Specifically, these cells are STRO-3 positive, delineating them as nonhematopoietic perivascular bone marrow stromal stem cells, and demonstrate an extensive capacity for proliferation and differentiation.11 MPCs are non-cycling cells which lack the phenotypic characteristics of leukocytes and mature stromal cells but maintain the functionality to differentiate into multiple stromal cell types, including smooth muscle cells. Potential advantages of MPCs include accessibility in bone marrow, allogenicity which negates the need for immunosuppression, and ease of ex vivo expansion.3 Previous studies in large animal models of MI have found MPCs capable of improving LV function and attenuating infarct expansion following myocardial delivery.4 However, the underlying mechanisms which may contribute to the effect of MPC implantation on post-MI remodeling and whether these are concentration dependent remain unexplored. Therefore, the present study utilized this specific MPC line in order to examine how MPC injection may modify the extracellular environment post-MI.
While a number of cellular and extracellular events feed into the LV remodeling process, matrix metalloproteinases (MMPs) and MMP tissue inhibitors (TIMPs) have been identified as a common end-effector system in post-MI LV remodeling.12-14 Moreover, in an adult sheep model of post-MI LV remodeling, a clear association has been demonstrated between the emergence of certain MMP types and MI expansion in the borderzone region.12 Accordingly, using a well established post-MI sheep model,4,12,15 the present study was designed to test the hypothesis that myocardial MMP and TIMP levels will change as a function of MPC concentration delivered to the borderzone and be directly related to the post-MI remodeling process.
METHODS
Overview
This study utilized an adult sheep MI model with the following specific objectives: First, to quantify LV remodeling and infarct expansion following delivery of MPCs in varying doses. Second, to harvest the myocardium at 8 weeks following MI in order to quantify MMP and TIMP levels. Third, to measure relative myocardial collagen content, as well as an index of collagen degradation.
Animal Model
An MI was surgically induced in 47 female sheep using a well described method.15 Briefly, an anteroapical infarction was produced by ligating the left anterior descending artery and its diagonal branches, resulting in an infarction of approximately 20% of the LV mass. This technique has been shown to reproducibly create MI of consistent magnitude.15 Subsequently, the sheep were randomized to receive either vehicle alone or a specific concentration of MPCs. For the purposes of referent control values for the subsequent myocardial biochemical analysis, LV myocardial samples were collected from referent normal female sheep (n=6). All animals were treated and cared for in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (National Research Council, Washington, DC, 1996).
MPC prep/injection
The specific fraction of MPCs used in this experiment were perivascular STRO-3 positive cells derived from bone marrow aspirates from male crossbred sheep using techniques similar to those previously described.11,16
Cells were cryopreserved in 42% ProfreezeTM/50% Alpha MEM/7.5% DMSO in 4ml ampoules. Recovery of the frozen MPC preparations was achieved by thawing on ice. Sheep were randomized to receive the following MPC concentrations (106;M); 25, 75, 225, or 450 or cell medium only (MI only). All animals except for the 450M group received twenty mid-myocardial (2-3mm depth) borderzone injections of 0.2 ml (4 ml total). The 450M cell group received twenty 0.4 ml injections (8ml total). Male MPCs or cell medium alone were delivered by direct injection into the clearly identifiable borderzone region adjacent to the infarct on the anterior wall of the LV within 1 hour of coronary occlusion.
Left Ventricular Function and Myocardial Sampling
At baseline (prior to MI induction), immediately following infarction, and at 8 weeks post-MI quantitative two dimensional echocardiograms were obtained (Philips 7500 ultrasound system using a 5-MHz probe) using a sub-diaphragmatic technique. From these recordings, end-diastolic volume, ejection fraction, and MI length were determined.17 After echocardiography a blood sample was collected and the serum was separated and stored at -80°C. Subsequently, the sheep were euthanized and the heart was rapidly harvested. Transmural sections from the MI, remote and borderzone regions were fixed in 10% neutral buffered formalin or flash frozen.
Myocardial Collagen Content and Telopeptide Studies
Plasma samples were collected prior to and 8 weeks following MI induction. A carboxyterminal telopeptide region type I collagen (ICTP) enzyme-immunoassay kit (06099, Orion Diagnostica) was utilized in order to measure collagen degradation as previously described.18 Collagen content in myocardial tissue samples was determined using a hydroxyproline assay.19
LV Myocardial MMP/TIMP Analysis
LV myocardial samples were homogenized (3- to 30-second bursts) in 5 mL of an ice-cold extraction buffer (1:3 wt/vol) containing cacodylic acid (10 mmol/L), NaCl (0.15 mol/L), ZnCl (20 mmol/L), NaN3 (1.5 mmol/L), and 0.01% Triton X-100 (pH 5.0). The homogenate was then centrifuged (4°C, 10 minutes, 800g) and the supernatant decanted and saved on ice. Final protein concentration of the myocardial extracts was determined with a standardized colorimetric assay (BCA Protein Assay, Pierce). The extracted samples were then aliquoted and stored at -80°C.
