Abstract
Background
Previous research has reported that inhibition of breathing can be observed in hypertensive patients at rest during the daytime, as well as in sleep at night. The present study hypothesized that the variability of breathing and end tidal CO2 (PetCO2) in seated women at rest is positively associated with their 24-hr blood pressure level.
Methods
Breath-to-breath measures of breathing rate and tidal volume were recorded via inductive plethysmography in each of 54 women during two 20 min sessions of seated rest, and in 32 women during nighttime sleep. PetCO2 was also recorded during these sessions via a respiratory gas monitor. Ambulatory blood pressure was recorded for 24 hr between the two clinic sessions via oscillometry.
Results
Breath pauses > 10 sec were observed significantly more often in women in the upper than the lower tertile of 24-hr systolic blood pressure. Breath-to-breath variability in breathing rate, tidal volume, and minute ventilation were greater in the higher blood pressure tertile women. Variability in PetCO2 was also greater in high blood pressure tertile. These associations were independent of age, weight, and body surface area. Breathing variability was inversely correlated with heart rate variability.
Conclusion
Greater variability in breathing at rest that is independent of metabolic activity characterizes women with elevated blood pressure. The linear association of breathing variability with 24-hr blood pressure level is consistent with the hypothesis that intermittent breathing inhibition may predispose to the development of some forms of hypertension.
Keywords: Apnea, blood pressure, breathing, end tidal CO2, hypertension
INTRODUCTION
Intermittent inhibition of breathing during sleep is a well-documented risk factor for primary hypertension1,2. Sleep apnea in hypertension is most often due to mechanical obstruction of the upper airway, but is sometimes of central nervous system origin. Even in obstructive sleep apnea, however, its intermittent character suggests the participation of central factors. Congestive heart failure, on the other hand, is more often associated with central sleep apnea3,4. In advanced heart failure, a phenomenon of periodic breathing can be observed, in which episodes of hypoventilation alternate with hyperventilation5. Periodic breathing can in fact be observed in heart failure patients while they are awake as well as during sleep5, and is a risk factor for cardiovascular mortality6.
Episodes of breathing inhibition during daytime seated rest have been reported in one previous study of hypertensive patients7. These apneic episodes were accompanied by transient blood pressure instability and increased spectral power of heart rate at low frequencies. However, these episodes did not result in decreased mean breathing rates, relative to those of matched normotensive controls. This intriguing finding was reported in the context of other findings that hypertensive patients at seated rest exhibit hyperventilatory breathing8.
The present study investigated the incidence of spontaneous apneic events (breath pauses > 10 sec) during seated rest in a cohort of otherwise healthy women whose 24-hr blood pressures varied from low normal to mildly hypertensive. It was hypothesized that those with higher 24-hr blood pressure would show a greater incidence of apneic events during seated rest than those with lower 24-hr blood pressure. On the assumption that spontaneous apneic events would be accompanied by compensatory hyperventilation, it was also hypothesized that breathing variability of those with higher blood pressure would be greater than those with lower blood pressure.
Increased variability in breathing rate and tidal volume could be the result of increased variability in metabolic activity. By contrast, increased variability in ventilation accompanied by increased variability of end tidal CO2 (PetCO2) would indicate an independence of this respiratory phenomenon from metabolic activity. Therefore, the study also assessed variability of PetCO2 at the same time that breathing variability was determined.
Finally, previous studies have reported that heart rate variability (HRV) is decreased in hypertensive patients9,10. The present study examined the association of HRV at seated rest with 24-hr blood pressure, and the possible mediating role of breathing variability in this association.
METHODS
Study population
Eighty one women were initially recruited from the surrounding community through local advertisement. Fifty four met the qualifying criteria, and completed the study. The sample whose data were analyzed in this manuscript included healthy female volunteers and hypertensive patients enrolled in two successive randomized clinical trials11 (ClinicalTrials.gov ID # NCT00328016) with different objectives than those that were the purpose of the present investigation. In those studies, the numbers of women enrolled were substantially larger than the numbers of men, resulting in a male sample of inadequate size to generate reliable conclusions about the relationships reported here with women.
