Abstract
Despite the increased prevalence of type 2 diabetes mellitus (T2DM) in the pediatric population, there is limited information about the relative effectiveness of treatment approaches. This article describes the rationale and design of a National Institutes of Health-sponsored multi-site, randomized, parallel group clinical trial designed to test the hypothesis that aggressive reduction in insulin resistance early in the course of T2DM is beneficial for prolongation of glycemic control, as well as improvement in associated abnormalities and risk factors. Specifically, the trial compares treatment with metformin with two alternate approaches, one pharmacologic (combining metformin treatment with rosiglitazone) and one combining metformin with an intensive lifestyle intervention program. The in Adolescents and Youth (TODAY) study recruits 800 patients over a 4-yr period and follows them for a minimum of 2 yr and maximum of 6 yr. Patients are 10–17 yr of age, within 2 yr of diagnosis of diabetes at the time of randomization, lack evidence of autoimmunity, and have sustained C-peptide secretion. The primary outcome is time to loss of glycemic control, defined as a hemoglobin A1c >8% for 6 consecutive months. Secondary outcomes include the effect of the alternative treatments on insulin secretion and resistance, body composition, nutrition, physical activity and fitness, cardiovascular risk monitoring, microvascular complications, quality of life, depression, eating pathology, and resource utilization. TODAY is the first large-scale, systematic study of treatment effectiveness for T2DM in youth. When successfully completed, this study will provide critical new information regarding the natural history of T2DM in youth, the benefits of initiating early aggressive treatment in these patients, and the efficacy of delivering an intensive and sustained lifestyle intervention to children with T2DM.
Keywords: adolescents, lifestyle change, metformin, obesity, thiazolidinedione, type 2 diabetes
Obesity has dramatically increased in prevalence world-wide among children and adolescents, accompanied by the appearance and increasing prevalence of type 2 diabetes mellitus (T2DM). Before the 1990s, it was rare for most pediatric centers to have patients with T2DM. However, by 1994, T2DM patients represented up to 16% of new cases of childhood diabetes in urban diabetes centers (1), and by 1999, depending on geographic location, T2DM accounted for 8–45% of new cases of childhood diabetes, with disproportionate prevalence among minority populations (2, 3). Indeed, over the past decade, the increase of T2DM among children and adolescents has been labeled an ‘epidemic’ (4).
Despite this increased prevalence and the potential short- and long-term risks associated with early onset of T2DM, the most effective approaches to treat youth with T2DMare not known and pediatric diabetologists have had to rely on treatment paradigms derived from research and experience in the care of adults. However, youth with T2DM differ in ways that may have an impact on potential treatment. First, as the environment and care of children with T2DM are highly influenced by parent attitudes, beliefs, and actions, treatment requires a family-centered approach not typical of studies of T2DM in adults. Moreover, the psychological and emotional turmoil and changing family interactions that normally occur during adolescence, as well as the adverse socio-economic conditions frequent in this population, may make achievement of stringent treatment goals especially difficult (5, 6). Furthermore, while lifestyle interventions directed at reversing markedly sedentary behaviors and deleterious dietary habits are widely thought to be an important part of therapy for T2DM, the ability to deliver such an intervention to a population of adolescents has never been demonstrated. In addition, the waning efficacy of oral hypoglycemic agents is a concern for youth, given the expected long duration of the condition. The young age at onset and increased risk for later cardiovascular complications heighten the urgency to not only achieve but also sustain optimal metabolic control of diabetes. Finally, the potential for differential efficacy and/or adverse effects of diabetes treatments in youth require specific assessment in this target population.
This article describes the rationale and design of a National Institutes of Health (NIH)-sponsored multi-site trial designed to test the hypothesis that an aggressive approach of reducing insulin resistance early in the course of T2DM is beneficial for prolongation of glycemic control, as well as improvement in associated abnormalities and risk factors. Specifically, the trial compares treatment with metformin, the most commonly used agent for the treatment of T2DM in youth (7, 8), with two alternate approaches providing additional reduction in insulin resistance, one pharmacologic (combining metformin treatment with rosiglitazone) and one combining metformin with an intensive lifestyle intervention program.
Research design and methods
Study design
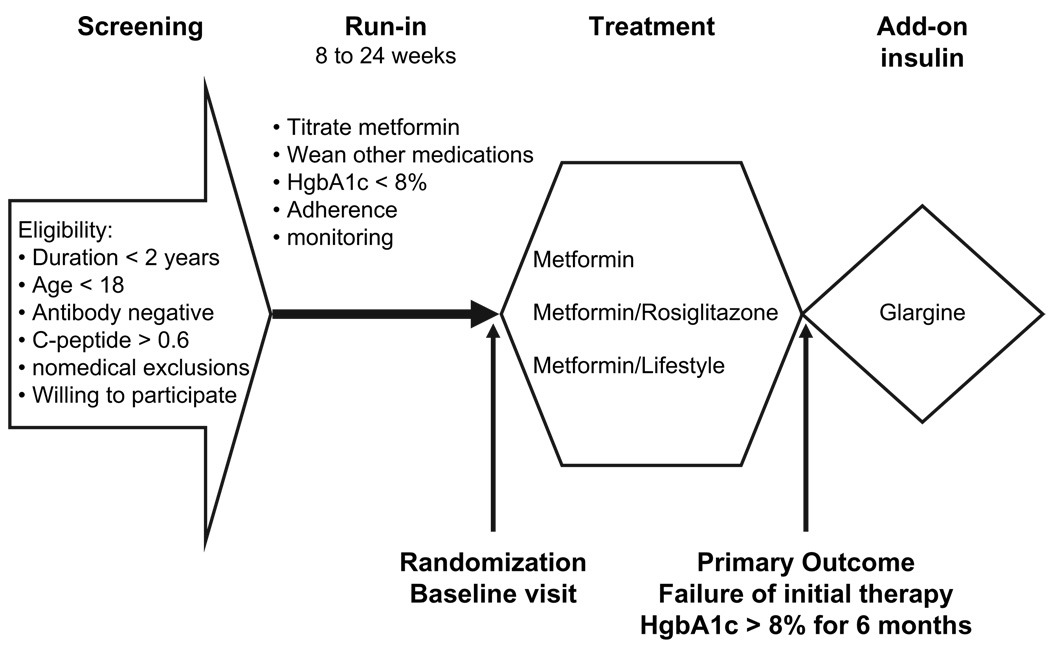
Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) is a randomized parallel-group trial consisting of a screening visit, a 2- to 6-month single-blind run-in period, and a treatment period of up to 5 yr (Fig. 1). Participants meeting eligibility criteria at the end of run-in are randomized 1:1:1 to (i) metformin alone, (ii) metformin plus rosiglitazone, or (iii) metformin plus an intensive lifestyle intervention called the TODAY Lifestyle Program (TLP). TODAY intends to recruit 800 patients over a 4-yr period and follow patients for a minimum of 2 yr and maximum of 6 yr.
Fig. 1.
Schematic of TODAY trial.
Primary outcome
The primary outcome of TODAY is the time to treatment failure, defined in one of two following ways: (i) all regularly scheduled hemoglobin A1c (HbA1c) values ≥8% over a 6-month period or (ii) the inability to wean from temporary insulin therapy within 3 months following acute metabolic decompensation.
Study population
Eligibility criteria include the following: (i) age10–17 yr inclusive; (ii) diagnosis of T2DM by standard laboratory criteria (9) for less than 2 yr by the time of randomization – for asymptomatic patients with a normal fasting glucose but elevated 2-h glucose during an oral glucose tolerance test (OGTT), HbA1c must be ≥6%, and subjects being treated with diabetes medication for whom diagnostic serum glucose documentation is not available are eligible if HbA1c was ≥8% at the time of diagnosis; (iii) body mass index (BMI) ≥85th percentile; (iv) fasting C-peptide at screening >0.6 ng/mL and absence of pancreatic autoimmunity (both GAD and ICA512); (v) a family member or adult closely involved in the daily activities of the child must consent to participate in the child’s treatment [family support person (FSP)] – the inclusion of a parent or caregiver has been shown to be important to the success of lifestyle change (10), particularly in ethnic minority groups (11), and in addition, parental weight change is associated with child weight change (12); and (vi) fluency in English or Spanish due to the intensive personal interactions required for the run-in and lifestyle intervention.