For this study, the relative abundances of MMPs corresponding to each class were examined by quantitative zymograpy or immunoblotting (10 μg myocardial protein), which has been described in detail previously.20-22 Abundance of the gelatinase MMP-2 was determined by zymography, as described previously.22 For immunoblotting, following electrophoresis the separated proteins were transferred to a nitrocellulose membrane. After a blocking and washing step, the membranes were incubated in antisera (0.4 μg/mL) corresponding to the regional myocardial abundance of the interstitial collagenases MMP-1 (IM35, Calbiochem) and MMP-13 (AB8114, Chemicon International), the gelatinase MMP-9 (AB804, Chemicon International), the matrilysins MMP-3 (Ab38907, Abcam) and MMP-7(Ab38996, Abcam), or the membrane-type MMP MT1-MMP (AB815, Chemicon). The TIMPs were measured in identical fashion using specific antisera for TIMP-1 (AB8116, Chemicon), TIMP-2 (Ab38973, Abcam), and TIMP-4 (AB816, Chemicon). After incubation with a secondary antibody, immunoreactive signals were detected by chemiluminescence (Western Lightning Chemiluminescence Reagent Plus, Perkin Elmer). Recombinant standards (Chemicon or Oncogene) were included in all immunoblots as positive controls. The zymograms and immunoblots were analyzed by densitometry (Gel Pro Analyzer, Media Cybernetics), as previously described.22
Myocardial Histology
After dehydration in a graded series of ethanol and infiltration with paraffin, LV sections 4 μm in thickness were cut from the specimens and mounted on glass slides. The slides were then rehydrated and stained using hematoxylin and eosin (H&E) histochemical techniques. Cross sectional myocyte area and percent collagen analysis were then performed following digitization at a magnification of 40X, using an image-analysis system (Sigma Scan, SPSS). Only those myocytes in which the nucleus was centrally located within the cell were digitized and analyzed to ensure that the short axis of the myocyte was perpendicular to the microscope objective. A minimum of 25 myocytes from each high-power field and a minimum of 2 fields per specimen were analyzed to obtain an average cross-sectional area for each myocardial region. For the purposes of MPC identification, staining of representative sections for antibody against a Y chromosome epitope (Sigma anti-RBMY1A2 (1:250)) was performed using sections of testis as a positive control. A second set of immunohistochemistry was performed in which parallel sections were stained for both Y chromosome and alpha smooth muscle actin (ASMA) (Abcam Alpha Smooth Muscle Actin, ab5694(1:100)). Within the targeted injection region 40 high power fields per image were scanned at 20 and 40 × magnification, digitized, and analyzed.
Assessment of MPC Engraftment
Genomic DNA was extracted from frozen tissue samples using QIAmp® DNA Mini Kit (Qiagen, Valencia, CA). SYR2 and SYR3 primer pairs were selected to amplify genes from the ovine male-specific region.4,23 The detection limit for SRY2 and SRY3 was 500pg, equivalent to approximately 85 cells,24 as described in greater detail previously.4
Data Analysis
The LV function and MI length measurements were subjected to analysis of variance (ANOVA) in which the treatment variables were MI and MPC treatment. Following the ANOVA, pair-wise comparisons were performed by Bonferroni bounds. For the ICTP plasma assay results, the data was not normally distributed, therefore log transformation was performed prior to comparing individual group means by T-test. For hydroxyproline values, a two-way ANOVA was performed in which the main treatment was MI and the sub-plot effect was MPC treatment. The densitometry results obtained from the MMP and TIMP zymography and immunoblotting were converted to a normalized integrated optical density (IOD) and then analyzed by two-way ANOVA. Values were considered significant at the p<0.05 level and values reported as the mean ± SEM. The authors had full access to the data and take full responsibility for its integrity.
Results
The baseline LV functional characteristics and final sample sizes for all of the groups are shown in Table 1. One animal in the 225M treatment group died one hour after MI induction and MPC injection. Myocardium harvested from this animal was incorporated in the histology and MSC identification experiments.
Table 1.
LV Geometry, Function, Infarct and Myocyte Size at 8 weeks following surgical myocardial infarction and injection of increasing concentrations of MPCs or cell medium to the borderzone region.
| LV EDV (mL) Baseline=70.0±3.5 | LV EF (%) Baseline=40.4±2.9 | MI length (cm) Baseline=6.86±0.23 | Myocyte Cross Sectional Area(μm2) Normal=216±3 | ||
|---|---|---|---|---|---|
| Borderzone | Remote | ||||
| MI Only (n=14) | 102.8±8.0* | 13.7±0.9* | 9.20±0.32* | 459±24* | 275±21* |
| 25 M (n=7) | 85.0±7.9*§ | 23.8±3.0*§ | 7.48±0.33§ | 485±18* | 260±24* |
| 75 M (n=7) | 84.6±5.4*§ | 23.6±2.4*§ | 7.88±0.16§ | 353±15*§# | 312±17* |
| 225 M (n=10) | 94.0±4.6* | 20.9±2.1*§ | 8.23±0.22*§ | 440±16* | 265±13* |
| 450 M (n=8) | 98.4±8.0* | 20.8±3.2*§ | 8.62±0.27*# | 392±26*# | 252±12* |
Values are means±SEM; LVEDV = Left ventricle end diastolic volume, LV EF = Left ventricle ejection fraction. MI Only = cell medium only, 25 M = 25 × 106 MPCs, 75 M = 75 × 106 MPCs, 225 M = 225 × 106 MPCs, 450 M = 450 × 106 MPCs, Normal = Referent Normal Control (n = 6).
p<0.05 vs. Baseline/Normal
p<0.05 vs. MI Only
p<0.05 vs. 25 M
LV Function and Geometry
The overall mean baseline LV end diastolic volume, prior to treatment randomization, was 60 ± 3.5 mL, which was not different between randomized treatment groups. LV end diastolic volume was increased from baseline in all post-MI groups (Table 1). LV end diastolic volume decreased in the 25 M and 75 M MPC treatment groups in comparison to the MI Only group. The mean baseline LV ejection fraction was 40 ± 3%, and LV ejection fraction decreased in all post-MI groups. The LV ejection fraction increased in all MPC treatment groups as compared to the MI Only group. Infarct length was reduced in the 25 M, 75 M, and 225 M treatment groups as compared to the MI Only group, whereas in the 450 M group infarct expansion was not different from the MI Only group. At the cellular level, myocyte cross sectional area for the remote and borderzone regions were increased from the referent control values in all post-MI groups, indicative of post-MI LV myocyte hypertrophy (Table 1). At higher MPC concentrations the borderzone myocyte cross sectional area decreased from MI Only values, with this being significant at the 75 and 450 M concentrations.