After telephone screening for medical history, potential participants were invited to the NIA-ASTRA Clinical Research Unit where informed consent was obtained. A physical examination was performed, and blood drawn for complete blood count with differential, comprehensive metabolic panel, including fasting glucose, liver and kidney function, lipid panel, and urinalysis, performed to ensure that the participants were free of respiratory, cardiovascular and renal diseases. Other exclusion criteria were tobacco use, and any medications that would interfere with respiration or blood pressure, including hormone replacement therapy. Qualifying criteria included ages 40–70, female gender, and European American race, since racial differences in blood pressure regulation have been reported12,13. Blood pressure inclusion criteria were based on five clinic measurements taken at six minute intervals, plus hourly recordings in the natural environment for 24-hr. Women with clinic blood pressure above 160 mmHg systolic or 100 mmHg diastolic pressure were excluded from the study. This protocol was approved by the Institutional Review Board of the Medstar Research Institute.
Physiological monitoring procedures
Breathing, PetCO2 and blood pressure were monitored between 9 am and 1 pm during two 20 min intervals of seated rest in a controlled environment within a one week period. (Some subjects were on a lower salt diet during the second session, but this had no effect on individual differences in breathing variability at rest). In each session, breathing rate, inter-breath interval and tidal volume were monitored continuously via an elasticized vest containing sinusoidal wires for inductive plethysmographic recording of thoracic and abdominal expansion (LifeShirt™, Vivometrics, Ventura, CA) 14. Tidal volume was calibrated before each session by exhaling a fixed volume of air into an inflatable bag, and minute ventilation was calculated as the product of breathing rate and tidal volume. Breath-to-breath measurements were recorded in a microprocessor and stored on a flash card that enabled subsequent downloading to a computer (Dell Computer, Model 740, Round Rock, TX). In addition, the vest included apparatus for continuous electrocardiographic recording.
During these sessions, PetCO2 was monitored continuously via a nasal cannula connected to a respiratory gas monitor (Datex-Ohmeda Model 5250, Fairfield, CT). Means of PetCO2 over successive 10-sec intervals were output to the computer.
Systolic and diastolic blood pressure and heart rate were automatically recorded at six min intervals during the clinic monitoring session and once per hour for 24-hr in the natural environment via an inflatable cuff applied to the non-dominant arm connected to an automated oscillometric device (Spacelabs, model 90207, Redmond, WA).
The last 32 of the 52 subjects also wore the Lifeshirt in bed at home during overnight sleep. The condition of night-time monitoring was added to the protocol after study onset when the frequency of daytime apnea was observed in the initial participants. A six-hr interval between midnight and 6 a.m. was selected for analysis of breathing pattern during sleep.
Data Analysis
Prior to data analysis, the Lifeshirt records were inspected for movement artifact. On the assumption that breathing does not occur faster than 30 breaths per minute at rest, any breaths recorded less than two seconds after a previous breath were deleted. In addition, any tidal volume values greater than two standard deviations above the individual mean were deleted. These procedures resulted in a removal of approximately 1% of the raw data, and had no significant effects on the results.
Means and standard deviations of breathing rate, tidal volume, minute ventilation and PetCO2 were calculated for tertiles of 24-hr systolic blood pressure, with 18 subjects in each tertile. The significance of group differences was determined by one-way analysis of variance.
An apneic event was defined as a breath pause > 10 sec. The significance of tertile differences in daytime and nighttime apneic events was also tested by one-way analysis of variance, and independence of significant associations with 24-hr systolic blood pressure from age, body mass index (BMI), and body surface area (BSA) were determined by stepwise multiple regression analysis with backwards elimination of non-significant variables.
The variability in each breathing measure was assessed by calculating the root mean square of successive differences (RMSSD) over consecutive breaths. RMSSD was used instead of standard deviation because the latter is sensitive to progressive changes over time, and the focus here was on breath-to-breath variability. Spectral analysis of the R-R interval series yielded low (LF) and high frequency (HF) components of HRV, and were transformed to natural logarithmic functions for statistical analysis. LF and HF components of HRV, and their ratio, have been previously identified as indices of sympatho-vagal influence on cardiac function in humans and laboratory animals15.