Exclusion criteria are any of the following: creatinine clearance < 70 mL/min; any hepatic transaminase > 2.5 the upper limit of normal; diabetic ketoacidosis at any time after diagnosis, except for a single episode related to a significant medical illness; use of inhaled glucocorticoids at dose above 1000 µg of daily fluticasone equivalent; oral glucocorticoids within the last 60 d or more than 20 d during the past year; use of medication(s) known to affect insulin sensitivity or secretion within the last 30 d, medication(s) known to cause weight gain within the last 30 d, anabolic steroids within the past 60 d, weight loss medication(s) within the last 30 d, or medication(s) known to affect the metabolism of study drug; participation in a formal weight loss program; participation in another interventional research study protocol in the past 30 d; abnormal reticulocyte count or HbA1c chromatogram indicating abnormal hemoglobin variants other than heterozygosity for S and C; genetic syndrome or disorder known to affect glucose; inability of either participant or family member to comprehend the lowest grade level at which lifestyle intervention materials are prepared; women who are pregnant, planning to become pregnant within 2 yr of enrollment, or who admit sexual activity without appropriate contraception; physical limitations preventing patient from being randomized to the lifestyle intervention; or other significant organ system illness or condition (including psychiatric or developmental disorder) that, in the opinion of the investigator, would prevent full participation.
In addition, patients are excluded from randomization if systolic blood pressure is ≥150 mmHg or diastolic blood pressure is ≥95 mmHg, despite appropriate medical therapy; total cholesterol >300 mg/dL, low density lipoprotein (LDL) > 190 mg/dL or triglycerides .800 mg/dL, despite appropriate medical therapy; hematocrit <30% or hemoglobin ,10 gm/dL, despite appropriate medical therapy; inability to demonstrate mastery of standard diabetes education (SDE) program during run-in; or an episode of diabetic ketoacidosis during run-in.
Treatment
Run-In
The run-in period has the following multiple goals: (i) provide uniform and high-quality SDE to all patients and their family members; (ii) titrate the dose of metformin (single-blind) as tolerated to a target dose of 1000 mg twice a day, with a minimum dose for randomization of 500 mg twice a day; (iii) discontinue all other diabetes medications; and (iv) document adherence to medication by pill count and study visit attendance. To proceed to randomization, patients must demonstrate 80% medication adherence for 6 wk, with no more than two missed study visits. They must also maintain an HbA1c ≤ 8% for a minimum of 2 months on metformin alone. Participants have a maximum of 6 months to complete all run-in goals prior to randomization. Standard diabetes education.
All eligible consenting participants receive SDE delivered by the TODAY certified diabetes educator. SDE was designed by TODAY investigators to provide participants and their family member with the knowledge, skills, and behaviors required for the successful management of T2DM and the ability to follow treatment guidelines in each of the treatment arms. Major topics include diabetes pathophysiology, medication action, nutrition and weight management, physical activity, diabetes self-care, and goal setting. SDE materials are provided in an easy-to-read, youth friendly, and culturally appropriate workbook format and delivered at a minimum of four sessions, each lasting 60–90 min. Both the youth and family member must demonstrate 80% mastery of the material on testing. After randomization, follow-up education is provided at each medical visit, based on the needs of the participants as they arise. Pharmacologic therapy.
Metformin is used in all treatment arms, and the standard treatment group receives metformin alone. The goal is to treat all participants with 1000 mg metformin twice a day, and participants are required to tolerate a minimum of 500 mg twice a day to be eligible for randomization. During run-in, metforminaive participants and participants treated with submaximal doses of metformin have metformin initiated and/or adjusted over 1–4 wk as tolerated. As all patients take two capsules (consisting of metformin and placebo or metformin and rosiglitazone) twice daily after randomization, patients also take two capsules (a combination of capsules containing metformin or placebo) twice daily during the run-in period and are masked to the metformin dose. The doses of insulin and/or other hypoglycemic agents are reduced with the goal of achieving HbA1c < 8% and no ketonuria for a minimum of 2 months on treatment with maximally tolerated doses of metformin alone.
Randomization
Both the investigators and the patients are masked to the pharmacologic treatment group. All patients receive two capsules twice a day containing an appropriate dose of metformin (provided by GlaxoSmithKline, Research Triangle Park, NC, USA) combined with either placebo or rosiglitazone (provided by GlaxoSmithKline) supplied by the Drug Distribution Center in a blister pack. All patients remain throughout the study on the maximum tolerated dose of metformin achieved during run-in. Patients randomized to the metformin plus rosiglitazone arm are started on a dose of 2 mg rosiglitazone twice a day, which is increased after 8 wk to 4 mg twice a day.
The TLP is a family-based, behavioral weight loss program with a sustained weight loss goal of 7–10% of initial body weight (13–16). The TLP is standardized but tailored to the developmental stage of the youth (17).
TLP is delivered by an interventionist referred to as the personal activity and nutrition leader (PAL) and consists of three phases of contact between: weekly inperson contact for the first 6 months, biweekly inperson visits alternating with phone contact for the next 6 months, and one monthly in-person contact and one monthly phone contact for the remainder of the participant’s involvement in TODAY (16, 18). Each TLP visit lasts 45–60 min and includes a combination of the PAL meeting separately and together with the youth and the youth’s FSP.
The key components of the TLP include psycho-education, along with dietary, physical activity, and behavior modifications. Concepts and skills previously shown to promote weight loss in youth and adapted for obese youth with T2DM are included in the psychoeducation component (14, 19). The dietary component (the traffic light eating plan) was adapted from The Traffic Light Diet (20, 21) and the Diabetes Food Guide Pyramid. Foods are divided into the colors of the traffic light; participants decrease the number of servings of red foods (foods that are high in fat and/or sugar) and choose healthy foods based on individual, family, and cultural preferences. Individualized calorie intake recommendations are also made to facilitate weight loss and typically range between 1200 and 1500 calories (22, 23). Once initial weight loss goals are achieved, the recommended caloric intake is gradually increased to allow for energy balance.
Two goals of 200 or 300 min per week of moderate-intensity physical activity are used depending on the initial fitness of the subject (24). Increased time spent in preferred physical activities and the gradual introduction of new forms of activity are encouraged, along with a decrease in the amount of time spent in sedentary activities (25) and increased involvement in lifestyle activities. Pedometers are used to help participants estimate and increase their lifestyle activities (24).
Self-monitoring, goal setting, behavioral skills training, and stimulus control have been used in previously successful pediatric weight loss interventions (26). PALs encourage FSPs to actively model healthy eating and physical activity and to restructure the home environment. Positive parenting or behavior change techniques are taught to FSPs, such as the use of praise and a family-based reward system (14). Initially, PALs model the use of contingent reinforcement by directly rewarding achievement of goals by the youth. Participants then transition to a system in which points received for achieving behavior change goals can be exchanged for family-based rewards.
Management of comorbid conditions
Treatment of dyslipidemia, hypertension, and microalbuminuria follow specified treatment algorithms (see www.todaystudy.org). An independent medical consultant reviews lipid, blood pressure, and urine results to assure that treatment goals are met according to the treatment algorithms and provides additional treatment recommendations for individual patients.
Treatment with insulin
When the patient reaches the defined ‘primary treatment failure’ (see Primary Outcomes below), treatment with insulin is instituted as add-on therapy, in combination with the subject’s assigned oral agent(s). In the metformin plus rosiglitazone group, the rosiglitazone dose is lowered to 2 mg twice daily by the central pharmacy. The patients and clinicians remain masked to the original treatment assignment but are unmasked to HbA1c. Initial insulin treatment is 0.2 U/kg glargine insulin each evening, which is increased up to 1.0 U/kg/d (maximum 100 U) until fasting blood glucose values between 70 and 150 mg/dL are achieved. If treatment goals are not achieved with glargine insulin alone, other insulins are used at the clinician’s discretion.
Patients may require temporary use of insulin due to hospitalization, intercurrent illness, or conditions leading to metabolic decompensation. In these circumstances, any type or dose of insulin can be used at the discretion of the treatment team. However, acute insulin therapy is weaned within 2–4 wk, and the inability to discontinue insulin within 3 months without decompensation (ketonuria and symptomatic hyperglycemia) is also defined as a ‘primary treatment failure’.
Schedule of medical care and research visits (Table 1)
Table 1.
