Abstract
Smooth Brucella abortus S2308 is virulent while rough derivatives are attenuated. Intracellular killing is often blamed for these differences. In the studies described, uptake kinetics and interaction of S2308 and S2308 manBA::Tn5 (CA180) rough mutants with macrophages were investigated. The results revealed that smooth B. abortus was rapidly internalized, achieving a maximum level in less than 5 minutes without additional uptake over the next 30 minutes. In contrast, continued uptake of the rough mutant was observed and only achieves a maximum level after 30 minutes. The results were confirmed by the differences in F-actin polymerization, lipid raft staining, early endosome colocalization and electron microscopic observations after smooth and rough Brucella infection. We also demonstrated for the first time that uptake of S2308, but not rough mutant CA180 was PI3-kinase and toll-like receptor 4 (TLR4) dependent. Differences in uptake were associated with differences in macrophage activation with regard to NF-κB translocation and cytokine production. These results provide evidence that the presence of B. abortus OPS dictates the interactions between Brucella and specific cell surface receptors minimizing macrophage activation and enhancing Brucella survival and/or persistence.
Keywords: Brucella, uptake, OPS, macrophage
1. Introduction
Brucella spp. are gram-negative, facultative intracellular bacteria that infect a variety of animals causing abortion and infertility. B. melitensis, B. abortus and B. suis also infect humans causing undulant fever. This worldwide zoonotic disease is endemic in many developing countries and is responsible for economic losses in livestock industries and public health concerns. The ability of this organism to cause disease in humans and animals following aerosol exposure has led to its inclusion on select agent and biodefense threat lists.
Macrophages and trophoblast cells are primary target cells in which the bacteria can multiply and cause persistent infection and abortion. The bacteria also infect a variety of non-professional phagocytes in culture, suggesting that Brucella have broad interactions with host cells [1]. Brucella invasion processes in epithelial cells and macrophages have been investigated. Early studies showed that M cells, macrophages and neutrophils ingest Brucella by zipper-like phagocytosis [2]. Rittig et al. (2001) recently confirmed that human monocytes and epithelial cells engulf B. melitensis and B. suis via conventional zipper-type mechanisms [3]. Invasion of HeLa cells by B. abortus requires small GTPase of the Rho subfamily [4] and requires energy input from the host cells [5]. It was also observed that Brucella invade macrophages through lipid raft microdomains [6]. In both epithelial cells and macrophages F-actin polymerization is involved in ingestion of Brucella, because inhibition of actin polymerization abolishes bacterial uptake [4, 5, 7].
Possible host cell surface receptors for Brucella have been recently identified [8-10], but the role of cellular prion protein in Brucella uptake remains questionable [11]. Brucella virulence factors involved in bacterial invasion have not been clearly defined. It has been reported that BvrS/BvrR two component regulatory system is important to Brucella penetration of professional and non-professional phagocytic cells [12]. The roles of type IV secretion system (T4SS) in Brucella invasion have been investigated and it is generally accepted that the T4SS is not involved [13]. More recent study showed that BMEI0216 gene of B. melitensis is required for internalization in HeLa cells [14]. Essential genes encoding invasins and adhesins have been identified by Brucella genome analysis, but those molecules have not been investigated on Brucella surface [15]. Brucella LPS has been recognized as an important virulence factor involved in bacterial adhesion and internalization [16]. Recent studies revealed that smooth Brucella invade phagocytes at lower number compared with rough organisms, and inhibit phagocyte apoptosis [17, 18]. In contract, Brucella rough strains attach and invade macrophages at higher efficiency and activate macrophage [6, 17, 19, 20]. However the mechanisms behind this have not been fully investigated.
In this study, the internalization processes and interaction of smooth and rough organisms with murine macrophages were monitored by a variety of methods. The results indicate that OPS dictates Brucella uptake by macrophages, and in doing so alters the outcome of infection by restricting macrophage activation.
2. Materials and Methods
2.1. Bacteria strains and growth conditions
Bacteria used in this experiment include B. abortus virulent S2308 strain and rough mutant S2308 manBA::Tn5 (CA180) [21]. The bacterial cultures were prepared as previously described [19].
2.2. Reagents
Mouse anti-Brucella and goat anti-Brucella sera were prepared in our laboratory. Donkey anti mouse IgG Alexa Fluor 594, donkey anti mouse IgG Alexa Fluor 488, donkey anti goat IgG Alexa Fluor 488, donkey anti goat IgG Alexa Fluor 594 and Alexa Fluor 488-phalloidin were purchased from Molecular Probes (Eugene, OR). Goat anti EEA1 and goat anti-NF-κB p65 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Cholera toxin B subunit-FITC and wortmannin were obtained from Sigma (St. Louis, MO).