The significance of the associations of each breathing measure and each HRV measure with 24-hr systolic blood pressure was examined by univariate correlational analysis, followed by multiple regression analysis that included age, BMI, and BSA.
Statistics were performed using SSPS, Version 11 (SPSS Inc., Chicago, Illinois) and SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina).
RESULTS
24-hr blood pressure group stratification
Table 1 shows means and standard deviations of age, physiometric, and physiological measures for all women, and for those in the lower, middle, and upper tertiles of 24-hr systolic blood pressure. Significant differences between upper and lower tertiles were observed for BSA (F2,51 = 9.293;p <.001), systolic blood pressure (F2,51 = 51.46; p <.0001), diastolic blood pressure (F2,51 = 13.13; p <.0001), and tidal volume (F2,17 = 5.85; p <.01), but not age, BMI heart rate, breathing rate, minute ventilation or PetCO2. When age, BMI, and BSA were included in a multiple linear regression of tidal volume on 24-hr systolic blood pressure, only BSA remained a significant individual determinant of 24-hr systolic blood pressure (F2,49 = 12.62; p <.001; R2 = .35).
Table 1.
Baseline resting means and standard deviations of demographic and physiological variables for all participants (n = 54) and those stratified by mean 24-hr systolic blood pressure (SBP) in the lower (SBP = 108.7±5.7), middle (SBP = 124.2±4.1) and upper (SBP = 139.7±8.7) tertiles.
| ALL (n=54) |
LOWER TERCILE (n=18) |
MIDDLE TERCILE (n=18) |
UPPER TERCILE (n=18) |
|
|---|---|---|---|---|
| Age (yr) | 53.4±7.7 | 50.2±9.2 | 54.9±6.9 | 54.2±6.3 |
| Body surface area | 1.79±14.47 | 1.68±0.12 | 1.79±0.15 | 1.91±0.20 |
| Body Mass Index | 26.82±4.55 | 25.30±3.74 | 26.34±3.49 | 28.44±5.83 |
| Systolic BP (mmHg) | 132.7 ± 15.1 | 112.6 ± 10.0 | 125.8 ± 7.4 | 142.4 ± 8.3 |
| Diastolic BP (mmHg) | 80.2 ± 9.3 | 73.0 ± 6.4 | 77.1 ± 6.4 | 85.1 ± 8.3 |
| Heart rate (bpm) | 66.4 ± 8.0 | 66.8 ± 4.9 | 66.8 ± 9.0 | 64.2 ± 6.1 |
| Breathing rate (per min) | 13.9±2.5 | 14.0±2.4 | 14.1±2.0 | 13.7±3.0 |
| Tidal volume (ml) | 418±133 | 367±128 | 386±116 | 497±125 |
| Min vent (L/min) | 5.12±1.37 | 4.78±1.58 | 5.18±1.49 | 5.41±1.00 |
| End tidal CO2 (mmHg) | 37.4±3.8 | 38.3±4.5 | 36.7±3.7 | 36.9±3.5 |
Breathing pauses as a function of 24-hr systolic blood pressure level
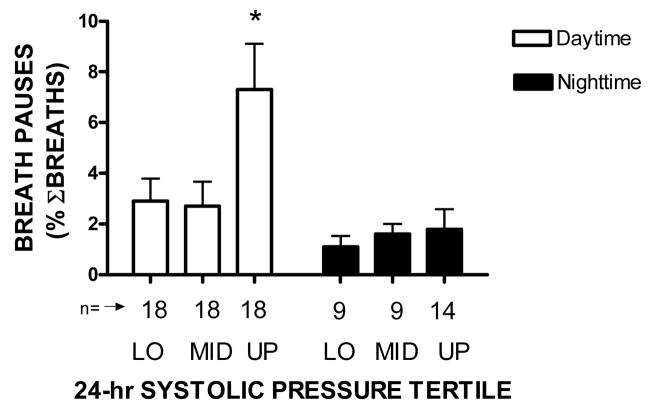
Figure 1 shows means and standard errors of apnea incidence during daytime rest and nighttime sleep for women in each tertile of 24-hr systolic blood pressure. Apeneas during daytime rest in women in the upper tertile were much more prevalent in those in the lower tertile (F3,104 = 8.23; p <.001).