Outcome data collection
| Eligibility | Baseline | Yr 1 | |||
|---|---|---|---|---|---|
| Measurement/assessment | Initial screen | Run-in | X = every 2 months | Post-Yr 1 follow-up | |
| Primary outcome | |||||
| Hemoglobin A1c | X | X | X | X | Quarterly |
| Primary treatment failure | |||||
| End of study | |||||
| Secondary outcomes | |||||
| Insulin sensitivity and secretion | X | 6, 12 | Annually | ||
| Primary treatment failure | |||||
| End of study | |||||
| Serum creatinine | X | X | 12 | Annually | |
| Primary treatment failure | |||||
| End of study | |||||
| Liver function tests | X | X | X | Quarterly | |
| Primary treatment failure | |||||
| End of study | |||||
| Hemoglobin and hematocrit | X | X | 2, 6, 12 | Annually | |
| Primary treatment failure | |||||
| End of study | |||||
| Anthropometrics | X | 6 | 24 months | ||
| Primary treatment failure | |||||
| End of study | |||||
| Dual-energy X-ray | X | 6 | 24 months | ||
| absorptiometry | Primary treatment failure | ||||
| End of study | |||||
| Lipids | X | X | 6, 12 | Annually | |
| Primary treatment failure | |||||
| End of study | |||||
| Fitness, nutrition, and activity | X | 6 | 24 months | ||
| Primary treatment failure | |||||
| End of study | |||||
| Psychosocial and quality of Life | X | 6 | 24 months | ||
| Primary treatment failure | |||||
| End of study | |||||
| Cardiovascular risk factors | X | 6, 12 | Annually | ||
| Primary treatment failure | |||||
| End of study | |||||
| Neuropathy (Michigan Neuropathy Screening Instrument) | X | 12 | Annually | ||
| Microalbumin | Primary treatment failure | ||||
| End of study | |||||
| Adverse events | X | X | X | Quarterly | |
| Primary treatment failure | |||||
| End of study | |||||
| Resource utilization costs | X | X | Quarterly | ||
| Primary treatment failure | |||||
| End of study | |||||
| Blood and urine for storage | X | 6, 12 | Annually | ||
| Primary treatment failure | |||||
| End of study | |||||
Participants have a medical visit every 2 months in the first year and every 3 months thereafter. Height, weight, blood pressure, Tanner stage, and degree of acanthosis nigricans are recorded. HbA1c and safety monitoring laboratory samples are obtained, and details of all adverse events are documented. Glucose meter memories are downloaded, and adherence estimated with pill counts. More extensive physiologic data are obtained at research visits, termed SuperVisits, which occur at randomization, 6 months, annually, ‘primary treatment failure’, and the end of study (Table 1). For SuperVisits, patients are studied following a 10- to 14-h fast and are asked to abstain from moderate to vigorous physical activity for 48 h.
Secondary outcomes
Insulin sensitivity and secretion
At SuperVisits, fasting laboratory samples are obtained followed by a standard 75-g OGTT, with glucose, insulin, and c-peptide samples collected every 30 min for 2 h. Insulin sensitivity and secretion are determined based on fasting glucose, insulin, C-peptide, and proinsulin levels, OGTT, homeostasis model assessment (HOMA), and quantitative insulin-sensitivity check index (QUICKI). HOMA-R (resistance) and B (secretion) are calculated using a computer-based model solution (27, 28). QUICKI is calculated as 1/(log[I0] 1 log [G0]) (29).
Body composition
Overweight status is assessed by standardized anthropometric measurements (height, weight, and BMI), as well as waist circumference and abdominal height measured laterally with the patient supine (30). Percent body fat is measured by dual-energy X-ray absorptiometry (DXA). Biological parent height and weight are collected at randomization. FSP weight and height are collected by direct measurement or self-report at randomization and at 2 yr.
Nutrition
Routine dietary habits and nutrient intake are evaluated by use of a food frequency questionnaire (FFQ) (31) that is interview administered and captures the last week of dietary intake.
Physical activity and physical fitness
Routine physical activity is assessed by use of the previous day physical activity recall (PDPAR), a questionnaire that assesses the previous day’s activities and their relative intensities for the after school hours (32). However, as lower intensity activities are harder to recall than higher intensity activities, the Computer Science Application (CSA) accelerometer is used to assess patterns of physical activity (33). Participants wear the accelerometer for 7 d and complete a diary of activities on the days that the monitor is worn.
The most popular methodologies to predict maximal oxygen uptake (VO2 max) in children utilize cycle ergometry to determine the physical work capacity (PWC) (34). In TODAY, participants undergo PWC 170 testing at SuperVisits. This test effectively predicts VO2 max in obese children and involves obtaining heart rates at three submaximal workloads, plotting these heart rates against the workload, extrapolating to determine the workload at a given heart rate or at maximal heart rate, and then converting that maximal workload to oxygen uptake.
Cardiovascular risk factors
Fasting blood samples are obtained for measurement of free fatty acids, lipoprotein subclass levels, average LDL particle density, total apolipoprotein B (ApoB), fibrinogen, highly sensitive c-reactive protein, plasminogen activator inhibitor (PAI)-1, homocysteine, vitamin B12, and interleukin-6.
Blood glucose monitoring
Participants are instructed to obtain blood glucose meter readings two times per day – in the morning on awakening (fasting) and at one additional time (e.g., before dinner, lunch, or 2 h after dinner) each day. At all clinic visits, meters are downloaded, and the number of tests performed per day, as well as the 14-d and 30-d average glucose levels, are recorded.
Microvascular complications
Spot urine measurement of microalbumin/creatinine ratio is obtained at randomization and annual visits. Abnormal values (>30 mg/mg creatinine) are confirmed with two additional spot urine samples within 3 months; diagnosis of microalbuminuria is made when there are two out of three positive tests. Estimated creatinine clearance is calculated at randomization and annual visits. The presence of peripheral neuropathy is evaluated using the Michigan Neuropathy Screening Instrument (35) annually. Identification and monitoring for retinopathy are not undertaken as a primary component of TODAY. It is expected that patients will receive retinopathy screening according to American Diabetes Association clinical practice guidelines and reports of retinal screenings are recorded.
Quality of life and psychological measures
Emotional status and quality of life are assessed by the Children’s Depression Inventory (up to age 16 yr) (36–40), the Beck Depression Inventory (BDI) (beginning at age 16 yr) (41–44), the Eating Disorders Examination Questionnaire (EDEQ), the Questionnaire on Eating and Weight Pattern – Revised (QEWP-R) (if indicated by the score on the EDEQ) (45). The Pediatric Quality of Life (PedsQL) (46) is used to evaluate health-related quality of life. The biological parent (where available) completes the BDI, EDEQ, and QEWP-R (only if indicated by the score on the EDEQ) at randomization. The FSP completes the BDI, EDEQ, QEWP-R (if indicated by the score on the EDEQ), PedsQL (adult proxy) and the Child Health Questionnaire (P28) at randomization and 2 yr.
Resource utilization and costs
Resource utilization for each of the treatment arms is assessed throughout the trial and considers the intensity of services used in providing care and assuring adherence to each treatment regimen. The frequency of contact with physicians, nurse educators, psychological support staff, dietitians, and the use of drugs, equipment, and supplies are captured through study forms. Information on the time spent by health professionals in treatment provision is obtained through surveys completed periodically by the providers. School absenteeism, work absenteeism (in children and adults), and the time spent in treatment activities by children and caregivers are measured utilizing previously validated questions, as well as academic and employment participation rates and progress. Measurement of quality-adjusted life years is obtained through the Health Utilities Index-2 (47), which provides a preference- based measure of quality of life.
Safety parameters
Data are collected regarding abnormalities in laboratory tests (hemoglobin/hematocrit, liver function tests, and calculated creatinine clearance), episodes of severe hypoglycemia, and incidence of side effects (e.g., gastrointestinal complaints, edema, and weight gain).
Laboratory methods
Samples are processed following standardized procedures and shipped on dry ice to the Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle, which serves as the Central Biochemistry Laboratory for the study. Details of the assays used are provided at www.todaystudy.org.
Statistical methods
The principal analyses of primary and secondary outcomes employ the ’intent-to-treat’ approach (48). The intent-to-treat analyses include all randomized patients, with all participants included in their randomly assigned treatment group; treatment group assignment is not altered based on the adherence to the assigned treatment regimen. All statistical tests are two sided, with the overall significance level of the primary outcome α = 0.05. The primary outcome analysis of time to treatment failure uses life-table methodology. Separate product-limit life-table estimated cumulative incidence curves are calculated for each treatment group, and the groups are compared using a log-rank test (49).
