Abstract
Adult hippocampal stem/progenitor cells (AHPs) continuously give rise to new neurons throughout life, which might be an important determinant of hippocampus-dependent function. Strikingly, the fate potential of AHPs is not restricted to the neuronal lineage because AHPs can be genetically induced to generate oligodendrocytes within their in vivo niche by AHP-specific ectopic expression of the basic-helix-loop-helix (bHLH) transcription factor achaete-scute complex-like 1 (ASCL1). Fate plasticity of AHPs is controlled by cell-autonomous and also niche-dependent mechanisms. Here, we discuss the biological importance and potential therapeutic applications of retained fate plasticity of AHPs in the adult mammalian brain in addition to the future scientific inquiries indicated by this finding.
Stem/progenitor cells in the adult hippocampus
Adult hippocampal stem/progenitor cells (AHPs) persist in the adult hippocampus and generate newborn cells throughout life [1]. The finding that neurogenesis occurs through the entire lifespan strongly influenced current concepts of hippocampal connectivity and hippocampus-dependent function [2]. Furthermore, discovery of the existence of AHPs in the adult hippocampus opened the possibility of AHP-mediated endogenous repair in neuro-psychiatric disease [3]. AHPs express a distinct set of stem-cell-associated proteins and are located in the sub-granular zone of the dentate gyrus [4]. The vast majority of cells that are born in the adult hippocampus are excitatory granule cells, the principle neuronal type of the dentate gyrus [5]. The number of newborn granule cells is not static but, rather, dynamically regulated by a variety of physiologic and pathologic stimuli [2]. Notably, alterations in hippocampal neurogenesis have been associated with several diseases, such as major depression, epilepsy and age-dependent cognitive decline, that affect hippocampal structure and/or function [6–8]. Although isolated AHPs retain the capability to generate all three neural lineages in the culture dish and thus show multipotency in vitro [9], the fate of newborn cells in vivo is largely restricted to the neuronal lineage. However, AHPs can be pushed toward the glial lineage and become capable of generating oligodendrocytes within their in vivo niche through overexpression of a single transcription factor, achaete-scute complex-like 1 (ASCL1; also named MASH1) [10] (Figures 1 and 2). We discuss the biological importance of directed fate specification, its implications for stem-cell diversity and potential therapeutic applications based on the fate plasticity of adult neural stem cells.
Figure 1.
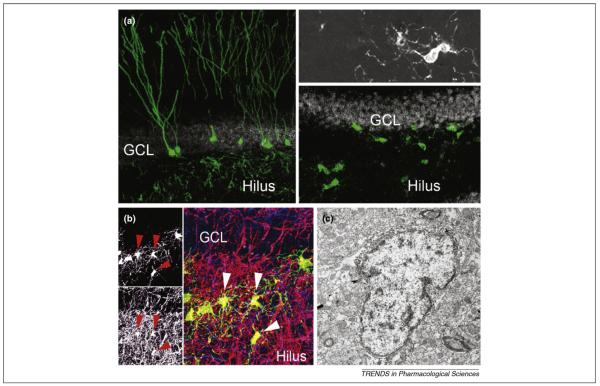
Ascl1 instructs AHPs to adopt an oligodendrocytic fate. (a) Newborn cells differentiate predominantly into granule cells in the adult hippocampus. Shown are retrovirus-labeled cells four weeks after injection of a green fluorescent protein (GFP)-expressing retrovirus (green). Retrovirus-mediated Ascl1 expression results in fate change (right panels; inset shows high-power view of an Ascl1-expressing newborn cell). (b) A fraction of Ascl1-induced, GFP-labeled oligodendrocytes (green) expresses myelin basic protein (MBP; red) wrapping around axonal processes (neurofilament 200 kD protein, blue). Left panels show single channels for GFP and MBP pointing toward GFP/MBP-co-labeled cells (red arrowheads in left panels; white arrowheads in right panels). (c) Ascl1-expressing cells have ultrastructural features of oligodendrocytic cells such as heterochromatin, a large nucleus and a large Golgi apparatus. For high-power confocal images and additional electron microscopy images supporting evidence for myelinating Ascl1-expressing cells, please refer to Ref. [10]. Abbreviations: GCL, granule cell layer.
Figure 2.