Figure 1.
Means and standard errors of percentage daytime resting and night-time sleep pauses in breathing for lower (LO), middle (MID), and upper (UP) tertiles of 24-hr systolic blood pressure level * = p <.05 compared with all other bars.
The association of apneic incidence with 24-hr systolic blood pressure level was independent of age, BMI and BSA (F2,46 = 7.05; p <.002), and apneic incidence was inversely correlated with individual mean breathing rate (r = −0.58; p <.001). The incidence of nighttime apneic events did not vary as a function of 24-hr systolic blood pressure (F =2.09; p > 0.05), and was significantly less than observed during the daytime (t = 3.43; p <.001). No such effects were observed for tertiles of 24-hr diastolic blood pressure.
Variability of breathing rate, tidal volume, minute ventilation, and end tidal CO2
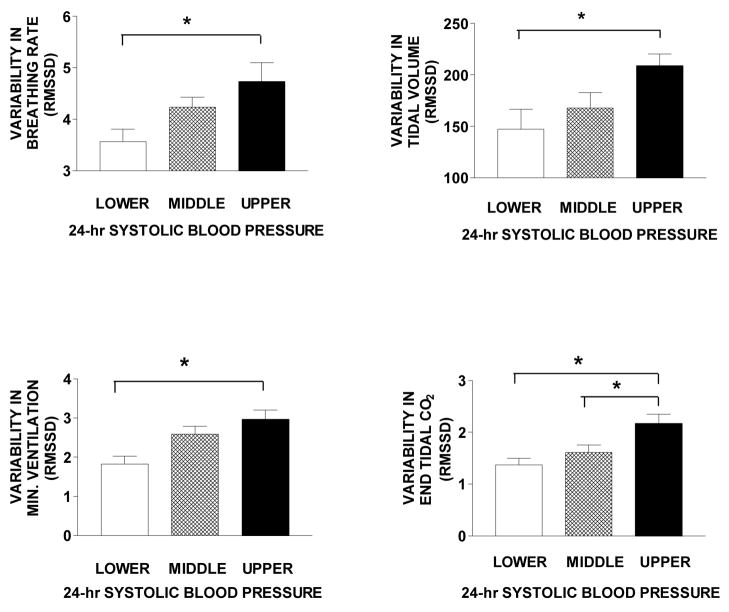
The upper panels of Figure 2 show that women in the higher systolic blood pressure tertile had greater variability in both breathing rate (F5,17 =4.37; p <.02) and tidal volume (F5,17 = 4.10; p <.03) than those in the lower tertile. A multiple linear regression of breathing rate variability, tidal volume variability, age, BMI, and BSA showed breathing rate variability and BSA to be independent determinants (F2,49 = 18.7; p <.001; R2 = .45). No such associations were found for 24-hr diastolic blood pressure.
Figure 2.
Means and standard errors in variability of breathing rate, tidal volume, minute ventilation, and end tidal CO2 for tertiles of 24-hr systolic blood pressure level * = p <.05 compared with other bars.
The lower panels of Figure 2 show that women in the higher systolic blood pressure tertile also had greater variability in minute ventilation (F5,17 =6.74; p <.01) and PetCO2 (F5,17 = 7.07; p <.01) than those in the lower tertile. A multiple linear regression of minute ventilation, PetCO2, age, BMI, and BSA on 24-hr systolic blood pressure showed PetCO2 and BSA to be significant independent determinants (F2,49 = 18.8; p <.003; R2 = .43). No such associations were found between variability in these breathing measures and 24-hr diastolic blood pressure.