2.3. Cell culture and infection
Murine macrophage-like cell J774.A1 (ATCC, TIB-67) was grown in complete Dulbecco’s modified Eagle’s medium (DMEM) with 10% (v/v) FBS and 0.1 mM non-essential amino acids (complete DMEM). The cells were passaged every 3 to 5 days and discarded after passage 15. Bone marrow derived macrophages (BMDM) were prepared from C3H/HeJ (TLR4d/d) and C3H/HeOuJ (TLR4+/+) mice as previously described [22]. Macrophages were seeded in 24-well or 6-well plates one day prior to infection with Brucella [19].
2.4. Immunofluorescent assay
J774.A1 cells cultured on glass coverslips with a thickness of 0.17 mm (Fisher Scientific, Pittsburgh, PA) were infected with S2308 or CA180 at an MOI of 10 as described previously [19]. The infected cells were fixed with 3% (w/v) paraformaldehyde immediately after a 5-minute centrifugation, and following 5, 10, 20, and 30 minutes incubations. To monitor bacterial infection within the first 5 minutes, J774.A1 cells cultured on coverslips were chilled on ice for 20 minutes. The cells were inoculated with S2308 and CA180 at 10 MOI, and centrifuged at 4°C for 5 minutes. Medium was replaced with pre-warmed complete DMEM (37°C) and the cells were fixed at 0.5, 1, 2, 3, 4, and 5 minutes.
For differential staining of intracellular and extracellular bacteria, fixed cells on coverslips were stained with a double antibody labeling procedure and observed as previously described [19]. At least 200 cells were detected to obtain each data.
To detect NF-κB translocation, J774.A1 macrophages cultured on coverslips were infected with S2308 or CA180 as described above. The cells treated with E. coli LPS (100 ng/ml) were used as positive control. The infected and control cells were fixed at 1 h p.i. and stained with goat anti p65 (1:500) and mouse anti Brucella (1:1000). NF-κB translocation and Brucella internalization was revealed by staining with donkey anti goat IgG Alexa Flour 594 and donkey anti mouse IgG Alexa Flour 488 (1:1000), respectively.
2.5. Drug treatment of macrophages before infection
To determine the roles of phosphoinositide 3-kinase (PI3-kinase) in the uptake of smooth and rough B. abortus, J774.A1 macrophages were treated with 100 nM of wortmannin for 30 minutes prior to infection. The cells were washed with complete DMEM before infection with S2308 and CA180 at an MOI of 100. Uptake of smooth and rough Brucella by the treated cells was determined using the gentamicin protection assay [19].
2.6. Cytokine ELISA
J774.A1 cells cultured in 24-well plates were infected with S2308 and CA180 at an MOI of 100 as described above. Cell culture supernatants were collected at various time points and cytokine levels in the supernatants were detected using sandwich ELISA kits (PeproTech Inc., Rocky Hill, NJ) following the manufacture’s instruction.
2.7. Electron microscopy
J774.A1 cells cultured on glass coverslips or in 6-well plates were infected with S2308 and CA180 as described above. The infected cells were fixed at various time points as previously described [23] and processed at Image Analysis Laboratory in College of Veterinary Medicine and Biomedical Sciences, Texas A&M University. Bacterial invasion was observed with JSM 6400 scanning electron microscopy in Electron Microscopy Center, Texas A&M University or with Carl Zeiss High Resolution Electron Microscope (EM 10 CA) at the Image Analysis Laboratory in the College of Veterinary Medicine and Biomedical Sciences, Texas A&M University.
2.8. Statistical analysis
Statistical significance was determined using Student’s t-test. A P value of < 0.05 was considered significant.
3. Results
3.1. B. abortus OPS affects binding and internalization
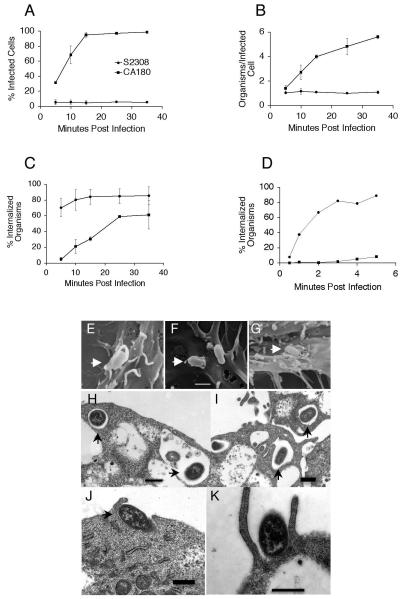
It has previously been reported that the uptake of rough organisms greatly exceeds that observed for smooth strains [6, 17, 19], and current experiments were conducted to reveal the dynamics of internalization. A double antibody labeling procedure was employed to differentiate external bacteria (red) from internalized organisms (green) (data not shown). In agreement with previous reports [6, 17, 19], CA180 has greatly elevated binding capacity compared with S2308. The dynamics of internalization were determined according to the following criteria: i) fraction of cells containing internalized bacteria; ii) average bacterial burden per infected cell; and iii) fraction of cell-associated bacteria that was internalized (Fig. 1). Using this approach it was demonstrated that the uptake of smooth organisms by J774.A1 cells was optimal within 5 minutes of exposure, while rough organisms take up to 35 minutes to be optimally internalized (Fig. 1). The fraction of the cells infected with S2308 reached 5.25 ± 3.32% at 5 minutes p.i. and remained unchanged up to 35 minutes p.i. However, the fraction of cells infected with CA180 was 31.42 ± 1.53% by 5 minutes p.i., and increased to 94.95 ± 3.04% by 15 minutes p.i. (Fig. 1A). The average number for S2308 remained about one bacterium per infected cell between 5 and 35 minutes p.i., while the number for the CA180 steadily increased from 1.41 ± 0.23/cell at 5 minutes to 5.62 ± 0.12/cell at 35 minutes p.i. (Fig. 1B). Analysis of the infected cells revealed that 80.27 ± 13.12% of the cell-associated smooth Brucella were internalized within 10 minutes. In contrast, only 21.1 ± 9.05% of cell associated rough Brucella were internalized at 10 minutes and by 25 minutes the macrophages had internalized 59.15 ± 1.63% of cell-associated rough organisms (Fig. 1C). These results confirm that Brucella OPS suppressed bacterial binding and uptake by macrophages, and reveal for the first time that the internalization of smooth Brucella S2308 by macrophages occurs much faster reaching saturation within 5 minutes, while rough Brucella mutant CA180 need more than 35 minutes.