MPC Engraftment Studies
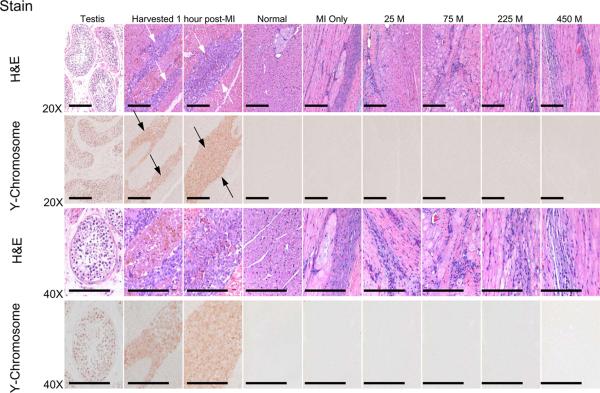
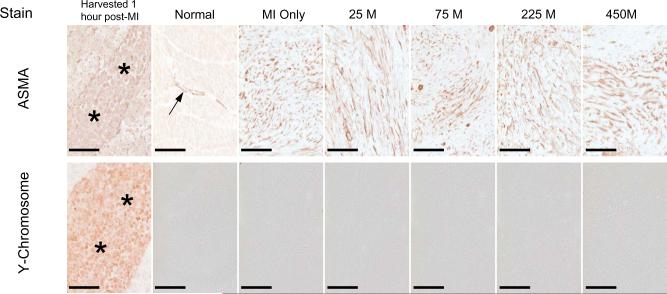
Parallel LV sections from the MI borderzone obtained at 1 hour post-MI and MPC injection, as well as at 8 weeks post-MI in which increasing concentrations of MPCs were injected within the borderzone at the time of MI induction, were stained with H&E and for the Y chromosome (Figure 1). A strong basophilic, linear array of cells were readily apparent within the borderzone at 1 hour following MPC injection. In sections obtained at 8 weeks post-MI a pleomorphic staining pattern occurred within the borderzone, containing cellular and extracellular components. Immunolocalization for the Y chromosome revealed a strong positive signal in the testis as well as in the linear array of cells within the injection site at 1 hour post-MI. Therefore, the positive Y chromosome staining was reflective of successful implantation of the MPCs within the borderzone following MI induction. However, an absence of Y chromosome staining was revealed after examination of over 240 high power fields within the borderzone at 8 weeks post-MI, thus indicating a failure of prolonged MPC engraftment. In order to investigate whether and to what degree MPC phenotypic changes may have occurred following MPC injection, co-localization studies were performed using parallel LV sections stained for ASMA and the Y chromosome (Figure 2). Cells staining positive for ASMA and Y chromosome, indicative of MPCs, were readily apparent within the targeted injection site at 1 hour following MPC injection. In normal referent control LV myocardial samples, ASMA staining was evident within the vasculature, consistent with vascular smooth muscle. At 8 weeks post-MI, robust staining for ASMA was observed within the borderzone, consistent with neovascularization and myofibroblasts. However, at this time point parallel LV sections were uniformly negative for the Y chromosome. Thus, while a heterogeneous ASMA cell population was present within the borderzone at 8 weeks post-MI, there was no evidence that these cells were residual MPCs. Additionally, using high sensitivity PCR technique, DNA extracts from the 225M animal that died 1 hour after cell injection did demonstrate the presence of male cells in the borderzone. However, extracts from the borderzone region in all treatment groups 8 weeks post-MI did not produce a PCR product consistent with the presence of the injected male cells. The positive control sample generated a PCR product of expected size and the negative control did not create a signal. Therefore, using cellular and PCR techniques, no significant engraftment of the successfully injected MPCs into the borderzone region occurred at 8 weeks post-MI.
Figure 1.
Parallel LV sections obtained from the MI borderzone were stained with hematoxylin and eosin (H&E) and the Y chromosome, were obtained at 1 hr following MI induction and MPC injection (225 M), as well as at 8 weeks post-MI in which increasing concentrations of MPCs were injected within the borderzone at the time of MI induction. Sections from testis as a positive control for the Y chromosome as well as referent normal sheep myocardium were included in this analysis. Sections were examined under low (20X) and high (40X) magnification and representative images are shown. A strong basophilic linear array of cells were readily evident within the targeted injection site at 1 hour following MPC injection (white arrows). Following 8 weeks of MI, a pleomorphic staining pattern was observed within the borderzone which included residual myocytes, inflammatory cells, myofibroblasts and bands of fibrillar collagen. Immunolocalization for the Y chromosome revealed a strong positive signal in the testis as well as in the linear array of cells within the injection site at 1 hour (black arrows). The positive Y chromosome staining is therefore reflective of successful implantation of MPCs within the myocardial borderzone following MI induction. However, examination of over 240 high power fields within the targeted MPC injection sites at 8 weeks post-MI revealed an absence of Y chromosome staining indicative of a failure of MPC engraftment. Treatment groups are labeled as follows: Normal = Referent Control (n=6), MI Only = cell medium only (n = 14), 25 M = 25 × 106 MPCs (n = 7), 75 M = 75 × 106 MPCs (n = 7), 225 M = 225 × 106 MPCs (n = 10), 450 M = 450 × 106 MPCs (n = 8). Scale bar = 200 μm.
Figure 2.
In order to more closely examine whether and to what degree MPC phenotypic changes may have occurred following MPC injections, parallel LV sections were stained for alpha smooth muscle actin (ASMA) and for the Y chromosome. ASMA and Y chromosome positive cells, indicative of MPCs, were readily evident within the targeted injection site at 1 hour following MPC injection (asteriks). In normal control LV myocardial samples, ASMA staining was evident within the vasculature, consistent with vascular smooth muscle (arrow). Following 8 weeks of MI, robust staining for ASMA was observed within the borderzone consistent with neovascularization and myofibroblasts. However, at this time point parallel LV sections were uniformly negative for the Y chromosome. Thus, while a heterogeneous ASMA cell population was present within the borderzone at 8 weeks post-MI, there was no evidence that these cells were residual MPCs. Treatment groups are labeled as follows: Normal = Referent Control (n = 6), MI Only = cell medium only (n = 14), 25 M = 25 × 106 MPCs (n = 7), 75 M = 75 × 106 MPCs (n = 7), 225 M = 225 × 106 MPCs (n = 10), 450 M = 450 × 106 MPCs (n = 8). Scale bar = 200 μm.