Associations of heart rate variability with 24-hr blood pressure
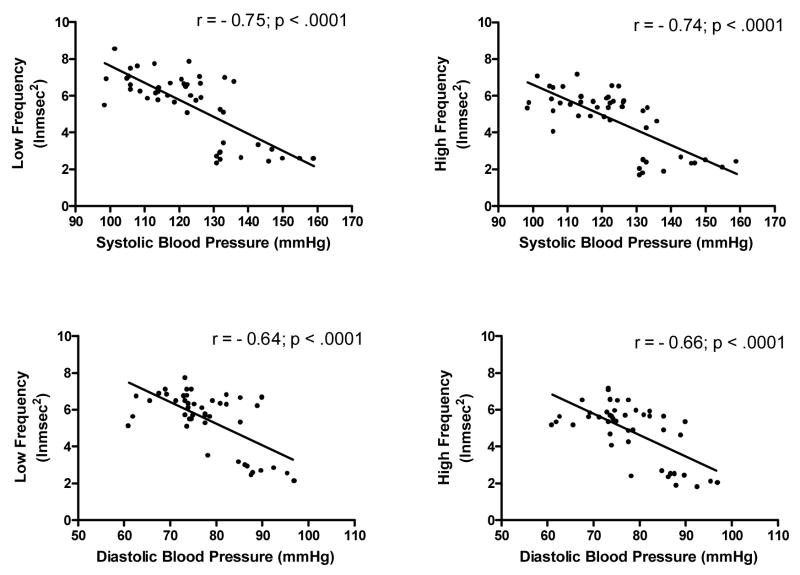
Figure 3 shows that both LF and HF frequency bands were significantly inversely correlated with 24-hr systolic and diastolic pressure. Table 2 shows the univariate linear correlations between measures of breathing variability and measures of HRV. In general, negative correlations were observed between breathing variability and HRV. When a multiple regression of age, BMI, BSA, all HRV measures, and all breathing variability measures on systolic blood pressure was performed, BSA and breathing rate variability were positive independent determinants, while LF was a negative independent determinant (F3,41 = 37.94; p <.001; R2 = .74). When a multiple regression of age, BMI, BSA, LF, HF, and variability in breathing rate, tidal volume, minute ventilation and PetCO2 on diastolic blood pressure was performed, only LF was an independent determinant (F3,41 = 27.92; p <.001; R2 = .37).
Figure 3.
Scatter plots of low and high frequency heart rate variability and 24-hr systolic and diastolic blood pressure.
Table 2.
Univariate linear correlations between breathing rate (RMSSD = root mean square of successive mean differences), tidal volume (TV), ventilation, end tidal CO2 (PetCO2), low frequency heart rate variability (LF HRV in lnmsec2), high frequency heart rate variability (HF HRV in lnmsec2), and the ratio of low to high frequency heart rate variability (LF/HF).
| TV (RMSSD) | Ventilation (RMSSD) | PetCO2 (RMSSD) | LF HRV (lnmsec2) | HF HRV (lnmsec2) | LF/HF | |
|---|---|---|---|---|---|---|
| Breathing rate (RMSSD) | .53** | .55** | .53** | −.16 | −.20 | .34* |
| Tidal Volume (RMSSD) | .83** | .44** | −.29 | −.40** | .37* | |
| Ventilation (RMSSD) | .42** | −.35* | −.39** | .14 | ||
| End tidal CO2 (RMSSD) | −.34* | −.43** | .34* | |||
| LF HRV (lnmsec2) | .93** | −.12 | ||||
| HF HRV (lnmsec2) | −.38** |
= p <.05;
= p <.01
DISCUSSION
The results of this study confirmed the hypothesis that women with higher blood pressure have a greater incidence of apneic events, and greater variability in breathing rate, tidal volume, minute ventilation, and PetCO2 than those with lower blood pressure.
These findings are consistent with those in a previous report describing intermittent breathing pauses during daytime rest in hypertensive persons7. We extend their findings by showing that increased variability in ventilatory activity can be seen at prehypertensive blood pressure levels, and is independent of variations in metabolic activity. Moreover, these findings appear to explain previous reports of hyperventilatory breathing in hypertensive patients at rest8. Higher tidal volume was observed in women with higher blood pressure, but this association was negated when the mediating role of greater BSA was taken into account. Thus, greater tidal volume could be expected in persons with larger body size and larger lungs. This contribution of BSA to the association between breathing variability and blood pressure was independent of that of BMI, which did not significantly influence the relationship.