Fig. 1.
B. abortus invasion dynamics and electron micrographs in murine macrophages. The invasion of B. abortus smooth strain S2308 and the rough derivative CA180 was monitored over time using double antibody staining and electron microscopy. Percentage of the cells infected at indicated time points (A). Average internalized bacteria per infected cell (B). A ratio (percent) of internalized bacteria to cell-associated bacteria (C). The results are means ± SD of three independent experiments. A ratio (percent) of internalized bacteria to cell-associated bacteria at early stages (less than 5 minutes) of infection (D). The data is a representative of two experiments with similar results. Scanning electron micrograph of macrophages infected with S2308 and fixed at 5 minutes p.i. (E), or infected with CA180 and fixed at 5 minutes (F) or 20 minutes p.i. (G). Transmission electron micrograph of macrophages infected with S2308 (H, I) and CA180 (J, K) and fixed at 5 min p.i. Bar = 1 μM.
To further investigate the kinetics of internalization of smooth Brucella, J774.A1 macrophage cells were chilled on ice prior to infection and fixed within 5 minutes of infection with S2308 and CA180, and intracellular and extracellular bacteria were enumerated as described above. More than 80% of the cell associated S2308 invaded the macrophage in less than 5 minutes, while only 8.26% of the CA180 was internalized (Fig. 1D). This rapid invasion by smooth organisms made visualization of the invasion processes using scanning electron microscopy difficult (Fig. 1E), while the rough Brucella can be easily detected by 5 minutes p.i. (Fig. 1F) and still be detected at 20 minutes p.i. (Fig. 1G). Transmission electron microscopy observation confirmed that smooth Brucella were internalized by five minutes (Fig. 1H, 1I). In contrast, most of the rough organisms still attached to cell surface or invading the cells (Fig. 1J, 1K).
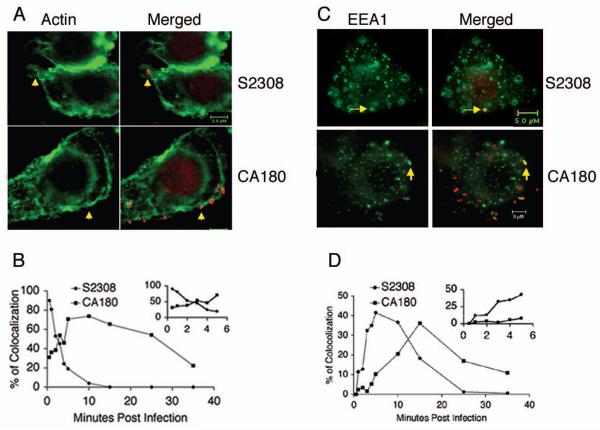
Actin polymerization has been identified as an essential part of the entry mechanism in phagocytosis of pathogens. Contact between Brucella and macrophages or HeLa cells induce transient local F-actin recruitment [4, 7]. To further determine the role of Brucella OPS in induction of F-actin polymerization, J774.A1 macrophages infected with B. abortus S2308 and the rough mutant CA180 was monitored by phalloidin Alexa Fluor 488 staining (Fig. 2). Both smooth and rough B. abortus contact with the macrophages elicited F-actin polymerization (Fig. 2A). Staining of the infected cells fixed at different time points indicated that both smooth and rough Brucella colocalized with F-actin at very early stages of infection and quickly depolymerized. More than 90% of the smooth organisms colocalized with F-actin by 30 seconds p.i. which dropped to 20% by 5 minutes p.i. (Fig. 2B insert). In contrast, CA180 recruited F-actin slowly, with a maximum colocalization of 70% detected by 5 to 10 minutes p.i. (Fig. 2B), which decreased gradually to 25% by 35 minutes p.i. These results suggest a role for Brucella OPS in restricting overall binding and uptake of the organism, while enhancing the kinetics of internalization.