The study is powered to allow all three possible comparisons between the treatment groups while maintaining the overall significance level at 0.05. If 10% of the metformin group has an HbA1c above the threshold, 250 participants per arm provides at least 90% power to detect a 50% reduction in the treatment failure rates in at least one combination therapy group (i.e., 5% with HbA1c above threshold during a 6-month period). If 20% of the metformin group has an HbA1c above the threshold, 250 participants per treatment arm provides at least 90% power to detect a 40% reduction in hazard rates in at least one of the combination therapy groups (i.e., 12% failing during a 6-month period). Sample size is adjusted for a 10% lost to dropout rate in each 6-month period.
Structure of the study group
The TODAY study group operates under the Studies to Treat or Prevent Pediatric Type 2 Diabetes (STOPP-T2D) cooperative agreement mechanism funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The group is composed of 13 clinical centers, a coordinating center, the project office, and central cores and laboratories. The protocol has been approved by the Institutional Review Board of each participating center. All participants provide written informed consent/assent according to the policies of each local center. A Data Safety Monitoring Board acts as an independent arbiter of study progress and patient safety.
Discussion
There have been no previous large-scale randomized intervention trials examining T2DM in youth. Although rising in prevalence, T2DM remains a relatively uncommon disorder in the pediatric population, and no single center has been able to identify sufficient eligible patients to complete meaningful studies independently. Therefore, the identification and funding of a consortium of centers to develop and undertake these studies represents a significant advance and a potential model for the development of consortia for the examination of other pediatric disorders.
In developing this protocol, the study group was confronted with a large number of unanswered questions. After wide-ranging discussion, investigators agreed that the most pressing issues related to the recognition that these young people can be expected to have a long duration of disease and that the insulin resistance characteristic of puberty provides an additional, although potentially transient, burden. In particular, investigators were interested in determining whether more aggressive therapy aimed at decreasing insulin resistance in adolescents would improve both glycemia and associated abnormalities relative to standard therapy and leads to improved metabolic state when the insulin resistance of puberty has waned. Therefore, TODAY was designed to focus on these primary issues while taking into account the psychosocial and familial characteristics unique to adolescents with T2DM.
Choice of primary outcome
The primary outcome of this study is defined in terms of HbA1c because this measure provides the best index of glycemic control and because previous large-scale studies have established it as a surrogate marker of long-term diabetic complications (50). Given the results of the Diabetes Control and Complications Trial (51), it is no longer acceptable to maintain fixed treatment regimens in the face of chronic elevations in HbA1c levels, and current treatment guidelines in adults with T2DM mandate add-on therapy with insulin or another oral agent if target HbA1c levels are not being met. Consequently, the primary outcome was chosen as the time to treatment failure (i.e., the need for add-on insulin therapy) rather than differences in HbA1c levels between the treatment groups over time. While the treatment target is an HbA1c of 6% or below, it was the consensus of the Study Group that a sustained HbA1c value of 8.0% or greater was a reasonable definition of treatment failure in this population. Acute management of patients with metabolic decompensation utilizes insulin treatment. However, if insulin treatment cannot be withdrawn after 3 months, this is also considered a treatment failure.
Choice of treatment arms
Many youth with T2DM present with marked hyperglycemia, with or without ketoacidosis, and require insulin to correct metabolic status. Nevertheless, following an initial period of insulin therapy, most of these youngsters, as well as those who are diagnosed at an earlier stage of T2DM, are able to achieve satisfactory glycemic control when treated with metformin alone. Metformin remains the most common monotherapy for T2DM in youth in North America (7, 8). Furthermore, metformin was the only oral hypoglycemic agent approved for use in children at the time of study design and has the added advantage of being associated with either weight loss or reduced weight gain in comparison to insulin, sulfonylureas or thiazolidinediones (TZDs). Thus, monotherapy with metformin was chosen as the ‘standard treatment’ against which other combination therapies would be compared in this trial.
Both metformin and TZDs improve glycemic control but by different mechanisms of action. Therefore, addition of a TZD to metformin offered the potential for additional reduction in insulin resistance relative to standard therapy. A further theoretical advantage in using a TZD as a therapeutic agent in T2DM is the potential that these agents will preserve pancreatic insulin secretion [Diabetes Prevention Program (DPP) and The Troglitazone in Prevention of Diabetes Study] (52–55). Preservation of beta-cell function is a critical aspect of long-term diabetes control, as retention of the ability to compensate for insulin resistance by producing adequate amounts of insulin is important for prevention of hyperglycemia.
Other combinations of drugs were considered but not selected by the Study Group because the estimated number of patients available for recruitment would not support a trial with more than three arms and because there was strong support for inclusion of an intensive lifestyle intervention group. Virtually all youth with T2DM are overweight as a result of the interplay between genetic factors, excess energy intake from high fat, high calorie, low nutrient diets, and sedentary behaviors with too much time spent watching TV and playing video games (56–59). Adding a third treatment group that combined an intensive lifestyle intervention with metformin was especially attractive because it provided a non-pharmacologic approach to improving insulin sensitivity, physical fitness, and body composition. Moreover, in a number of studies in adults, weight loss associated with improvements in eating behavior, diet, and physical activity has been shown to result in significant reductions in fasting plasma glucose and insulin levels, hepatic glucose output, peripheral insulin resistance, hypertension, and dyslipidemia (60–64). Three uncontrolled trials in adults with T2DM treated with oral agents have shown the benefit of weight loss associated with lifestyle modification on reducing mortality (63, 65, 66). As the epidemic of T2DM in children and youth is relatively recent, there is little controlled evidence regarding the use of lifestyle modification to improve insulin sensitivity and glycemic control, to induce weight loss, or affect other outcome measures, such as dyslipidemia and hypertension, in pediatric patients with T2DM.
Secondary outcomes
In designing the study, the TODAY Study Group faced a dilemma. On one hand, the study provides a unique opportunity to learn more about the pathophysiology, natural history, and psychosocial impact of T2DM in youth. On the other hand, the burdens of completing secondary outcome assessments could adversely affect patient recruitment and retention. The measures selected provide insight into the mechanism by which the treatment regimens affect durable glycemic control (e.g., effects on insulin resistance, sensitivity, diet, and physical fitness) and provide information concerning the differential risks and benefits of the three treatment arms (e.g., studies of microvascular complications and cardiovascular risk).
Insulin resistance
The efficacy and durability of the successful treatment of T2DM in adults are determined to a great extent by the ability to ameliorate insulin resistance. Yet, little is currently known about how successful drug and lifestyle approaches will be in improving insulin sensitivity in children and youth. In particular, significant questions remain about the impact of pubertal progression on insulin resistance and how changes in insulin sensitivity affect beta-cell function in pediatric patients with T2DM. Therefore, an important component of TODAY is to determine (i) the influence of baseline insulin sensitivity and secretion on the response to therapy and (ii) the effect of each therapy on the progression of changes in insulin sensitivity and secretion.
The glucose clamp technique and the frequently sampled intravenous glucose tolerance test with minimal model analysis are considered the ‘gold standard’ for determination of insulin sensitivity and secretion. However, these are labor intensive, time-consuming, and costly procedures that are not easily implemented in large clinical trials. A number of simpler methods have been developed that are preferable in the context of large clinical trials such as TODAY. The OGTT, HOMA, QUICKI are used to compare insulin secretion and sensitivity among the three treatment groups. Use of these multiple measures helps compensate for not using the ‘gold standard’ measures as listed above. Values of glucose and insulin/C-peptide derived from the OGTT, when combined with anthropometric parameters, can predict insulin sensitivity and secretion indices derived from clamp measurements with reasonable accuracy (67–69). The first-phase insulin secretion is estimated with the insulinogenic index (28). The indices, based on fasting measurement of glucose and glucose-regulating hormones such as insulin (including C-peptide) and proinsulin, have also been proven to closely correlate with corresponding clamp-derived indices of insulin sensitivity and secretion in diverse pediatric populations (28, 29, 69–71).
Body composition
The development of insulin resistance during puberty is most closely correlated to fat mass, independent of insulin, sex steroids, leptin, or insulin-like growth factor-1 (IGF-1) status (72). Weight loss in overweight adolescents improves both insulin sensitivity (73) and blood pressure (74). However, there are scarce data describing the effects of weight loss on these parameters in children or adolescents with diabetes. Therefore, TODAY will investigate how changes in adiposity relate to glycemic control, insulin sensitivity, cardiovascular risk factors, and blood pressure in children and adolescents with T2DM.