The increased breathing variability observed in the present study is distinct from the disordered breathing in hypertensive patients during sleep and in heart failure. For example, occurrences of apnea during sleep can result in hypoxia, chemoreceptor-mediated sympathetic arousal and consequent pressor responses1. The extent to which hypoxia participates in the development of sodium-sensitive forms of hypertension remains to be clarified, since mild to moderate hypoxia actually increases renal sodium excretion16.
The physiology of central sleep apnea presents a different picture3,4. Regular alternation of hypoventilation followed by hyperventilation can occur over approximately one min intervals. That the hypoventilation is the primary event is suggested by the finding that a negative interaction between central and peripheral chemoreceptors is stimulated by hypercapnia17. The hypercapnia appears to be mediated by increased parasympathetic activity, since electrical stimulation of the vagus nerve in a patient with intractable epilepsy was found to engender periodic breathing18. In the present study, none of the participants had either central sleep apnea or heart failure, and no evidence of periodic breathing was observed. Rather, women with higher blood pressure tended to show apneic events non-systematically with respect to time, balanced by compensatory hyperventilation that maintained means of various breathing measures that were not significantly different from those of women with lower blood pressure.
The finding that LF and HF HRV were lower in women with higher than lower 24-hr blood pressure extends those in previous research with normotensive and hypertensive patients. Lucini et al9 found that LF was increased while HF was decreased in a cohort that included both men and women, and a larger blood pressure range than in the present study. They noted that the effects were already evident in prehypertensive patients. Subsequently, increased plasma renin activity was found to be an independent determinant of reduced HF power and reduced RMSSD in a group of hypertensive patients10.
The finding that the inverse association between HRV and blood pressure level was independent of the association of breathing variability suggests that their respective contributions to long-term blood pressure regulation are mediated by different neuroendocrine pathways. A number of previous studies have concluded that hypertension is associated with increased sympathetic and/or decreased parasympathetic activity19,20. To what extent prehypertension or hypertension is associated with variably increased parasympathetic influence on respiratory function remains to be clarified.
A recent review of the literature concluded that vagal activity in conscious humans serves multiple behavioral functions, including an evolutionary older, unmyelinated component that mediates behavioral inhibition, suppressed emotion, and hypervigilant attention to the environment, and a more recent myelinated component that facilitates prosocial behavior21. Numerous previous studies have described transient inhibition of breathing as part of an integrated cardiorespiratory response to demands of the environment for vigilant attention22. In contrast to the “fight or flight” response, which is mediated by sympathetic arousal, vigilant attention involves decreased skeletal muscle blood flow and increased cerebral blood flow, as well as momentary inhibition of breathing.
Slower breathing by women at rest has been associated with high perceived chronic life stress23, and is a risk factor for salt sensitivity of blood pressure11. Voluntary hypoventilation that results in sustained hypercapnia has been associated in humans with decreased renal sodium excretion24 and increased concentrations of circulating vasconstrictor factors that are responsive to plasma volume expansion25. Moreover, intermittent behavioral stress that suppressed respiration chronically was found to potentiate a sodium-sensitive form of experimental hypertension in genetically-normotensive dogs26. Taken together, these findings are consistent with the hypothesis that stress-induced hypoventilatory breathing could play a causal role in the development of salt-sensitive forms of human hypertension.
These findings may seem surprising in light of recent studies showing that device-guided slow breathing can decrease blood pressure in hypertensive patients27. However, if slowed breathing by these patients is compensated by proportional increases in tidal volume, the percentage of lung surface in contact with the circulatory system could result in increased gas transfer and decreased pCO2. It remains to be determined whether device-guided breathing operates through this mechanism, and therefore might be most relevant to sodium-sensitive forms of hypertension.