Fig. 2.
F-actin polymerization and early endosome marker colocolization during B. abortus invasion. J774.A1 macrophages cultured on coverslips were infected with S2308 and CA180 at an MOI of 10 and fixed with 3% (w/v) paraformaldehyde at selected time points. The bacteria (red) were stained with mouse anti Brucella serum and detected with donkey anti mouse IgG Alexa Fluor 594. F-actin (A) or EEA1 (C) was detected as described in the Materials and Methods. The dynamics of Brucella colocalization with F-actin (B) or EEA1 (D) were revealed in time course. The insert shows the dynamics in early stage of the infection (5 minutes or less). The data shown is a representative of two experiments with similar results.
Early endosomes are cellular compartments that receive and sort endocytosed material to late endosomes and lysosomes. To examine whether the early interactions of Brucella with the endocytic compartment was changed by OPS, J774.A1 macrophages were infected with S2308 and CA180. Staining of the infected macrophages with early endosome associated antigen (EEA1) revealed that both Brucella smooth and rough organisms invade macrophages through early endosomes (Fig. 2C). Examination of the infected cells fixed at various time points indicated that the interactions were transient. However, smooth Brucella S2308 entered early endosomes faster than the rough mutant CA180 (Fig. 2D), which is in agreement with the uptake kinetics described above.
3.2. Smooth Brucella uptake by macrophages is associated with lipid rafts
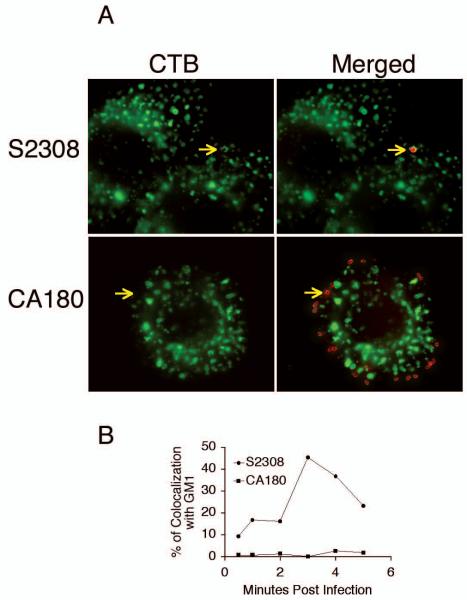
Recent studies have shown that lipid rafts or caveolae play an important role in bacterial entry. Lipid raft-associated molecules, such as cholesterol, ganglioside GM1, and glycosylphosphatidylinositol (GPI)-anchored protein, are selectively incorporated into macropinosomes containing Brucella. Among those molecules, membrane cholesterol and ganglioside GM1 have been shown to be important in establishing Brucella entry and infection [24]. Methyl-β-cyclodextrin or filipin treatments of J774.A1 cells inhibited uptake of smooth Brucella in a dose dependent manner, while the treatments had limited effect on the uptake of rough Brucella [6]. In the current report, colocolization of Brucella with lipid rafts was visualized using fluorescent microscopy. J774.A1 cells infected with S2308 and CA180 were stained with goat anti-Brucella antibody. Colocalization of the bacteria and GM1 was determined after staining with CTB-FITC conjugate and donkey anti goat IgG Alexa Fluor 594. In agreement with previous report [6], S2308 but not CA180 colocalized with GM1 (Fig. 3A). Maximum colocalization with S2308 is detected at 3 min p.i. and rapidly declines (Fig. 3B), which is consistent with rapid internalization and trafficking of this smooth organism .
Fig. 3.
Brucella smooth strain invades macrophages using lipid rafts as revealed by GM1 colocalization. J774.A1 macrophages cultured on coverslips were infected with S2308 and CA180 at an MOI of 10 and fixed with 3% (w/v) paraformaldehyde at various time points. (A) The bacteria (red) were stained with mouse anti Brucella serum and detected with donkey anti mouse IgG-Alexa Fluor 594. Ganglioside GM1 (green) was detected by cholera toxin B subunit-FITC staining. (B) Dynamics of Brucella colocalization with GM1. The result is a representative of two experiments with similar results.
3.3. Effect of PI3-kinase inhibitors on the invasion of smooth and rough B. abortus
It has been shown that PI3-kinase plays an important roles in phagocytosis and phagosome trafficking [25]. Reports of the effect of PI3-kinase on Brucella uptake have been controversial. Guzman-Verri et al. reported that PI3-kinase is involved in the uptake of Brucella S2308 by HeLa cells [4], while others showed that wortmannin (PI3-kinase inhibitor) treatment did not inhibit wild type Brucella uptake [26, 27]. To determine the roles of PI3-kinase in the uptake of smooth and rough B. abortus, J774.A1 macrophages were treated with 100 nM of wortmannin before Brucella infection. Wortmannin treatment significantly inhibited the uptake of S2308 strain, but had limited effect on the uptake of the rough mutant (Fig. 4). These results demonstrated that PI3-kinase activity is important for the uptake of smooth Brucella, but appears to play a limited role (if any) in the uptake of rough mutants.