A cross-calibration study of different body composition techniques versus DXA in children has shown that skinfold thickness measurements, bioelectrical impedance, and various combinations of anthropometric measures are highly inaccurate when compared with DXA (75). DXA is also easy to perform and provides minimal radiation exposure. Therefore, DXA was selected as the measure of adiposity. Use of magnetic resonance imaging to assess differential subcutaneous and visceral fat partitioning was considered but felt to be too burdensome and expensive for use in this large cohort.
Nutrition/diet
Diet change has been correlated with a decrease in diabetes risk, as well as weight loss, in a number of prevention and intervention studies, including the DPP (76). Therefore, evaluation of baseline diet characteristics, as well as the effect of treatment interventions on dietary change in participants, will allow us to evaluate the impact of the lifestyle intervention on eating behaviors within this treatment group and in comparison with the other two treatment groups. In addition, dietary assessment over the duration of the trial provides insight into which components of the intervention lead to the most significant and durable change in lifestyle.
A number of tools can be used to assess dietary intake, a variety of which were considered for this trial. Although there are few validity studies of food frequency approaches in adolescent populations, the available data suggest that validity and reproducibility are comparable to results obtained in adults (77). Recently, the Block Kid’s Questionnaire was validated in low-income African-American children. This instrument has been further modified to incorporate common food choices among ethnically and regionally diverse youth aged 10–19 yr participating in another large childhood diabetes study, SEARCH [Search for Diabetes in Youth, Centers for Disease Control/NIH], based on previously validated work with FFQ methodology in ethnically diverse populations (31).
Fitness/activity
Physical activity is defined as any bodily movement produced by skeletal muscles that results in energy expenditure. Physical fitness, on the other hand, characterizes the physiologic state of an individual, including aerobic power, muscular endurance, muscular strength, body composition, and/or flexibility. Both physical activity and fitness are independently related to changes in insulin sensitivity and improvement in glycemic control and other abnormalities associated with T2DM. Those who are more active and expend more energy have higher fitness levels. However, there is only a moderate relationship between physical activity and physical fitness (78). Therefore, the effect of the treatment interventions on both of these measures is compared.
Physical activity is comprised of activities that span a spectrum of intensity levels and can be assessed using activity recall questionnaires and/or objective measures of activity, such as pedometers and accelerometers. The most popular activity questionnaire currently used with youth is the PDPAR (32). However, the PDPAR analyzes the activity level over a short time frame and may not reflect activity levels that vary with seasons or as a result of an acute illness or time commitment (79). The activity questionnaire may also not accurately quantify changes in lower intensity lifestyle activities. However, in combination with accelerometer data, it offers a valuable comparison of differential activity levels across treatment arms.
The accelerometer allows the collection and storage of daily patterns of physical activity and is a more complex instrument than the pedometer. In adults, correlation coefficients ranging from r = 0.66 to r = 0.89 between CSA counts and metabolic measures (33) have been obtained. In 7- to 15-yr-old boys and girls, the CSA was validated against heart rate telemetry, with correlation coefficients between the two ranging from r = 0.50 to 0.74 (80). Comparisons with oxygen consumption during treadmill exercise and self-selected speed on a track found that the CSA was highly related to both and was highly sensitive to change in speed but not changes in grade (81). As the CSA has been found to successfully detect bouts of moderate-intensity physical activity such as brisk walking (82), this monitor was chosen for TODAY where moderate-intensity activity is the goal.
Cardiorespiratory fitness is defined as the ability to absorb, transport, and use oxygen. Higher VO2 max values indicate better cardiorespiratory fitness. However, measurement of VO2 max is a challenging task in children (83, 84). Motivating youth to exercise maximally is difficult, they may have difficulty obtaining VO2 max, the tests require expensive metabolic measuring systems, and take approximately an hour to complete.
In contrast, the PWC method of predicting VO2 max has several advantages (34). First, the PWC equipment and space needs are modest. Second, the PWC can be administered in 12–15 min and requires only the measurement of heart rate. Third, the PWC is ideal to use in a test–retest protocol because learning, practice, or training have little effect on results.
Cardiovascular markers
The development of cardiovascular risk is an important consideration in comparing the efficacy and long-term implications for the treatment interventions in TODAY. To address this, the differential effects of the three treatment arms on both traditional and non-traditional markers are compared.
Obesity, T2DM, hypertension, and dyslipidemia are associated with a significantly increased risk of cardiovascular morbidity and mortality in adults, and insulin resistance has been considered the underlying pathologic mechanism (85–87). However, these traditional risk factors account for only approximately 50% of the cardiovascular risk associated with hyper-insulinemia and insulin resistance, an observation that has led to the identification of a number of ‘non-traditional’ risk factors (88), including alterations in hemostasis and markers of acute and chronic systemic inflammation.
Very little is known about the relationship between obesity, insulin resistance, T2DM, and non-traditional cardiovascular risk factors in children. Several large epidemiologic studies in youth have shown an association between obesity, traditional risk factors (e.g., hypertension and dyslipidemia), and cardiovascular disease (89, 90). However, less is known about non-traditional risk factors. In small series, obese children and adolescents have been found to have increased levels of fibrinogen, PAI-1, and D-dimer, as well as abnormalities in factor VIIc, von Willebrand factor, PAI-1, fibrinogen, and tissue plasminogen activator (91–93). Following weight loss interventions, decreased levels of PAI-1 and interleukin-6 (IL-6) have been demonstrated (92–94), suggesting that lifestyle interventions may be able to alter cardiovascular risk in young patients.
Microvascular complications associated with diabetes produce significant burdens for the individual patient and are responsible for considerable healthcare costs. Microvascular complications are more common among children with T2DM at the time of presentation than among those with type 1 diabetes mellitus (95, 96). There is evidence, although limited, which suggests that progression rates of microvascular complications are also greater in youth with T2DM. Therefore, a comparison of the effect of the treatment interventions on the prevention and slowing of rates of development of microvascular complications associated with T2DM is an important secondary outcome of TODAY and could significantly modify the interpretation of the primary outcome results.
Psychosocial outcomes
The psychological, emotional, and social status of a patient interacts with his or her chronic illness in complex ways. Difficulties in the family social or psychological structure can hasten onset of lifestyle-related disorders, such as T2DM, and then further interfere with treatment. Onset of a chronic illness, particularly one that requires significant personal change, can adversely affect many aspects of a patient’s and family’s emotional wellbeing. This trial examines whether psychological characteristics of patients and families influence treatment outcome and whether the interventions have an effect on psychological problems and quality of life. The following questions are of interest: (i) whether the participant’s and/or parents’ psychological characteristics at baseline affect the success of the three treatment arms, (ii) whether the treatment assignment has an effect on psychological outcomes related to the diagnosis of diabetes, and (iii) whether changes in the family FSP’s psychological status during the intervention affect outcomes.
Cost
Rising healthcare costs and limited healthcare resources have increased the focus placed on the economic aspects of health care. Analyses of resource utilization and costs contribute to decisions made by health service providers and policy makers regarding diabetes care. By incorporating measures of resource utilization, cost, and an intervention’s effectiveness, cost-effectiveness analysis has become an important tool in decision making about the use of different treatments for the same condition. Several large trials of diabetes care interventions have demonstrated that enhanced treatment of diabetes improves health at an acceptable cost (97–99).
Therefore, an important secondary outcome of TODAY is an investigation of the resources, related costs, and cost-effectiveness of the three treatment arms. The cost-effectiveness analysis considers the resources used and associated costs for each treatment arm throughout the time of the trial. The time frame of the analysis is short term. The main analysis is based on the primary glycemia outcome in the trial and, thus, examines the costs of each treatment relative to the glycemia benefits obtained. By incorporating costs incurred by participants and caregivers, the analysis adopts a societal perspective, following the accepted standard for such studies (100, 101).
Conclusions
TODAY is the first large-scale, systematic study of treatment effectiveness for T2DM in youth, an emerging disease with significant long-term healthcare and social implications for this population. When successfully completed, TODAY will provide critically needed new information regarding broad questions of treatment approach in youth T2DM, particularly regarding the benefits of initiating early aggressive treatment in these patients. Furthermore, TODAY will address the efficacy and feasibility of adapting intensive and sustained lifestyle interventions previously shown to be successful in weight loss programs to the needs of children with T2DM.