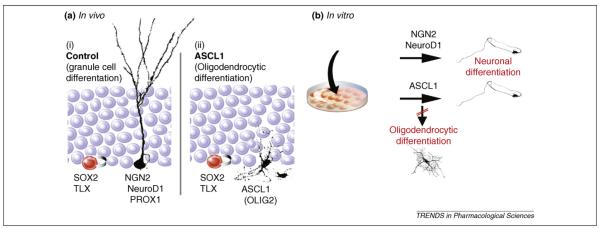
Context-dependent fate specification induced by Ascl1. (a) Dividing AHPs that are located in the inner border of the granule cell layer expressing certain transcription factors such as SOX2 and TLX almost exclusively generate excitatory granule cells under control conditions (i). Even though direct evidence is scant, a sequence of bHLH and proneural transcription factors, such as neurogenin 2 (NGN2), neurogenic differentiation 1 (NeuroD1) and prospero homeobox 1 (PROX1), is thought to be required for proper neuronal differentiation. AHP-specific expression of Ascl1 within the in vivo niche redirects the fate of the progeny from a neuronal to an oligodendrocytic lineage (ii). This fate specification is accompanied by oligodendrocyte lineage transcription factor 2 (OLIG2) expression but potentially involves other transcription factors that are induced in the course of fate specification. (b) The effect of ASCL1 is context dependent because isolated AHPs differentiate into neuronal cells and not oligodendrocytes when challenged with Ascl1 in the culture dish; this is similar to the overexpression of proneural genes such as NGN2 and NeuroD1. The distinct response to Ascl1 in vitro and in vivo indicates context-dependent effects of ASCL1 that are specific to the endogenous niche of AHPs, which could be explained by factors expressed within the dentate niche or alterations in neural stem/progenitors upon isolation and growth factor exposure
Directed differentiation of AHPs in vivo
Several laboratories successfully isolated dividing precursors from the adult rodent and human hippocampus [9,11–13]. Isolated cells can self-renew and differentiate into all three neural lineages within the culture dish providing in vitro evidence that they are neural stem cells. However, most culture conditions use media that are supplemented with high amounts of growth factors that can substantially alter the cellular properties of isolated cells compared with their behavior in their natural environment within the brain [14]. Despite recent data that indicate self-renewal of AHPs in vivo [15], definite proof of self-renewal and multipotency is still missing because appropriate experimental strategies, such as serial transplantation of isolated AHPs or longitudinal observation of dividing cells within their endogenous niche, have not yet been published. An indirect approach to test the potency of AHPs within the adult brain is to alter the fate of AHP progeny. With this is mind, we cell-type-specifically challenged AHPs with a battery of transcription factors that are crucially involved in cellular differentiation programs during embryonic development. Strikingly, overexpression of Ascl1 redirected the fate of AHP-generated cells from a neuronal to an oligodendrocytic lineage [10]. ASCL1-induced oligodendrocytes matured and seemed to participate in myelination of axonal processes. However, further experiments are needed to prove myelination in vivo at a single-cell level, which is technically challenging (Figure 1). Notably, our experiments showed that AHPs retained the ability to change their fate upon expression of a master regulatory gene. This finding might not be surprising given the fact that AHPs behave as stem cells in the culture dish; the unexpected observation, however, is that the niche of the adult dentate gyrus apparently provides sufficient cues to support maturation of a neural cell type (oligodendrocytes) that is not generated under normal conditions in the adult hippocampus.
Directed differentiation of AHPs: in vivo reprogramming?
Ascl1 is a basic-helix-loop-helix (bHLH) transcription factor that has been previously implicated in both the generation of GABAergic interneurons and oligodendrocytes[16,17]. Notably, Ascl1 seems to be dispensable for the generation of dentate gyrus granule cells, at least during embryonic development [18]. As outlined earlier, ASCL1 converts the fate of AHP progeny from neurogenic to oligodendrogenic when expressed in AHPs. A series of recent studies showed that somatic cells can be reprogrammed to a pluripotent, embryonic stem-cell-like state with the expression of only four transcription factors [19–21]. Reprogramming with these four transcription factors is certainly an extreme example of fate plasticity, with each of the factors leading to a specific cellular response that, when combined, results in an undifferentiated, embryonic stem cell-like state of transduced cells [22]. In this context, Ascl1-mediated fate direction of AHPs could be interpreted as in vivo reprogramming, which has also been recently shown for adult pancreatic exocrine cells into β-cells [23]. In fact, there is no strict definition of what ‘reprogramming’ actually means, for example, to which undifferentiated state a cell has to revert. By definition, AHPs are rather undifferentiated and express stem-cell-associated genes such as SRY (sex determining region Y)-box 2 (Sox2) and Nestin. Therefore, it is currently not possible to judge if AHPs de-differentiate to a less restricted state before they switch to an oligogenic fate or if Ascl1 initiates the differentiation program toward the oligodendrocytic lineage without affecting the ‘initial’ differentiation state of transduced AHPs. Profiling of the epigenetic state of ‘normal’ AHPs versus Ascl1-induced cells might help to solve this question [24]. However, these experiments are extremely challenging experimentally now because the number of available tissue is rate limiting for these types of experiments.