Collagen Studies
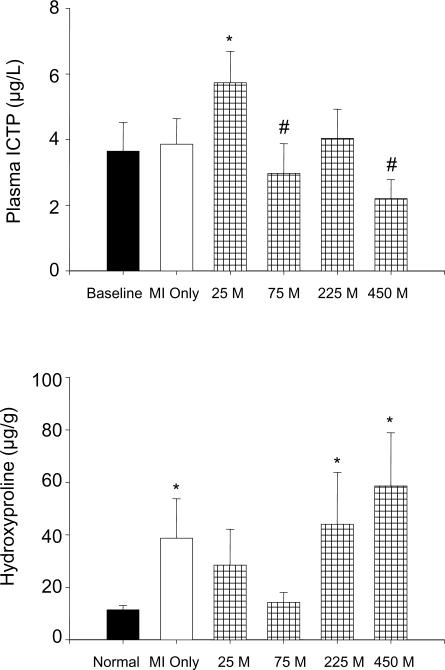
An index of collagen degradation, as measured by plasma telopeptides (ICTP), increased from mean baseline values in the 25 M treatment group (Figure 3). ICTP levels in both the 75 M and 450 M treatment groups were lower in comparison to the 25 M group. Myocardial collagen, as measured by hydroxyproline levels, was 11.4±1.7 μg/g in the referent control group. Within the remote region, hydroxyproline increased from the referent control value to 16.7±2.5 μg/g (p<0.05) in the MI Only group and was similarly increased in all MPC treatment groups. Within the MI region, hydroxyproline was increased from the referent control value to 253.2±29.5 μg/g (p<0.05) in the MI Only group and was again similarly increased in all MPC treatment groups. The greatest differential changes in hydroxyproline levels were in the borderzone region. Specifically, the MI Only, 225 M and 450 M groups were increased from the referent control value (p<0.05).
Figure 3.
Top: Collagen degradation as measured by plasma ICTP levels, mean baseline vs. at 8 weeks post-MI with variable MPC concentrations. Bottom: Collagen content of the borderzone region as measured by hydroxyproline at 8 weeks post-MI with increasing concentrations of MPCs. Treatment groups are labeled as follows: Normal= Referent Control (n=6), MI Only = cell medium only (n = 14), 25 M = 25 × 106 MPCs (n = 7), 75 M = 75 × 106 MPCs (n = 7), 225 M = 225 × 106 MPCs (n = 10), 450 M = 450 × 106 MPCs (n = 8). Values are presented as means ± standard error of the mean. *p<0.05 vs. Baseline/Normal, #p<0.05 vs. 25 M
MMP and TIMP Myocardial Levels
MMP and TIMP Regional Distribution (Table 2)
Table 2.
Regional Abundance of Myocardial MMPs at 8 Weeks After MI
| MMP Class | Referent Control | Region | MI Only | MPC Concentration (per million cells) |
|||
|---|---|---|---|---|---|---|---|
| 25 M | 75 M | 225 M | 450 M | ||||
| Collagenases | |||||||
| MMP-1 | 1319±74 | Remote | 3749±839* | 1293±186§ | 1172±261§ | 1884±653§ | 2421±595 |
| BZ | 4025±2058 | 1175±162 | 935±232 | 1325±266 | 3385±1200 | ||
| MI | 604±253* | 84±30* | 120±26* | 222±70* | 691±337*# | ||
| MMP-13 | 242±31 | Remote | 1074±430* | 705±349 | 387±138 | 507±213 | 331±78§ |
| BZ | 1082±280* | 640±354 | 405±109§ | 327±97§ | 460±188§ | ||
| MI | 2897±954* | 1650±546 | 1737±772 | 1832±360 | 828±337§ | ||
| Gelatinases | |||||||
| MMP-2 | 26047±3312 | Remote | 39750±6760 | 20155±5036§ | 21884±1370§ | 23923±1718§ | 28420±7800 |
| BZ | 50932±11021* | 37294±11758 | 30272±7792 | 42229±8193 | 30079±8625 | ||
| MI | 203450±24810* | 190152±6468* | 173293±11222* | 142759±18349§* | 162343±21567* | ||
| MMP-9 | 553±69 | Remote | 1133±220* | 1030±312 | 891±54 | 869±177 | 883±111 |
| BZ | 804±90 | 1336±243§* | 719±121# | 859±197# | 888±166# | ||
| MI | 33±17* | 56±33* | 14±2* | 81±26* | 142±85* | ||
| Stromelysin/Matrilysin | |||||||
| MMP-3 | 1140±93 | Remote | 837±162 | 922±124 | 931±61 | 1358±335 | 1431±263 |
| BZ | 1188±185 | 936±95 | 1298±146 | 854±124 | 1271±145 | ||
| MI | 1246±176 | 1430±84 | 1320±65 | 1510±219 | 1658±213* | ||
| MMP-7 | 527±79 | Remote | 1139±370* | 295±66§ | 259±55§ | 313±58§ | 449±101§ |
| BZ | 617±137 | 259±33§ | 189±44*§ | 215±73*§ | 570±141# | ||
| MI | 188±32* | 98±17* | 96±30* | 215±80* | 117±33* | ||
| Membrane-type | |||||||
| MT1-MMP | 355±43 | Remote | 980±380* | 505±93 | 413±61 | 795±208 | 597±83 |
| BZ | 1400±664* | 513±109 | 438±103 | 470±87§ | 633±138 | ||
| MI | 6931±2611* | 2790±566 | 4862±1513* | 3508±770 | 2162±298§ | ||
Values reported in integrated optic densities (IOD), presented as mean±SEM. MI Only = cell medium only, 25 M = 25 × 106 MPCs, 75 M = 75 × 106 MPCs, 225 M = 225 × 106 MPCs, 450 M = 450 × 106 MPCs.
p<0.05 vs. Normal Referent Control
p<0.05 vs. MI Only
p<0.05 vs. 25 M. BZ=Borderzone Region, MI=Infarct Region
Remote Region
MMP-1 increased in the MI Only group from referent control values and decreased in the 25, 75, and 225 M groups. MMP-13 increased in the MI Only group, and was decreased from this value in the 450 M group. MMP-2 decreased in the 25, 75, and 225 M groups as compared to the MI Only group. MMP-9 increased in the MI Only group, whereas MMP-9 remained unchanged in all MPC groups. MMP-3 levels were unchanged, while MMP-7 was increased in the MI Only group and decreased in all MPC treatment groups. Membrane-type MMP, MT1-MMP, increased in the MI Only group but remained unchanged in all MPC groups. TIMP-1 increased in the MI Only and 25 M groups compared to referent control values. TIMP-2 increased in the MI Only group and remained unchanged in all MPC groups. TIMP-4 increased in the MI Only group and fell from this value in the 75, 225, and 450 M groups.