At the same time, TODAY will capitalize on the recruitment of this cohort of patients to examine in relative depth the pathophysiology of patients with recently diagnosed T2DM, the natural history of early stages of the disorder, the effect of alternative treatment interventions on T2DM physiology, and the pathophysiology of treatment failure. This information promises to provide an improved foundation for informed clinical management of children with T2DM and has the potential to allow analysis of patient characteristics, including both biologic and behavioral/social, that determine differential benefit of different treatment approaches. Finally, an extensive parallel analysis of cost of the intervention will provide policy makers with critical information regarding the efficacy and cost-effectiveness of intensive medical and lifestyle interventions in these young patients.
TODAY was designed as an efficacy trial, and the Study Group recognizes that clinicians do not generally have the means available to study sites for delivery of the labor- and resource-intensive interventions described. However, it is hoped that successful proof of principle by TODAY, along with the analysis of secondary outcomes that may allow delineation of which components of the intervention were most critical, will provide the basis for future effectiveness studies examining the translation of these findings into routine clinical practice.
Acknowledgements
This work was completed with funding from NIDDK/NIH grant numbers U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254 and from the National Center for Research Resources General Clinical Research Centers Program grant numbers M01-RR00036 (Washington University School of Medicine), M01-RR00043-45 (Children’s Hospital Los Angeles), M01-RR00069 (University of Colorado Health Sciences Center), M01-RR00084 (Children’s Hospital of Pittsburgh), M01-RR01066 (Massachusetts General Hospital), M01-RR00125 (Yale University), and M01-RR14467 (University of Oklahoma Health Sciences Center).
Footnotes
The following individuals and institutions constitute the TODAY Study Group (the symbol ‘*’ indicates principal investigator or director):
Clinical centers
Baylor College of Medicine: M. Haymond,* B. Anderson, P. Barrera, P. Brosnan, C. Bush, T. Green, H. Holden, G. Jeha, M. Jones, S. McGirk, S. McKay, D. Miller, B. Schreiner, and M. Zarate.
Case Western Reserve University: W. Dahms,* T. Casey, L. Cuttler, D. Drotar, E. Frieson, C. Levers-Landis, P.McGuigan, M. Palmert, S. Sundararajan, and D. Witherspoon.
Children’s Hospital Los Angeles: M. Geffner,* N. Chang, D. Dreimane, S. Estrada, J. Fabian, M. Halvorson, B. Hollen, F. Kaufman (Study Chair), C. Munoz, A. Ward, and P. Yasuda.
Children’s Hospital of Philadelphia: L. Katz,* R. Allegretto, C. Carchidi, J. Kaplan, C. Lassiter, S. Magge, S. Sababu, B. Schwartzman, and S. Willi.
Children’s Hospital of Pittsburgh: S. Arslanian,* F. Bacha, S. Foster, T. Hannon, K. Kolenc, A. Kriska, I. Libman, M. Marcus, D. Rofey, P. Scanlon, T. Songer, E. Venditti, and V. Weisbrod.
Columbia University Medical Center: R. Goland,* J. Belle, R. Cain, P. Kringas, N. Leibel, D. Ng, M. Ovalles, K. Robbins, D. Seidman, and L. Siegel-Czarkowski.
Massachusetts General Hospital/Joslin Diabetes Center: D. Nathan,* L. Laffel,* A. Angelescou, L. Bissett, C. Ciccarelli, K. Corrales-Yauckoes, L. Delahanty, A. Goebel-Fabbri, V. Goldman, L. Higgins, K. Hood, M. Larkin, M. Lee, L. Levitsky, M. Malloy, R. McEachern, K. Milaszewski, D. Norman, B. Nwosu, L. Orkin, S. Park-Bennett, D. Richards, A. Rodriguez-Ventura, M. Sheehy, L. Soyka, and B. Steiner.
State University of New York Upstate Medical University: R. Weinstock,* D. Bowerman, K. Bratt, S. Carusone, S. Doolittle, K. Duncan, R. Franklin, J. Hartsig, R. Izquierdo, J. Kearns, S. Meyer, R. Saletsky, and P. Trief, PhD.
University of Colorado Health Sciences Center: P. Zeitler* (Steering Committee Chair), K. Bellon, C. Cain, N. Celona-Jacobs, L. Gaskell, J. Glazner, D. Hanze, J. Higgins, F. Hoe, G. Klingensmith, T. McCann, K. Nadeau, B. Van Dorsten, and N. Walders.
University of Oklahoma Health Sciences Center: K. Copeland,* R. Brown, J. Chadwick, C. Macha, A. Nordyke, D. Olson, T. Poulsen, L. Pratt, J. Preske, J. Schanuel, and S. Sternlof.
University of Texas Health Science Center at San Antonio: D. Hale,* N. Amodei, R. Favela-Prezas, D. Gonzalez, S. Haffner, C. Hernandez, R. Lozano, J. Lynch, S. Rivera, M. Rodriguez, G. Rupert, and A. Wauters.
Washington University School of Medicine/Saint Louis University Medical Center: N. White,* S. Tollefsen,* A. Arbel&áez, S. Carnes, D. Dempsher, D. Flomo, M. Harris, T. Jones, V. Kociela, M. Sadler, T. Whelan, and B. Wolff.
Yale University: S. Caprio,* E. Estrada, M. Grey, C. Guandalini, S. Lavietes, P. Rose, A. Syme, and W. Tamborlane.
Coordinating center
George Washington University Biostatistics Center: K. Hirst,* L. Coombs, S. Drilea, S. Edelstein, N. Grover, A. Lau, C. Long, and L. Pyle.
Project office
NIDDK: B. Linder.*
Central units
Central Blood Laboratory (Northwest Lipid Research Laboratories): S. Marcovina* and G. Strylewicz.
DEXA Reading Center (University of California at San Francisco): J. Shepherd* and M. Sherman.
Diet Assessment Center (University of South Carolina): E. Mayer-Davis.*
Drug Distribution Center (Eminent Services): P. Thadikonda.*
Lifestyle Program Core (Washington University): D. Wilfley,* K. Franklin, D. O’Brien, J. Patterson, T. Tibbs, D. Van Buren, and R. Welch.
Others
State University of New York at Buffalo: L. Epstein.
Centers for Disease Control: P. Zhang.