Context-dependent effects of ASCL1
The effect of viral-mediated Ascl1 expression in AHPs revealed a striking context dependency: AHPs that were cultured in vitro differentiated into neurons upon Ascl1-induction instead of adopting an oligodendrocytic fate [10] (Figure 2). The distinct response of AHPs in vitro versus in vivo indicates that ASCL1 functions in association with other factors or transduction pathways that are altered by removing the cells from their endogenous niche. The context-dependent effect of ASCL1 could be due either to: (i) factors expressed by neighboring cells within the dentate gyrus whose signals are lost after AHP isolation or (ii) alterations of cellular properties owing to growth-factor exposure and single-cell dissociation under in vitro conditions [25]. The identification of factors that influence the state of AHPs is crucial to understanding the effects of ASCL1 under normal conditions, in which AHPs generate neurons. In addition, it will be important to discover the molecular mechanisms that make the dentate gyrus neurogenic, in contrast to other regions such as the substantia nigra or spinal cord that contain cells with stem-cell characteristics (e.g. showing self-renewal and multipotency) when grown in the culture dish, even though no new neurons are generated in these areas under normal conditions [26–29].
The effect of ASCL1 differed not only between AHPs in vivo and in vitro; cell-type specific Ascl1 expression in dividing stem/progenitor cells of the second neurogenic region in the adult brain, the subventricular zone (SVZ) of the lateral ventricles from which newborn cells migrate toward the olfactory bulb, was not sufficient to induce the formation of new oligodendrocytes [10]. However, two important differences between the two neurogenic areas are that a large number of newborn cells in the SVZ express endogenously high levels of ASCL1 and that ASCL1 is required for at least early postnatal formation of the olfactory bulb [30]. When stem/progenitor cells were isolated from the SVZ and transduced with Ascl1-expressing retroviral vectors, they differentiated into neuronal cells, as did AHPs in vitro. However, Ascl1-induced neuronal cells showed distinct morphological features depending on whether they were derived from AHPs or stem/progenitor cells from the SVZ (S.J. and J.H.G., unpublished), underscoring the context dependency of ASCL1 effects and the diversity of the adult neural stem/progenitor cells from the two neurogenic regions of the adult mammalian brain.
Therapeutic applications of AHP fate plasticity
Although there are efforts to determine how to replace or supplement lost neuronal cells with endogenous AHPs in a large variety of neuro-psychiatric diseases, glial cell replacement or supplementation will also probably be beneficial for several illnesses.
Multiple sclerosis (MS) is a prevalent autoimmune disease in which the immune system attacks the myelin-sheath-wrapping axonal processes. Eventually, MS results in several physical and mental symptoms and often progresses to physical and cognitive disability [31]. Neuronal cells also become affected and eventually die, secondary to myelin damage. Previous studies showed that experimental autoimmune encephalitis, a commonly used rodent model of MS, initially enhances hippocampal neurogenesis [32]. However, at later stages in the disease process the number of newborn neurons strongly declines [32]. Because demyelination precedes neuronal loss, a promising strategy might be to replace lost oligodendrocytes with the activation of endogenous AHPs.
Current therapeutic strategies of directed fate specification in human disease are far from any kind of clinical application because intracerebral injections of retroviral vectors cannot be considered safe in humans at this time. However, this approach would have the potential advantage that only a restricted number of generations of AHP progeny would be altered, leaving a large number of progenitors unaffected (those that did not divide at the time of viral injection). Alternatively, small molecules that trigger certain transcriptional programs might be utilized to direct the fate of AHP progeny. Genetic or drug-mediated fate specification can also be envisioned, on the progenitor cell level or alternatively by manipulating the dentate niche. Furthermore, it seems unlikely that the fate of AHP progeny is restricted to the neuronal and oligodendrocytic lineages. Future studies will determine whether directed fate specification toward the astrocytic lineage is possible.