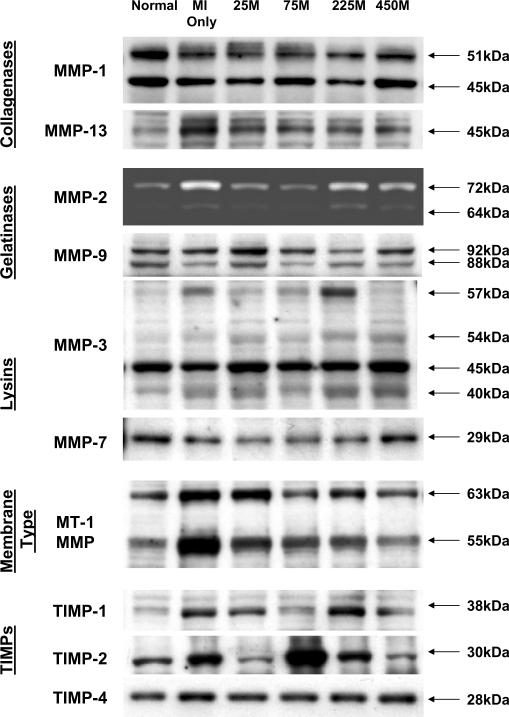
Borderzone Region (Figure 4)
Figure 4.
Relative abundance of representative MMP types and TIMPs in the borderzone region were examined by immunoblotting in myocardium obtained from normal referent control, MI Only, and 25 M, 75 M, 225 M, and 450 M MPC treatment groups. MMP/TIMP classifications are shown at left and molecular weights are shown at right. A robust increase in MMP-9 was displayed in the 25 M group, with decreased levels in the 75, 225, and 450 M groups. MMP-7 decreased in the 25, 75, and 225 M groups. MT-1 MMP decreased with MPC treatment as compared to MI Only. While TIMP-2 appears elevated in the 75 M group, quantitative results did not show statistical significance. Quantitative data are presented in Tables 2 and 3.
MMP-1 increased in the MI Only and 450 M groups. MMP-13 increased in the MI Only group, and the 75, 225 and 450 M groups decreased compared to the MI Only group. MMP-2 increased in the MI Only group. MMP-9 increased in the 25M group compared to the referent control values, and decreased in the 75, 225, and 450 M groups as compared to the 25 M group. MMP-3 levels were unchanged by MPC injection. MMP-7 decreased in the 75 and 225 M groups, with the 25, 75, and 225 M groups decreased from the MI Only group. Membrane type 1 MMP, MT1 - MMP, increased in the MI Only group, with all MPC treatment groups unchanged from control values. TIMP-1 increased in the MI Only group and remained unchanged in the MPC groups. TIMP-4 increased in the MI Only group and decreased from this value in all MPC groups.
MI Region
MMP-1, MMP-9, and MMP-7 decreased in all groups as compared to referent control values. MMP-13 increased in the MI Only group and all MPC groups were unchanged from control values. MMP-2 increased in all treatment groups. MMP-3 increased in the 450 M group. MT1-MMP increased in the MI Only and 75 M groups. TIMP-1, TIMP-2, and TIMP-4 levels decreased in all treatment groups as compared to the respective referent control values.
DISCUSSION
A common occurrence following MI is adverse LV remodeling, defined as infarct expansion, LV dilation, and ultimately LV dysfunction. One potential approach to interrupt adverse LV remodeling is cellular therapy, notably mesenchymal precursor cells (MPCs).1-10 The present study utilized a large animal model in order to examine whether and to what degree different concentrations of MPCs injected into the borderzone at the time of MI induction would differentially alter indices of LV cellular and extracellular remodeling at two months post-MI. The important findings of this study were three-fold. First, MPC delivery decreased MI expansion and LV dilation but these effects were realized at lower MPC concentrations. Second, LV myocyte hypertrophy and collagen accumulation were differentially affected by the MPC concentration. Third, MMP and TIMP levels, critical determinants of collagen turnover, were influenced in a MPC concentration dependent manner. Thus, MPC injection at the time of MI induction caused concentration dependent effects on LV cellular and extracellular remodeling at two months post-MI. However, the greatest affects on these processes were identified at lower MPC concentrations, thus suggesting a therapeutic threshold for this particular cell therapy.
Initial studies regarding the use of stem cells for cardiac repair in murine models purported stem cells having the ability to localize to the heart and yield new myocardium.25 However, more recent clinical studies utilizing MPCs have failed to demonstrate significant effects on LV remodeling and function.8-10 The reasons for the failure of translation from basic stem cell studies to the clinical context are likely multifactorial, but would include a lack of consensus regarding optimal stem cell type and preparation, delivery method, delivery location, and cell concentration. The present study focused upon one of these potential factors by examining cellular and extracellular effects of differing concentrations of MPCs in a large animal MI model. This study clearly identified MPC concentration dependent effects occurred with regard to infarct expansion, borderzone myocyte hypertrophy, and determinants of collagen dynamics. Therefore, this study confirms that MPC concentration is a critical determinant with regard to altering the post-MI LV remodeling process.