Fig. 4.
PI3-kinase in the uptake of Brucella in macrophages. J774.A1 was treated with 100 nM wortmannin for 30 minutes before infection and continuously thereafter. Gentamicin protection assay was used to monitor the uptake of S2308 and CA180 in the presence and absence of wortmannin. The data shown represent the means ± SD of three independent experiments. □p<0.05 when compared with untreated control.
3.4. TLR4 is important for smooth Brucella uptake in murine BMDM
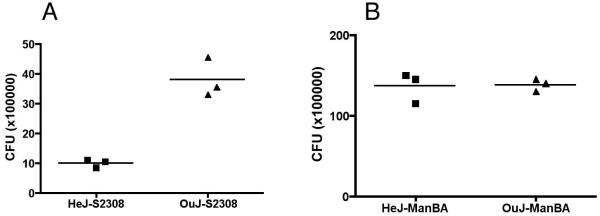
Signaling through TLRs has been shown to activate macrophages including enhanced bacterial phagocytosis and phagosome maturation, nucleus translocation of NF-κB and proinflammatory cytokine production [28]. To determine the role of TLR4 in Brucella invasion, uptake of smooth and rough B. abortus was evaluated in BMDMs from C3H/HeJ and C3H/HeOuJ mice. The results revealed that the defect in TLR4 resulted in almost 75% decrease in the internalization of smooth B. abortus S2308: 1 × 106 CFU/well in HeJ BMDM vs. 3.8 × 106 CFU/well in OuJ macrophages (Fig. 5A). In contrast, the defect in TLR4 had no apparent effect on internalization of rough mutant CA180: 1.37 × 107 CFU/well in HeJ BMDM vs. 1.38 × 107 CFU/well in OuJ BMDM (Fig. 5B). These results indicate that TLR4 is important for smooth B. abortus internalization in macrophages. The failure to significantly affect the internalization of rough mutant CA180 is consistent with the observations that smooth and rough B. abortus are internalized via different pathways.
Fig. 5.
TLR4 is important for smooth Brucella uptake in murine macrophage. BMDMs cultured from (A) TLR4 deficient (HeJ) or (B) TLR4 sufficient control (OuJ) mice were infected with smooth and rough Brucella at an MOI of 100. Uptake of the bacteria was determined using gentamicin protection assay. The results are representatives of four experiments
3.5. Macrophages are activated by rough, but not smooth Brucella infection
In order to elaborate on the mechanistic basis for enhanced uptake of rough organisms, the activation state of macrophages was evaluated. Translocation of NF-κB p65 was monitored 1 h p.i. using fluorescence microscopy. In these experiments, nucleus translocation of NF-κB was easily detected in J774.A1 macrophages exposed to rough organisms at an MOI of 100 (Fig. 6A) and an MOI of 10 (data not shown). In contrast, no translocation was evident in cells exposed to smooth organisms at an MOI of 100 (Fig. 6B). E. coli LPS (100 ng/ml) induced strong translocation of p65 (Fig. 6C), while no translocation was detected in untreated cells (Fig. 6D). In order to verify the overall activation state of these macrophages, cell culture supernatants collected at 8, 24, 48 and 72 h p.i. were examined for IL1-α and TNF-α production using sandwich ELISA. The results revealed that rough Brucella stimulated high levels of TNF-α (Fig. 6E) and IL1-α (Fig. 6F) production compared with S2308, indicating that macrophages were activated after rough Brucella, but not smooth Brucella infection.
Fig. 6.
Rough Brucella infection induces macrophage activation. J774.A1 macrophages cultured on coverslips were infected with CA180 (A) or S2308 (B) at an MOI of 100. Control cells were treated with 100 ng/ml E. coli LPS (C) or left untreated (D). The cells were fixed with 3% (w/v) paraformaldehyde 1 h p.i. and detected for p65 (red staining) translocation and Brucella (green) using IX70 fluorescence microscopy (Olympus) after immunofluorescence staining. TNF-α (E) and IL1-α (F) were detected using sandwich ELISA after macrophages were infected with smooth and rough B. abortus at an MOI of 100. The supernatants were collected at 8, 24, 48 and 72 h p.i. The results (mean ± SD) are representatives of three experiments with similar results.
4. Discussion
As an important innate immune component and primary target cell of Brucella, macrophage plays a central role in the pathogenesis of brucellosis. Interaction of the pathogen with macrophages determines the outcome of infection [29]. Unlike other pathogenic bacteria, Brucella sp. do not have classical virulence factors. Their virulence is determined by their abilities to invade, survive and reach the replicative niche in the host cells [16, 30]. OPS is considered an important virulence factor in this organism [6, 21, 31, 32]. The current study provides more evidence that Brucella OPS is essential for uptake by murine macrophages leading to a productive infection. With OPS, smooth Brucella S2308 invade macrophages via lipid rafts, which requires PI3-kinase and TLR4 signal and directs intracellular trafficking through a unique pathway to the replication niche [16]. Without OPS, rough Brucella mutant CA180 invade macrophages through a different pathway and are either destroyed as a result of macrophage activation or released prematurely as a result of cell death [17, 19].