Conclusions
AHPs are not restricted in their fate and can be redirected from the neuronal to the oligodendrocytic lineage by overexpression of a single gene, Ascl1. To analyze the behavior and potential usefulness of Ascl1-induced oligodendrocytes generated from AHPs in demyelinating disease will be an important proof-of-principle experiment as to whether directed fate specification of AHPs can be utilized for glial cell replacement. These experiments will have to provide unambiguous evidence that the progeny of fate-directed AHPs are capable of generating functional myelin sheaths under demyelinating conditions and that these myelin wraps are able to promote electrical axonal conduction after lesion. Furthermore, future experiments should aim to identify niche-expressed factors and region-specific differences in adult neural stem/progenitor cells that are responsible for the context-dependent effects of ASCL1. E-proteins, forming functionally important heterodimers with bHLH transcription factors, might have a crucial role with respect to the context-dependent effects of ASCL1 because they are required for the full transcriptional activity of bHLH transcription factors [33]. It is interesting that the mRNA of one of the E-proteins (E47) is highly expressed in the SVZ and the rostral migratory stream but to a lesser extent in the adult dentate area (www.brain-map.org). The expression levels of E-proteins in AHPs are unknown, but isolation and growth factor exposure of isolated cells could (in contrast to the situation within the endogenous niche) alter the expression of E-proteins and, thus, explain the observed differences of ASCL1 action in vivo versus in vitro. However, experiments will be needed to identify differences in the molecular characteristics between the two neurogenic areas on a more global and unbiased level. For example, so far, hardly anything is known about the molecular differences or similarities between neural stem/progenitor cells in the SVZ and their counterparts in the subgranular zone of the dentate gyrus. The picture has become even more complicated after the recent finding that the adult SVZ apparently contains a diversity of neural stem/progenitor cells [34]. However, expression data, including mRNA, protein and small non-coding RNAs in neural stem/progenitor cells and immature neurons in the two neurogenic areas, will set the stage for a better understanding of fate restriction and fate plasticity of neural stem/progenitor cells within the adult brain.
Acknowledgements
We thank M.L. Gage for editing this manuscript. Support was provided by NCCR Neural Plasticity and Repair (www.nccr-neuro.ethz.ch), Swiss National Science Foundation (to S.J.; www.snf.ch), National Institute on Aging (www.nia.nih.gov), Lookout Fund and McDonnell Foundation (to F.H.G.; www.jsmf.org).
References
- 1.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 2.Zhao C, et al. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Goldman SA. Disease targets and strategies for the therapeutic modulation of endogenous neural stem and progenitor cells. Clin. Pharmacol. Ther. 2007;82:453–460. doi: 10.1038/sj.clpt.6100337. [DOI] [PubMed] [Google Scholar]
- 4.Kempermann G, et al. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 5.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parent JM, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempermann G, et al. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann. Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 8.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 9.Palmer TD, et al. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol. Cell. Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 10.Jessberger S, et al. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat. Neurosci. 2008;11:888–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker TL, et al. Latent stem and progenitor cells in the hippocampus are activated by neural excitation. J. Neurosci. 2008;28:5240–5247. doi: 10.1523/JNEUROSCI.0344-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babu H, et al. Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS One. 2007;2:e388. doi: 10.1371/journal.pone.0000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer TD, et al. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411:42–43. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- 14.Gabay L, et al. Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron. 2003;40:485–499. doi: 10.1016/s0896-6273(03)00637-8. [DOI] [PubMed] [Google Scholar]
- 15.Suh H, et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petryniak MA, et al. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battiste J, et al. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–293. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- 18.Galichet C, et al. Neurogenin 2 has an essential role in development of the dentate gyrus. Development. 2008;135:2031–2041. doi: 10.1242/dev.015115. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Q, et al. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meissner A, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman SA, Natesan S. A niche-defying feat: induced oligoneogenesis in the adult dentate gyrus. Cell Stem Cell. 2008;3:125–126. doi: 10.1016/j.stem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Horner PJ, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J. Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frielingsdorf H, et al. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10177–10182. doi: 10.1073/pnas.0401229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song H, et al. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 29.Lie DC, et al. The adult substantia nigra contains progenitor cells with neurogenic potential. J. Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parras CM, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keegan BM, Noseworthy JH. Multiple sclerosis. Annu. Rev. Med. 2002;53:285–302. doi: 10.1146/annurev.med.53.082901.103909. [DOI] [PubMed] [Google Scholar]
- 32.Aharoni R, et al. Neurogenesis and neuroprotection induced by peripheral immunomodulatory treatment of experimental autoimmune encephalomyelitis. J. Neurosci. 2005;25:8217–8228. doi: 10.1523/JNEUROSCI.1859-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murre C, et al. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 34.Merkle FT, et al. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]