In the present study, several approaches were taken to identify MPCs in the myocardium, all of which were negative at 8 weeks post-MI. The fact that MPCs were not detected within the injected myocardium at prolonged intervals post-MI is not without precedent.4,5 For example, Zeng et al. failed to show significant mesenchymal cell engraftment at 1 month post-MI, yet reported improved LV pump function. A different study which tracked the injection of mesenchymal precursor cells in vivo demonstrated the vast majority of cells were depleted after 72 hours.26 The present study builds upon these observations in several important ways. First, all MPC concentrations failed to engraft at eight weeks post-MI, demonstrating that lack of engraftment is not a concentration dependent phenomenon. Second, despite failure of engraftment, long-term effects were concentration dependent, implying paracrine effects had lasting consequences for LV cellular and extracellular remodeling. Furthermore, it is unlikely that prolonged engraftment would be desirable, given reports of cardiac teratoma formation with long-term engraftment of some stem cells.27
In regards to post-MI LV remodeling, infarct expansion and LV dilation were reduced with MPC treatment. However, these effects upon LV remodeling were not uniform across all MPC concentrations utilized. At the cellular level, myocyte cross sectional area increased, indicative of hypertrophy within the borderzone and remote regions. The degree of borderzone myocyte hypertrophy was unaffected at low MPC concentrations but was attenuated at high MPC concentrations. Using high MPC concentrations injected into the borderzone would result in higher oxygen and nutrient demand, thereby resulting in an increased metabolic burden. In addition, higher concentrations may elicit a greater inflammatory response, which may have altered the myocyte microenvironment and affect myocyte growth. While remaining speculative, the results of the present study suggest that high MPC concentrations may affect myocyte adaption in the post-MI period.
A fundamental structural protein within the extracellular matrix is fibrillar collagen, and the structure and content of this matrix protein significantly changes in a heterogeneous fashion post-MI. In the present study, fibrillar collagen increased in all regions post-MI with respect to normal referent myocardium. With respect to MPC treatment, relative collagen content was differentially affected in the borderzone. Specifically, borderzone collagen content was reduced at lower MPC concentrations. Consistent with this observation, an index of collagen degradation, plasma ICTP levels, were elevated with low MPC concentrations. These findings suggest that MPC placement within the borderzone following MI altered collagen dynamics, most likely with respect to collagen degradation. Accordingly, we next examined determinants of collagen degradation.
A family of proteolytic enzymes which play an important role in the process of collagen turnover, and have been reported to be significantly altered post-MI, are the MMPs.11,17,18 A previous study by this laboratory reported regional differences in MMP levels within the post-MI myocardium of a sheep model at the eight week timepoint.12 The present study examined whether and to what degree MPC injection altered this MMP portfolio. With respect to regional changes, the general trend in the remote myocardium was for a relative reduction of MMP levels by MPC injection. While these findings would imply that lower MMP levels with MPC injection may stabilize the extracellular matrix post-MI, this interpretation is difficult without knowledge of activational status and interactions with other proteins such as TIMPs. With respect to concentration dependent effects of MPCs on MMP levels, the most notable changes were observed in the borderzone region. Specifically, increased MMP-9 levels at the 25 M concentration paralleled reductions in relative collagen content and an increased level of collagen degradation. Another MPC concentration dependent change was observed in the borderzone with respect to MMP-7 levels. Specifically, MMP-7 was reduced at lower MPC concentrations and remained unaltered from MI Only at high MPC concentrations. In addition to proteolytic enzymes, MMP-7 processes cytokines such as TNF-alpha, which may in turn alter extracellular matrix remodeling processes.28 While the mechanisms and resultant effects of MPCs on MMP levels in the context of post-MI remodeling remain speculative, the present study demonstrates that alterations in MMPs occur at both regional and concentration dependent levels, which would alter the extracellular matrix and in turn influence LV chamber remodeling.
MMP proteolytic activity is a highly regulated process, which includes post-translational changes and inhibitory processes. Thus, while the present study measured the relative content of MMP levels, this is not reflective of changes in endogenous proteolytic activity which may have occurred. Accordingly, the present study measured levels of the TIMPs, the predominant endogenous inhibitors of MMPs. Consistent with past reports using this sheep MI model,12 region specific differences in TIMP levels were observed. Specifically, relative TIMP levels were reduced in the MI region and increased in the remote region at 8 weeks post-MI. With regard to MPC injections, TIMP-1 levels were reduced at higher concentrations in the remote region and at all concentrations in the borderzone. When considered with concordant decreased remote region MMP levels, this suggests a preservation of MMP/TIMP stoichiometry. Conversely, when considering decreased borderzone TIMP-1 levels at the 25 M concentration with concordantly increased MMP-9 levels, a greater net proteolytic potential is suggested, and would concur with the observed increase in collagen degradation. Relative borderzone TIMP-4 levels, which are highly expressed in the cardiovascular system,14 were reduced with MPC injection. This observation may hold relevance in several ways. First, MPC injection alters MMP/TIMP stoichiometry and therefore alters the overall proteolytic potential. Moreover, TIMPs have multiple biologic functions with respect to growth and apoptosis,29 and therefore differential effects of relative borderzone TIMP levels may have secondarily influenced myocyte growth and viability.
Limitations and Future Directions
The present study was performed in a cardiac surgical context, allowing direct visualization of MPC injection into a targeted region. Thus, while the open chest technique was a pragmatic approach for this study, the preponderance of clinical methodology has utilized trans-catheter MPC delivery,30 making comparisons between techniques difficult. A factor which could potentially have compromised MPC engraftment is the degree of immune privilege of the MPCs. Also, it should be recognized that the use of a one-time bolus treatment of MPCs at an early post-MI time point may not be the optimal method for this cellular therapy. Cell delivery at later post-MI time points may decrease the inflammatory/rejection response the cells are subjected to, potentially altering engraftment results.