The internalization dynamics of S2308 and its rough derivative CA180 demonstrated that OPS limited overall binding and enhanced internalization dynamics. Uptake of smooth strains in macrophages reached saturation faster than the uptake of rough strains. The results were confirmed by F-actin polymerization, colocalization of the bacteria with EEA1 and electron microscopic observations. Saturated internalization was achieved within 3 minutes for S2308 and did not change for next 30 minutes, which has not been previously demonstrated [7]. In contrast to smooth bacteria, rough mutant CA180 invaded the cells for extended periods. The different internalization dynamics resulted in significant difference in percentages of infected cells by these organisms (Fig. 1A). One interpretation of these results may be that OPS blocks the interaction between Brucella and macrophage. Without OPS expression, bacterial membrane proteins are more exposed [33]. This hypothesis is supported by our recent observation that LPS isolated from B. melitensis 16M inhibits latex bead uptake in macrophage (J. Pei and T.A. Ficht, unpublished data). Another possibility is that loss of OPS expression may affect the conformation and function of various surface proteins [34]. In these cases, interactions between rough Brucella and macrophages could be enhanced. The third interpretation is that changes occur in cells following smooth Brucella infection or contact with the cells that restrict additional uptake. However, such changes (if they occur) do not prevent rough Brucella uptake in mixed infection with smooth and rough organisms, or in super-infection by rough B. abortus after smooth Brucella infection (data not shown). These results appear to support the hypothesis that Brucella smooth and rough strains invade macrophage via different pathways [6, 35]. Furthermore, increased uptake is observed at elevated MOIs and prolonged infection time (data not shown). The fourth possibility is that Brucella infection, especially rough Brucella infection activates the macrophages and up-regulate receptor expression [36]. This assumption is supported by the recent report that the presence of smooth or rough Brucella LPS decreased smooth Brucella uptake, which suggested that, as a legand, LPS compete with Brucella cells for binding sites on macrophages [10, 20]. The fact that smooth organisms exhibit increased uptake at either elevated MOIs or as a result of prolonged infection time is most likely an artifact resulting from the presence of spontaneous rough mutants in the culture or simply due to the overwhelming numbers of organisms interacting with the cell, and such experimental conditions should be avoided.
Although S2308 and rough mutant CA180 invade macrophages via different pathways, transient early endosomal association appeared to be similar, suggesting that the organisms start their intracellular trafficking by phagocytic pathway. The difference in smooth and rough Brucella association dynamics is due to the difference in uptake dynamics.
Phagocytes express a variety of cell surface receptors that participate in bacterial recognition and internalization. The cell receptors and molecular mechanisms involved in non-opsonized Brucella uptake have not been clearly identified, although sialic acid-containing molecules and class A scavenger receptor may serve as receptors as recently reported [8, 10]. It has also been reported that opsonized Brucella are internalized via complement and Fc receptors in macrophages and monocytes [16]. However, the participation of such pathways in the establishment or persistence of infection remains to be elucidated. In the current report, B. abortus interaction with target cells was evaluated in the absence of opsonization.
Phagocytosis is extremely complex and many cell receptors, signaling molecules and pathways are involved. In terms of bacterial internalization, different microbes may recognize different receptors, activate different signaling pathways, and trigger diverse cellular processes such as cytoskeletal rearrangement, membrane ruffling, cytokine and chemokine production, and microbial killing mechanisms. Among these molecules, PI3-kinase is known to be involved in phagocytosis and phagosome maturation [25]. However, controversial results have been reported regarding the role of PI3-kinase in Brucella phagocytosis [4, 26, 27]. Our results revealed that PI3-kinase is essential for smooth, but not rough B. abortus uptake by murine macrophage J774.A1, suggesting that uptake mechanisms of smooth and rough Brucella are different in these cells.
Recent studies showed controversial results regarding the role of TLR signals in bacteria uptake and phagosome maturation [28, 37]. Blander et al. reported that TLR signals are essential for bacteria uptake and bacterial phagosome maturation in macrophages [28]. However, Yates and Russell demonstrated that TLR signals are not required for phagosome maturation [37]. Our current study revealed that the uptake of smooth but not rough organisms is TLR4-dependent. The link between TLR4 and lipid raft formation has been documented [38] and the impact of the loss of TLR4 signal is suspected to be a direct result of the failure to form lipid rafts necessary for the uptake of smooth Brucella. Another finding in this study is that smooth B. abortus failed to activate NF-κB translocation to the macrophage nucleus and is undoubtedly responsible for the observed difference in production of cytokines and nitric oxide after smooth and rough Brucella infection [20, 35]. This is consistent with the stealth nature of smooth Brucella [22].