Writing group
P. Zeitler, L. Epstein, M. Grey, K. Hirst, F. Kaufman, W. Tamborlane, and D. Wilfley.
References
- 1.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin dependent diabetes mellitus among adolescents. J Pediatr. 1996;128:608–615. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Pettitt DJ, Jones KL, Arslanian SA. Type 2 diabetes mellitus in minority children and adolescents. An emerging problem. Endocrinol Metab Clin North Am. 1999;28:709–729. doi: 10.1016/s0889-8529(05)70098-0. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:1–9. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 5.Pinhas-Hamiel O, Standiford D, Hamiel D, Dolan LM, Cohen RM, Zeitler P. The type 2 family: a setting for development and treatment of adolescent type 2 diabetes. Arch Pediatr Adolesc Med. 1999;153:1063–1067. doi: 10.1001/archpedi.153.10.1063. [DOI] [PubMed] [Google Scholar]
- 6.Pinhas-Hamiel O, Zeitler P. Barriers to the treatment of adolescent type 2 diabetes – a survey of provider perceptions. Pediatr Diabetes. 2003;4:24–28. doi: 10.1034/j.1399-5448.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2002;25:89–94. doi: 10.2337/diacare.25.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Silverstein JH, Rosenbloom AL. Treatment of type 2 diabetes mellitus in children and adolescents. J Pediatr Endocrinol Metab. 2000;13:1403–1409. doi: 10.1515/jpem-2000-s614. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Diagnosis and classification of diabetes. Diabetes Care. 2005;28:S37–S42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 10.Epstein LH, Valoski A, Wing RR, Mccurley J. Ten-year follow-up of behavioral family-based treatment for obese children. JAMA. 1990;264:2519–2523. [PubMed] [Google Scholar]
- 11.Dounchis JZ, Hayden HA, Wilfley DE. Obesity, body image, and eating disorders in ethnically diverse children and adolescents. In: Thompson JK, Smolak L, editors. Body Image, Eating Disorders, and Obesity in Youth: Assessment, Prevention, and Treatment. Washington, D.C: American Psychological Association; 2001. pp. 76–98. [Google Scholar]
- 12.Wrotniak BH, Epstein LH, Paluch RA, Roemmich JN. Parent weight change as a predictor of child weight change in family-based behavioral obesity treatment. Arch Pediatr Adolesc Med. 2004;158:342–347. doi: 10.1001/archpedi.158.4.342. [DOI] [PubMed] [Google Scholar]
- 13.Epstein LH, Wilfley DE. Today Lifestyle Program Youth and FSP Handbooks and Treatment Manuals. 2005 [Google Scholar]
- 14.Faith MS, Saelens BE, Wilfley DE, Allison DB. Behavioral treatment of childhood and adolescent obesity: current status, challenges, and future directions. In: Thompson JK, Smolak L, editors. Body Image, Eating Disorders, and Obesity in Youth: Assessment, Prevention, and Treatment. Washington, D.C: American Psychological Association; 2001. pp. 313–340. [Google Scholar]
- 15.Morgan CM, Tanofsky-Kraff M, Wilfley DE, Yanovski JA. Childhood obesity. Child Adolesc Psychiatr Clin N Am. 2002;11:257–278. doi: 10.1016/s1056-4993(01)00007-4. [DOI] [PubMed] [Google Scholar]
- 16.Wing RR. Treatment of obesity in patients with type 2 diabetes. In: Fairburn CG, Brownell KD, editors. Eating Disorders and Obesity. New York: Guilford Press; 2002. pp. 578–582. [Google Scholar]
- 17.Obesity evaluation and treatment: Expert Committee recommendations. Barlow SE, Dietz WH. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. 1998;102:E29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed]
- 18.Perri MG, Corsica JA. Improving the maintenance of weight loss in behavioral treatment of obesity. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York: Guilford Press; 2002. pp. 357–379. [Google Scholar]
- 19.Epstein LH, Myers MD, Raynor HA, Saelens BE. Treatment of pediatric obesity. Pediatrics. 1998;101:554–570. [PubMed] [Google Scholar]
- 20.Epstein LH. Development of evidence-based treatment for pediatric obesity. In: Kazdin AE, Weisz JR, editors. Evidence-based Psychotherapies for Children and Adolescents. New York: Guildford Publications; 2003. pp. 374–388. [Google Scholar]
- 21.Goldfield GS, Raynor HA, Epstein LH. Treatment of pediatric obesity. In: Stunkard AJ, Wadden TA, editors. Obesity: Theory and Therapy. New York: Guilford Press; 2002. pp. 532–555. [Google Scholar]
- 22.American Diabetes Association, National Institutes Of Diabetes, Digestive And Kidney Disease. The prevention or delay of type 2 diabetes. Diabetes Care. 2002;25:742–749. doi: 10.2337/diacare.25.4.742. [DOI] [PubMed] [Google Scholar]
- 23.Goldfield GS, Epstein LH. Can fruits and vegetables and activities substitute for snack foods. Health Psychol. 2002;21:299–303. [PubMed] [Google Scholar]
- 24.Corbin CB, Pangrazi RP, Lemasurier GC. Physical activity for children: current patterns and guidelines. President’s Council on Physical Fitness and Sports Research Digest. 2004;5:1–8. [Google Scholar]
- 25.Epstein LH, Valoski AM, Vara LS, Mccurley J, Wisniewski L, Kalarchian MA. Effects of decreasing sedentary behavior and increasing activity on weight change in obese children. Health Psychol. 1995;14:109–115. doi: 10.1037//0278-6133.14.2.109. [DOI] [PubMed] [Google Scholar]
- 26.Robinson TN. Behavioral treatment of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999;23:S52–S57. doi: 10.1038/sj.ijo.0800860. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Hermans MP, Levy JC, Morris RJ, Turner RC. Comparison of tests of b-cell function across a range of glucose tolerance from normal to diabetes. Diabetes. 1999;48:1779–1786. doi: 10.2337/diabetes.48.9.1779. [DOI] [PubMed] [Google Scholar]
- 29.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate, method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 30.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal saggital diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 31.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9:314–324. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 32.Weston AT, Petosa R, Pate RR. Validation of an instrument for measurement of physical activity in youth. Med Sci Sports Exerc. 1997;29:138–143. doi: 10.1097/00005768-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Melanson EL, Freedson PS. Validity of the Computer Science and Applications, Inc. (CSA) activity monitor. Med Sci Sports Exerc. 1995;27:934–940. [PubMed] [Google Scholar]
- 34.Boreham CA, Paliczka VJ, Nichols AK. A comparison of the PWC1709 and 20-MST tests of aerobic fitness in adolescent schoolchildren. J Sports Med Phys Fitness. 1990;30:19–23. [PubMed] [Google Scholar]
- 35.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs M. Rating scales to assess depression in schoolage children. Acta Paedopsychiatr. 1981;46:305–310. [PubMed] [Google Scholar]
- 37.Kovacs M. The childrens depression inventory. Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 38.Kovacs M, Beck AT. An empirical-clinical approach toward a definition of childhood depression. In: Schulterbrandt JG, Raskin A, editors. Depression in Childhood: Diagnosis, Treatment, and Conceptual Models. New York: Raven Press; 1997. pp. 1–25. [Google Scholar]
- 39.Kovacs M. Children’s Depression Inventory Manual. North Tonawanda, NY: Multi-Health systems, Inc; 1992. [Google Scholar]
- 40.Reynolds WM. Depression in children and adolescents. In: Reynolds WM, editor. Internalizing Disorders in Children and Adolescents. New York: John Wiley & Sons, Inc; 1992. [Google Scholar]
- 41.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 43.Beck AT, Steer RA. Beck Depression Inventory II. San Antonio: Psychological Corp; 1996. [Google Scholar]
- 44.Kendall PC, Hollon SD, Beck AT, Hammern CH, Ingram RE. Issues and recommendations regarding the use of the Beck Depression Inventory. Cognit Ther Res. 1987;11:289–299. [Google Scholar]
- 45.Landgraf JM, Abetz L, Ware JE. The CHQ User’s Manual. 1st edn. Boston, MA: The Health Institute New England Medical Center; 1996. [Google Scholar]
- 46.Varni Jw, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life inventory generic core scales and type 1 diabetes module. Diabetes Care. 2003;26:631–637. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- 47.Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies. Ann Med. 2001;33:375–384. doi: 10.3109/07853890109002092. [DOI] [PubMed] [Google Scholar]
- 48.Peduzzi P, Wittes J, Detre K, Holford T. Analysis as-randomized and the problem of non-adherence: an example from the Veterans Affairs Randomized Trial of Coronary Artery Bypass Surgery. Stat Med. 1993;12:1185–1195. doi: 10.1002/sim.4780121302. [DOI] [PubMed] [Google Scholar]
- 49.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley & Sons, Inc; 1980. pp. 219–224. [Google Scholar]
- 50.The DCCT Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 2005;44:968–983. [PubMed] [Google Scholar]
- 51.The DCCT Research Group. The effect of intensive diabetes treatment on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial. N Engl J Med. 2005;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 52.Girard J. Fatty acids and beta cells. Diabetes Metab. 2000;26:S6–S9. [PubMed] [Google Scholar]