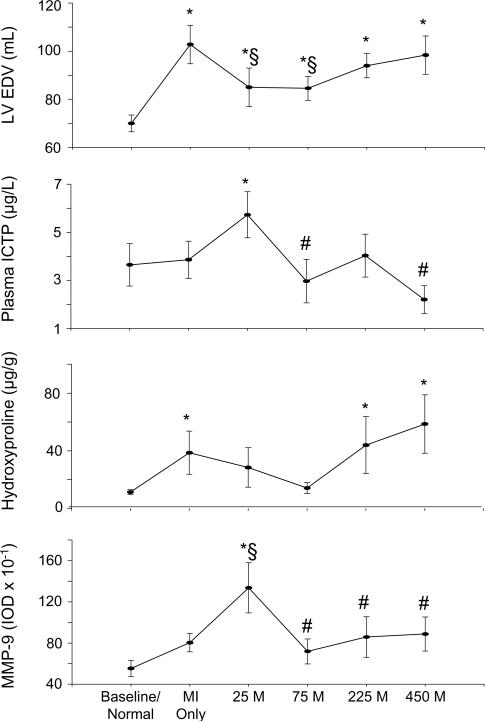
The present study focused on one possible variable with respect to MPCs and post-MI remodeling, and demonstrated that MPC concentration influenced the degree of LV remodeling, collagen structure, and underlying determinants of extracellular matrix turnover. An example of these cellular and extracellular variants are summarized in Figure 5, which depicts decreased LV dilation, increased collagen degradation, decreased borderzone collagen content, and increased borderzone MMP-9 levels at the lowest MPC concentration while the highest MPC concentration displays opposing trends. Future MPC studies will be needed to address questions regarding the optimal cell preparation, delivery method, and delivery location, while taking into account the influence of MPC concentration.
Figure 5.
Graphic representation of LV EDV at 8 weeks post-MI, collagen degradation as measured by plasma ICTP levels, collagen content as measured by hydroxyproline, and levels of the gelatinase MMP-9 at 8 weeks after surgical MI with increasing concentrations of MPCs vs. cell medium only. Treatment groups are labeled as follows: Normal = Referent Control (n = 6), MI Only = cell medium only (n = 14), 25 M = 25 × 106 MPCs (n = 7), 75 M = 75 × 106 MPCs (n = 7), 225 M = 225 × 106 MPCs (n = 10), 450 M = 450 × 106 MPCs (n = 8), all injected following surgical MI. Decreased LV EDV, increased collagen degradation and MMP-9 levels, and decreased collagen content is highlighted in the 25 M treatment group, in contrast to the opposing trends seen in the 450 M group. MMP levels are measured in integrated optic densities (IODs). Values are presented as means ± standard error of the mean. *p<0.05 vs. Control, §p<0.05 vs. MI Only, #p<0.05 vs. 25 M
Table 3.
Regional Abundance of Myocardial TIMPs at 8 Weeks After MI
| Referent Control | Region | MI Only | MPC Concentration (per million cells) |
||||
|---|---|---|---|---|---|---|---|
| 25 M | 75 M | 225 M | 450 M | ||||
| MMP Tissue Inhibitors | |||||||
| TIMP-1 | 880±612 | Remote | 2543±431* | 2155±339* | 1474±354 | 1973±279 | 1844±347 |
| BZ | 2193±351* | 1622±213 | 1763±390 | 1659±233 | 1527±307 | ||
| MI | 10±2* | 11±3* | 21±9* | 10±4* | 8±3* | ||
| TIMP-2 | 155±65 | Remote | 1057±502* | 396±255 | 342±251 | 874±179 | 467±189 |
| BZ | 976±307 | 137±64 | 819±736 | 664±214 | 460±202 | ||
| MI | 30±6* | 9±2* | 47±27* | 28±8* | 35±20* | ||
| TIMP-4 | 1386±37 | Remote | 2131±296* | 1673±101 | 1438±213§ | 1493±62§ | 1479±35§ |
| BZ | 2319±238* | 1541±196§ | 1469±133§ | 1411±172§ | 1529±142§ | ||
| MI | 186±37* | 127±56* | 231±58* | 62±19*§ | 139±36* | ||
Values reported in integrated optic densities (IOD), presented as mean±SEM. BZ=Borderzone. MI Only = cell medium only, 25 M = 25 × 106 MPCs, 75 M = 75 × 106 MPCs, 225 M = 225 × 106 MPCs, 450 M = 450 × 106 MPCs.
p<0.05 vs. Normal Referent Control
p<0.05 vs. MI Only
p<0.05 vs. 25 M
Acknowledgments
Funding Sources This research was supported by NIH grants HL63954, HL71137, HL73021, HL59165, a Merit Award from the Veterans' Affairs Health Administration, and by Angioblast Systems, New York, NY. Dr. J. Gorman and Dr. R. Gorman are supported by individual Established Investigator Awards from the American Heart Association (Dallas, TX). Mr. Schuster, Dr. Martens and Dr. Itescu are employees of Angioblast System, Inc.
Footnotes
Conflict of Interest Disclosures Jennifer A. Dixon MD: None, Robert C. Gorman MD: None, Robert E. Stroud MS: None, Shenikqua Bouges BS: None, Hamamoto Hirotsugu MD: None, Joseph H. Gorman III MD: None, Timothy P. Martens MD: None, Silviu Itescu MBBS: None, Michael D. Schuster MD: None, Theodore Plappert CVT: None, Martin G St. John-Sutton MBBS: None, Francis G. Spinale, MD, PhD: None.