In conclusion, we have provided evidence that Brucella OPS controls the organism uptake process and restricts macrophage activation to establish a replicating niche. In contrast, rough Brucella mutants invade macrophage via different pathways activating macrophage and leading to bacterial destruction [19, 23].
5. Acknowledgements
We thank Helga Sittertz-Bhatkar from Image Analysis Laboratory for her excellent technical help with electron microscopy analysis. This work was supported by grants to TAF from USDA/CSREES (99-35204-7550) and NIH/AID (AI48496).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Pizarro-Cerda J, Moreno E, Gorvel JP. Invasion and intracellular trafficking of Brucella abortus in nonphagocytic cells. Microbes Infect. 2000;2(7):829–35. doi: 10.1016/s1286-4579(00)90368-x. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann MR, Cheville NF, Deyoe BL. Bovine ileal dome lymphoepithelial cells: endocytosis and transport of Brucella abortus strain 19. Vet. Pathol. 1988;25:28–35. doi: 10.1177/030098588802500104. [DOI] [PubMed] [Google Scholar]
- 3.Rittig MG, Alvarez-Martinez MT, Porte F, Liautard JP, Rouot B. Intracellular survival of Brucella spp. in human monocytes involves conventional uptake but special phagosomes. Infect Immun. 2001;69(6):3995–4006. doi: 10.1128/IAI.69.6.3995-4006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzman-Verri C, Chaves-Olarte C, von Eichel-Streiber C, Lopez-Goni I, Thelestam M, Arvidson S, Gorvel JP, Moreno E. GTPases of the Rho subfamily are required for Brucella abortus internalization in nonprofessional phagocytes: direct activation of Cdc42. J Biol Chem. 2001;276(48):44435–43. doi: 10.1074/jbc.M105606200. [DOI] [PubMed] [Google Scholar]
- 5.Detilleux PG, Deyoe BL, Cheville NF. Effect of endocytic and metabolic inhibitors on the internalization and intracellular growth of Brucella abortus in Vero cells. Am J Vet Res. 1991;52(10):1658–64. [PubMed] [Google Scholar]
- 6.Porte F, Naroeni A, Ouahrani-Bettache S, Liautard JP. Role of the Brucella suis Lipopolysaccharide O Antigen in Phagosomal Genesis and in Inhibition of Phagosome-Lysosome Fusion in Murine Macrophages. Infect Immun. 2003;71(3):1481–90. doi: 10.1128/IAI.71.3.1481-1490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusumawati A, Cazevieille C, Porte F, Bettache S, Liautard JP, Sri Widada J. Early events and implication of F-actin and annexin I associated structures in the phagocytic uptake of Brucella suis by the J-774A.1 murine cell line and human monocytes. Microb Pathog. 2000;28(6):343–52. doi: 10.1006/mpat.2000.0354. [DOI] [PubMed] [Google Scholar]
- 8.Castaneda-Roldan E, Avelino-Flores F, Dall’Agnol M, Freer E, Cedillo L, Dornand J, Giron J. Adherence of Brucella to human epithelial cells and macrophages is mediated by sialic acid residues. Cellular Microbiology. 2004;6(5):435–445. doi: 10.1111/j.1462-5822.2004.00372.x. [DOI] [PubMed] [Google Scholar]
- 9.Watarai M, Kim S, Erdenebaatar J, Makino S, Horiuchi M, Shirahata T, Sakaguchi S, Katamine S. Cellular prion protein promotes Brucella infection into macrophages. J Exp Med. 2003;198(1):5–17. doi: 10.1084/jem.20021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S, Watarai M, Suzuki H, Makino S, Kodama T, Shirahata T. Lipid raft microdomains mediate class A scavenger receptor-dependent infection of Brucella abortus. Microb Pathog. 2004;37(1):11–9. doi: 10.1016/j.micpath.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Fontes P, Alvarez-Martinez MT, Gross A, Carnaud C, Kohler S, Liautard JP. Absence of evidence for the participation of the macrophage cellular prion protein in infection with Brucella suis. Infect Immun. 2005;73(10):6229–36. doi: 10.1128/IAI.73.10.6229-6236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sola-Landa A, Pizarro-Cerda J, Grillo MJ, Moreno E, Moriyon I, Blasco JM, Gorvel JP, Lopez-Goni I. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol Microbiol. 1998;29(1):125–38. doi: 10.1046/j.1365-2958.1998.00913.x. [DOI] [PubMed] [Google Scholar]
- 13.Celli J, Gorvel JP. Organelle robbery: Brucella interactions with the endoplasmic reticulum. Curr Opin Microbiol. 2004;7(1):93–7. doi: 10.1016/j.mib.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Castro R, Verdugo-Rodriguez A, Luis Puente J, Suarez-Guemes F. The BMEI0216 gene of Brucella melitensis is required for internalization in HeLa cells. Microb Pathog. 2007 doi: 10.1016/j.micpath.2007.08.008. in press. [DOI] [PubMed] [Google Scholar]