These findings should be qualified by several considerations. First, the cohort was limited to women, and studies are needed to determine whether similar associations occur in men. Second, the cohort was limited to European Americans, because previous studies have shown that differences in resting PetCO2, as well as renal sodium regulation exist between European and African Americans28. Third, these observations were made under conditions of seated rest in a clinical setting, and the extent to which comparable results would be observed in ambulatory subjects in their natural environments remains to be determined.
In summary, this study found that episodes of apneic breathing during seated rest occur in women, whose incidence is positively related to their 24-hr blood pressure level. Increased variability in minute ventilation was accompanied by increased variability in PetCO2, suggesting that the episodes were independent of variations in metabolic activity. The increased breathing variability in women with higher blood pressure was accompanied by decreased heart rate variability. It remains for future studies to determine the extent to which this form of disordered breathing is a cause or a consequence of long-term blood pressure elevation.
Acknowledgments
This work was supported by the National Institute on Aging Intramural Research Program, National Institutes of Health. The authors hereby thank Beverly Parsons for her expert assistance with subject recruitment and testing, Denis Muller for help with statistical analysis of data, and S. Morteza “Mo” Farasat for his careful reading of the manuscript.
Sole source of support: National Institute on Aging (NIH) Intramural Research Program.
References
- 1.Narkiewicz K, Wolf J, Lopez-Jimenez F, Somers VK. Obstructive sleep apnea and hypertension. Curr Cardiol Rep. 2005;7(6):435–40. doi: 10.1007/s11886-005-0061-z. [DOI] [PubMed] [Google Scholar]
- 2.Parati G, Lombard C, Narkiewicz K. Sleep apnea:epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Reg Integr Comp Physiol. 2007;293(4):R1671–83. doi: 10.1152/ajpregu.00400.2007. [DOI] [PubMed] [Google Scholar]
- 3.Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;13(2):595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepin JL, Chouri-Pontarollo N, Tamisier R, Levy P. Cheyne-Stokes respiration with central sleep apnea in chronic heart failure: proposals for a diagnostic and therapeutic strategy. Sleep Med Rev. 2006;10(1):33–47. doi: 10.1016/j.smrv.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Mortara A, Sleight P, Pinna GD, Maestri R, Prpa A, LaRovere MT, Cobelli F, Tavazzi L. Abnormal awake respiratory patterns are common in chronic heart failure and may prevent evaluation of autonomic tone by measures of heart rate variability. Circ. 1997;96:246–252. doi: 10.1161/01.cir.96.1.246. [DOI] [PubMed] [Google Scholar]
- 6.La Rovere MT, Pinna GD, Meastri R, Robbi E, Mortara A, Fanfulla F, Febo O, Sleight P. Clinical relevance of short-term breathing disorders in chronic heart failure patients. European J Heart Fail. 2007;9(9):949–54. doi: 10.1016/j.ejheart.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Novak V, Novak P, de Champlain J, Nadeau R. Altered cardiorespiratory transfer in hypertension. Hypertens. 1994;23:104–113. doi: 10.1161/01.hyp.23.1.104. [DOI] [PubMed] [Google Scholar]
- 8.Almazov VA, Fedoseev GB, Degtyareva ZY, Katyukhin VN. Disorders in external respiration in patients with essential hypertension. Therapevt Archiv. 1981;53:121–123. [Google Scholar]
- 9.Lucini D, Mela GS, Malliani A, Pagani M. Impairment in Cardiac Autonomic Regulation Preceding Arterial Hypertension in Humans. Circ. 2002;106:2673–2679. doi: 10.1161/01.cir.0000039106.89299.ab. [DOI] [PubMed] [Google Scholar]
- 10.Virtanen R, Jula A, Kuusela T, Heleniu H, Voipio-Pukki LM. Reduced heart rate variability in hypertension: associations with life-style factors and plasma rennin activity. J Hum Hypertens. 2003;17:171–179. doi: 10.1038/sj.jhh.1001529. [DOI] [PubMed] [Google Scholar]