REFERENCES
- 1.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 2.Psaltis PJ, Zannettino A, Worthley SG, Gronthos S. Mesenchymal stromal cells - potential for cardiovascular repair. Stem Cells. 2008 Jul 3; doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 3.Amado LC, Saliaris AP, Schuleri KH, John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–9. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamamoto H, Gorman JH, Ryan LP, Hinmon R, Martens TP, Schuster MD, Plappert T, Kiupel M, John-Sutton MG, Itescu S, Gorman RC. Allogeneic STRO-3 positive mesenchymal precursor cell therapy to limit post infarction ventricular remodeling: the effect of cell dosage. Ann Thorac Surg. 2009;87:794–801. doi: 10.1016/j.athoracsur.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, Zhang G, Suntharalingam P, Boozer S, Mhashilkar A, Panetta CJ, Swingen C, Deans R, From AH, Bache RJ, Verfaillie CM, Zhang J. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–75. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 6.Makkar RR, Price MJ, Lill M, Frantzen M, Takizawa K, Kleisli T, Zheng J, Kar S, McClelan R, Miyamota T, Bick-Forrester J, Fishbein MC, Shah PK, Forrester JS, Sharifi B, Chen PS, Qayyum M. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J Cardiovasc Pharmacol Ther. 2005;10:225–33. doi: 10.1177/107424840501000403. [DOI] [PubMed] [Google Scholar]
- 7.Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV, Kögler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–8. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 8.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–94. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 9.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grøgaard HK, Bjørnerheim R, Brekke M, Müller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 10.Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, Ingels M, Jacobs A, Geukens R, Dendale P, Vijgen J, Dilling D, Steels P, Mees U, Rummens JL. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114:I101–7. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 11.Gronthos S, Fitter S, Diamond P, Simmons PJ, Itescu I, Zannettino AC. A Novel Monoclonal Antibody (STRO-3) Identifies an Isoform of Tissue Nonspecific Alkaline Phosphatase Expressed by Multipotent Bone Marrow Stromal Stem Cells. Stem Cells and Development. 2007;16:953–963. doi: 10.1089/scd.2007.0069. [DOI] [PubMed] [Google Scholar]
- 12.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, John-Sutton MG, Gorman JH, 3rd, Edmunds LH, Jr, Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107:2857–63. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 13.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99:3063–70. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 14.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006;69:604–13. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Markovitz LJ, Savage EB, Ratcliffe MB, Bavaria JE, Kreiner G, Iozzo RV, Hargrove WC, 3rd, Bogen DK, Edmunds LH., Jr Large animal model of left ventricular aneurysm. Annals of Thoracic Surgery. 1989;48:838–845. doi: 10.1016/0003-4975(89)90682-6. [DOI] [PubMed] [Google Scholar]
- 16.Martens TP, See F, Schuster MD, Sondermeijer HP, Hefti MM, Zannettino A, Gronthos S, Seki T, Itescu S. Mesenchymal lineage precursor cells induce vascular network formation in ischemic myocardium. Nature Clinical Practice Cardiovascular Medicine. 2006;3:S18–S22. doi: 10.1038/ncpcardio0404. [DOI] [PubMed] [Google Scholar]
- 17.Moainie SL, Gorman JH, 3rd, Guy TS, Bowen FW, 3rd, Jackson BM, Plappert T, Narula N, John-Sutton MG, Narula J, Edmunds LH, Jr, Gorman RC. An ovine model of postinfarction dilated cardiomyopathy. Ann Thorac Surg. 2002;74:753–60. doi: 10.1016/s0003-4975(02)03827-4. [DOI] [PubMed] [Google Scholar]
- 18.López B, González A, Querejeta R, Díez J. The use of collagen-derived serum peptides for the clinical assessment of hypertensive heart disease. J Hypertens. 2005;23:1445–51. doi: 10.1097/01.hjh.0000173780.67308.f1. [DOI] [PubMed] [Google Scholar]
- 19.Stroud JD, Baicu CF, Barnes MA, Spinale FG, Zile MR. Viscoelastic properties of pressure overload hypertrophied myocardium: effect of serine protease treatment. Am J Physiol Heart Circ Physiol. 2002;282:H2324–35. doi: 10.1152/ajpheart.00711.2001. [DOI] [PubMed] [Google Scholar]
- 20.Nagatomo Y, Carabello BA, Coker ML, McDermott PJ, Nemoto S, Hamawaki M, Spinale FG. Differential effects of pressure or volume overload on myocardial MMP levels and inhibitory control. Am J Physiol. 2001;278:H151–H161. doi: 10.1152/ajpheart.2000.278.1.H151. [DOI] [PubMed] [Google Scholar]
- 21.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000;102:1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 22.Coker ML, Thomas CV, Clair MJ, Hendrick JW, Krombach RS, Galis ZS, Spinale FG. Myocardial matrix metalloproteinase activity and abundance with congestive heart failure. Am J Physiol. 1998;274:H1516–H1523. doi: 10.1152/ajpheart.1998.274.5.H1516. [DOI] [PubMed] [Google Scholar]
- 23.Meadows JR, Hawken RJ, Kijas JW. Nucleotide diversity on the ovine Y chromosome. Anim Genet. 2004;35:379–85. doi: 10.1111/j.1365-2052.2004.01180.x. [DOI] [PubMed] [Google Scholar]
- 24.Weikard R, Pitra C, Kühn C. Amelogenin cross-amplification in the family Bovidae and its application for sex determination. Mol Reprod Dev. 2006;73:1333–7. doi: 10.1002/mrd.20554. [DOI] [PubMed] [Google Scholar]
- 25.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 26.Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva F. Fate of cultureexpanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res. 2009;104:398–402. doi: 10.1161/CIRCRESAHA.108.187724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–57. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 28.Rosendahl MS, Ko SC, Long DL, Brewer MT, Rosenzweig B, Hedl E, Anderson L, Pyle SM, Moreland J, Meyers MA, Kohno T, Lyons D, Lichenstein HS. Identification and characterization of a pro-tumor necrosis factor-alphaprocessing enzyme from the ADAM family of zinc metalloproteases. J Biol Chem. 1997;272:24588–93. doi: 10.1074/jbc.272.39.24588. [DOI] [PubMed] [Google Scholar]
- 29.Lovelock JD, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, Sivasubramanian N, Mann DL. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2005;288:H461–8. doi: 10.1152/ajpheart.00402.2004. [DOI] [PubMed] [Google Scholar]
- 30.Reffelmann T, Könemann S, Kloner RA. Promise of blood- and bone marrow-derived stem cell transplantation for functional cardiac repair: putting it in perspective with existing therapy. J Am Coll Cardiol. 2009;53:305–8. doi: 10.1016/j.jacc.2008.10.018. [DOI] [PubMed] [Google Scholar]