- 53.Greene DA. Rosiglitazone: a new therapy for type 2 diabetes. Expert Opin Investig drugs. 1999;8:1709–1719. doi: 10.1517/13543784.8.10.1709. [DOI] [PubMed] [Google Scholar]
- 54.Mcgarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42:128–138. doi: 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- 55.Unger RH, Orci L. Lipotoxic diseases of nonadipose tissues in obesity. Int J Obes Relat Metab Disord. 2000;24:S28–S32. doi: 10.1038/sj.ijo.0801498. [DOI] [PubMed] [Google Scholar]
- 56.Kimm SYS, Glynn NW, Kriska AM, et al. Longitudinal changes in physical activity in a biracial cohort during adolescence. Med Sci Sports Exerc. 2000;32:1445–1454. doi: 10.1097/00005768-200008000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Colditz GA, Willett WC, Stampfer MJ, London SJ, Segal MR, Speizer FE. Patterns of weight change and their relation to diet in a cohort of healthy women. Am J Clin Nutr. 1990;51:1100–1105. doi: 10.1093/ajcn/51.6.1100. [DOI] [PubMed] [Google Scholar]
- 58.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338:774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 59.West KM, Kalbfleisch JM. Influence of nutritional factors on prevalence of diabetes. Diabetes. 1971;20:99–108. doi: 10.2337/diab.20.2.99. [DOI] [PubMed] [Google Scholar]
- 60.Maggio CA, Pi-Sunyer FX. The prevention and treatment of obesity. Application to type 2 diabetes. Diabetes Care. 1997;20:1744–1766. doi: 10.2337/diacare.20.11.1744. [DOI] [PubMed] [Google Scholar]
- 61.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3:211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 62.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415. [PubMed] [Google Scholar]
- 63.Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med. 1987;147:1749–1753. [PubMed] [Google Scholar]
- 64.Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes. 1986;35:155–164. doi: 10.2337/diab.35.2.155. [DOI] [PubMed] [Google Scholar]
- 65.Lean ME, Powrie JK, Anderson AS, Garthwaite PH. Obesity, weight loss and prognosis in type 2 diabetes. Diabet Med. 1990;7:228–233. doi: 10.1111/j.1464-5491.1990.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 66.Chaturvedi N, Fuller JH The Who Multinational Study of Vascular Disease in Diabetes. Mortality risk by body weight and weight change in people with NIDDM. Diabetes Care. 1995;18:766–774. doi: 10.2337/diacare.18.6.766. [DOI] [PubMed] [Google Scholar]
- 67.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test and minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001;50:150–158. doi: 10.2337/diabetes.50.1.150. [DOI] [PubMed] [Google Scholar]
- 68.Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23:295–301. doi: 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 69.Guzzaloni G, Grugni G, Mazzilli G, Moro D, Morabito F. Comparison between beta-cell function and insulin resistance indexes in prepubertal and pubertal obese children. Metabolism. 2002;51:1011–1016. doi: 10.1053/meta.2002.34029. [DOI] [PubMed] [Google Scholar]
- 70.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 71.Uwaifo GI, Fallon EM, Chin J, Elberg J, Parikh SJ, Yanovski JA. Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care. 2002;25:2081–2087. doi: 10.2337/diacare.25.11.2081. [DOI] [PubMed] [Google Scholar]
- 72.Roemmich JN, Clark PA, Lusk M, et al. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution, and hormone release. Int J Obes Relat Metab Disord. 2002;26:701–709. doi: 10.1038/sj.ijo.0801975. [DOI] [PubMed] [Google Scholar]
- 73.Kay JP, Alemzadeh R, Langley G, D’angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism. 2001;50:1457–1461. doi: 10.1053/meta.2001.28078. [DOI] [PubMed] [Google Scholar]
- 74.Brownell KD, Kelman JH, Stunkard AJ. Treatment of obese children with and without their mothers: changes in weight and blood pressure. Pediatrics. 1983;71:515–523. [PubMed] [Google Scholar]
- 75.Goran MI, Driscoll P, Johnson R, Nagy TR. Cross-calibration of body composition techniques against dual-energy X-ray absorptiometry in young children. Am J Clin Nutr. 2005;63:299–305. doi: 10.1093/ajcn/63.3.299. [DOI] [PubMed] [Google Scholar]
- 76.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rockett HR, Breitenbach M, Frazier AL, et al. Validation of a youth/adolescent food frequency questionnaire. Prev Med. 1997;26:808–816. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- 78.Jacobs DR, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 79.Kriska AM, Knowler WC, Laporte RE, et al. Development of a questionnaire to examine the relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 80.Janz KF. Validation of the CSA accelerometer for assessing children’s physical activity. Med Sci Sports Exerc. 1994;26:369–375. [PubMed] [Google Scholar]
- 81.Nichols JF, Morgan CG, Chabot LE, Sallis JF, Calfas KJ. Assessment of physical activity with the Computer Science and Applications, Inc., accelerometer: laboratory versus field validation. Res Q Exerc Sport. 2000;71:36–43. doi: 10.1080/02701367.2000.10608878. [DOI] [PubMed] [Google Scholar]
- 82.Masse LC, Fulton JE, Watson KL, et al. Detecting bouts of physical activity in a field setting. Res Q Exerc Sport. 1999;70:212–219. doi: 10.1080/02701367.1999.10608041. [DOI] [PubMed] [Google Scholar]
- 83.Rowland TW, Cunningham LN. Oxygen uptake plateau during maximal treadmill exercise in children. Chest. 1992;101:485–489. doi: 10.1378/chest.101.2.485. [DOI] [PubMed] [Google Scholar]
- 84.Howley ET, Bassett DR, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27:1292–1301. [PubMed] [Google Scholar]
- 85.Reaven GM. 1988 Banting lecture: role of insulin resistance inhuman disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 86.Zavaroni I, Mazza S, Luchetti L, et al. Risk factors for coronary artery disease in healthy persons with hyperinsulinemia and normal glucose tolerance. N Engl J Med. 1989;320:702–706. doi: 10.1056/NEJM198903163201105. [DOI] [PubMed] [Google Scholar]
- 87.Zavaroni I, Mazza S, Luchetti L, et al. High plasma insulin and triglyceride concentrations and blood pressure in offspring of people with impaired glucose tolerance. Diabet Med. 1990;7:494–498. doi: 10.1111/j.1464-5491.1990.tb01430.x. [DOI] [PubMed] [Google Scholar]
- 88.Kullo IJ, Gau GT, Tajik AJ. Novel risk factors for atherosclerosis. Mayo Clin Proc. 2000;75:369–380. doi: 10.4065/75.4.369. [DOI] [PubMed] [Google Scholar]
- 89.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes. 2002;51:204–209. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- 90.Zieske AW, Malcolm GT, Strong JP. Natural history and risk factors of atherosclerosis in children and youth: the PDAY study. Pediatr Pathol Mol Med. 2002;21:213–237. doi: 10.1080/15227950252852104. [DOI] [PubMed] [Google Scholar]
- 91.Ferguson MA, Gutin B, Owens S, Litaker M, Tracy RP, Allison J. Fat distribution and hemostatic measures in obese children. Am J Clin Nutr. 1998;67:1136–1140. doi: 10.1093/ajcn/67.6.1136. [DOI] [PubMed] [Google Scholar]
- 92.Gallistl S, Sudi KM, Aigner R, Borkenstein M. Changes in serum interleukin-6 concentrations in obese children and adolescents during a weight reduction program. Int J Obes Relat Metab Disord. 2001;25:1640–1643. doi: 10.1038/sj.ijo.0801808. [DOI] [PubMed] [Google Scholar]
- 93.Sudi KM, Gallistl S, Payerl D, et al. Interrelationship between estimates of adiposity and body fat distribution with metabolic and hemostatic parameters in obese children. Metabolism. 2001;50:681–687. doi: 10.1053/meta.2001.22562. [DOI] [PubMed] [Google Scholar]
- 94.Estelles A, Dalmau J, Falco C, et al. Plasma PAI-1 levels in obese children - effect of weight loss and influence of PAI-1 promoter 4G/5G genotype. Thromb Haemost. 2001;86:647–652. [PubMed] [Google Scholar]
- 95.Takahashi C, Nagai N, Ujihara N, et al. Clinical profile of Japanese dialysis patients with diabetic nephropathy, diagnosed as having diabetes before the age of thirty. Diabetes Res Clin Pract. 1990;10:127–131. doi: 10.1016/0168-8227(90)90034-q. [DOI] [PubMed] [Google Scholar]
- 96.Yokoyama H, Okudaira M, Otani T, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000;58:302–311. doi: 10.1046/j.1523-1755.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- 97.DCCT Research Group. Lifetime benefits and costs of intensive therapy as practiced in the diabetes control and complications trial. JAMA. 1996;276:1409–1415. [PubMed] [Google Scholar]
- 98.United Kingdom Prospective Diabetes Study Group. Cost effectiveness of an intensive blood glucose control policy in patients with type 2 diabetes: economic analysis alongside randomised controlled trial (UKPDS41) BMJ. 2000;320:1373–1378. doi: 10.1136/bmj.320.7246.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.The Collaborative Study Group. An economic analysis of captopril in the treatment of diabetic nephropathy. Diabetes Care. 1996;19:1051–1061. doi: 10.2337/diacare.19.10.1051. [DOI] [PubMed] [Google Scholar]
- 100.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. pp. 5–9. [Google Scholar]
- 101.Drummond MF, O’brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmers. 2nd edn. New York: Oxford University Press; 1999. pp. 96–107. [Google Scholar]