- 11.Anderson DE, Parsons BA, McNeely JD, Miller ER. Salt sensitivity of blood pressure is accompanied by slow respiratory rate: Results of a clinical feeding study. J Amer Society Hypertens. 2007;1:256–263. doi: 10.1016/j.jash.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muntzel M, Drucke T. A comprehensive review of the salt and blood pressure relationship. Am J Hypertens. 1992;5:S1–S42. doi: 10.1093/ajh/5.4s.1s. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DE, Scuteri A, Agalakova N, Parsons DJ, Bagrov AY. Racial differences in resting end tidal CO2 and sodium pump inhibitor. American Journal of Hypertens. 2001;14:761–767. doi: 10.1016/s0895-7061(01)02163-x. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelm FH, Roth WT, Sackner MA. The LifeShirt. An advanced system for ambulatory measurement of respiratory and cardiac function. Behav Modif. 2003;27:671–91. doi: 10.1177/0145445503256321. [DOI] [PubMed] [Google Scholar]
- 15.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of Sympatho-vagal interaction in man and conscious dog. Cir Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 16.Honig A. Peripheral arterial chemoreceptors and reflex control of sodium and water homeostasis. Am J Physiol Integ Comp Physiol. 1989;257(26):R1282–R1302. doi: 10.1152/ajpregu.1989.257.6.R1282. [DOI] [PubMed] [Google Scholar]
- 17.Day TA, Wilson RJ. A negative interaction between central and peripheral respiratory chemoreceptors may underlie sleep-induced respiratory instability: a novel hypothesis. Adv Exp Med Biol. 2008;605:447–451. doi: 10.1007/978-0-387-73693-8_78. [DOI] [PubMed] [Google Scholar]
- 18.Papacostas SS, Myrianthopolou P, Dietis A, Papathanasiou ES. Induction of central type sleep apnea by vagus nerve stimulation. Electomyogr Clin Neirophysiol. 2007;47(1):61–63. [PubMed] [Google Scholar]
- 19.Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and blood pressure regulation. Exp Physiol. 2008;93(6):715–724. doi: 10.1113/expphysiol.2007.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julius S, Majahalme S. The changing face of sympathetic overactivity in hypertension. Ann Med. 2000;32(5):365–370. doi: 10.3109/07853890008995939. [DOI] [PubMed] [Google Scholar]
- 21.Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams RB. Patterns of reactivity and stress. In: Matthews K, Weiss SM, Detre T, Dembroski TM, Falkner B, Manuck SB, Williams RB, editors. Handbook of Stress, Reactivity and Cardiovascular Disease. New York: Wiley & Sons; 1986. pp. 109–125. [Google Scholar]
- 23.Anderson DE, Chesney MA. Gender specific association of perceived stress and inhibited breathing pattern. Intl J Behav Med. 2002;9:216–227. doi: 10.1207/s15327558ijbm0903_04. [DOI] [PubMed] [Google Scholar]
- 24.Anderson DE, Bagrov AY, Austin J. Inhibited breathing decreases renal sodium excretion. Psychosomatic Medicine. 1995;57:373–380. doi: 10.1097/00006842-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Bagrov AY, Fedorova OV, Austin-Lane J, Anderson DE. Endogenous marinobufagenin-like immunoreactive factors and Na+, K+,- ATPase inhibition during voluntary hypoventilation. Hypertension. 1995;26:781–788. doi: 10.1161/01.hyp.26.5.781. [DOI] [PubMed] [Google Scholar]
- 26.Anderson DE. Behavioral Factors in Hypertension. Vol. 9. New York: Elsevier; 1987. Experimental behavioral hypertension in laboratory animals. Handbook of Hypertension; pp. 226–245. [Google Scholar]
- 27.Parati G, Izzo JL, Gavish B. Respiration and blood pressure. In: Izzo JL, Sica D, Black HR, editors. Hypertension Primer. 4. Baltimore: Lippincott, Williams & Wilkins; 2008. pp. 136–138. [Google Scholar]
- 28.Anderson DE, Scuteri A, Agalakova N, Parsons DJ, Bagrov AY. Racial differences in resting end tidal CO2 and sodium pump inhibitor. American Journal of Hypertens. 2001;14:761–767. doi: 10.1016/s0895-7061(01)02163-x. [DOI] [PubMed] [Google Scholar]