- 15.DelVecchio VG, Kapatral V, Redkar RJ, Patra G, Mujer C, Los T, Ivanova N, Anderson I, Bhattacharyya A, Lykidis A, Reznik G, Jablonski L, Larsen N, D’Souza M, Bernal A, Mazur M, Goltsman E, Selkov E, Elzer PH, Hagius S, O’Callaghan D, Letesson JJ, Haselkorn R, Kyrpides N, Overbeek R. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc Natl Acad Sci U S A. 2002;99(1):443–8. doi: 10.1073/pnas.221575398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorvel JP, Moreno E. Brucella intracellular life: from invasion to intracellular replication. Vet Microbiol. 2002;90(14):281–97. doi: 10.1016/s0378-1135(02)00214-6. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Prada CM, Zelazowska EB, Nikolich M, Hadfield TL, Roop RM, 2nd, Robertson GL, Hoover DL. Interactions between Brucella melitensis and human phagocytes: bacterial surface O-Polysaccharide inhibits phagocytosis, bacterial killing, and subsequent host cell apoptosis. Infect Immun. 2003;71(4):2110–9. doi: 10.1128/IAI.71.4.2110-2119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross A, Terraza A, Ouahrani-Bettache S, Liautard JP, Dornand J. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect Immun. 2000;68(1):342–51. doi: 10.1128/iai.68.1.342-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pei J, Ficht TA. Brucella abortus rough muntants are cytopathic for macrophages in culture. Infection and immunity. 2004;72(1):440–450. doi: 10.1128/IAI.72.1.440-450.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rittig MG, Kaufmann A, Robins A, Shaw B, Sprenger H, Gemsa D, Foulongne V, Rouot B, Dornand J. Smooth and rough lipopolysaccharide phenotypes of Brucella induce different intracellular trafficking and cytokine/chemokine release in human monocytes. J Leukoc Biol. 2003;74(6):1045–55. doi: 10.1189/jlb.0103015. [DOI] [PubMed] [Google Scholar]
- 21.Allen CA, Adams LG, Ficht TA. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect Immun. 1998;66(3):1008–16. doi: 10.1128/iai.66.3.1008-1016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barquero-Calvo E, Chaves-Olarte E, Weiss DS, Guzman-Verri C, Chacon-Diaz C, Rucavado A, Moriyon I, Moreno E. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS ONE. 2007;2:e631. doi: 10.1371/journal.pone.0000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pei J, Turse JE, Wu Q, Ficht TA. Brucella abortus Rough Mutants Induce Macrophage Oncosis That Requires Bacterial Protein Synthesis and Direct Interaction with the Macrophage. Infect Immun. 2006;74(5):2667–75. doi: 10.1128/IAI.74.5.2667-2675.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watarai M, Makino SI, Michikawa M, Yanagisawa K, Murakami S, Shirahata T. Macrophage Plasma Membrane Cholesterol Contributes to Brucella abortus Infection of Mice. Infect Immun. 2002;70(9):4818–4825. doi: 10.1128/IAI.70.9.4818-4825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillooly DJ, Simonsen A, Stenmark H. Phosphoinositides and phagocytosis. J Cell Biol. 2001;155(1):15–7. doi: 10.1083/jcb.200109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S, Watarai M, Makino S, Shirahata T. Membrane sorting during swimming internalization of Brucella is required for phagosome trafficking decisions. Microb Pathog. 2002;33(5):225–37. doi: 10.1006/mpat.2002.0531. [DOI] [PubMed] [Google Scholar]
- 27.Pizarro-Cerda J, Moreno E, Sanguedolce V, Mege JL, Gorvel JP. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect Immun. 1998;66(5):2387–92. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304(5673):1014–8. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 29.Liautard JP, Gross A, Dornand J, Kohler S. Interactions between professional phagocytes and Brucella spp. Microbiologia. 1996;12(2):197–206. [PubMed] [Google Scholar]
- 30.Arellano-Reynoso B, Lapaque N, Salcedo S, Briones G, Ciocchini AE, Ugalde R, Moreno E, Moriyon I, Gorvel JP. Cyclic beta-1,2-glucan is a Brucella virulence factor required for intracellular survival. Nat Immunol. 2005;6(6):618–25. doi: 10.1038/ni1202. [DOI] [PubMed] [Google Scholar]
- 31.Ugalde JE, Czibener C, Feldman MF, Ugalde RA. Identification and characterization of the Brucella abortus phosphoglucomutase gene: role of lipopolysaccharide in virulence and intracellular multiplication. Infect Immun. 2000;68(10):5716–23. doi: 10.1128/iai.68.10.5716-5723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godfroid F, Taminiau B, Danese I, Denoel P, Tibor A, Weynants V, Cloeckaert A, Godfroid J, Letesson JJ. Identification of the perosamine synthetase gene of Brucella melitensis 16M and involvement of lipopolysaccharide O side chain in Brucella survival in mice and in macrophages. Infect Immun. 1998;66(11):5485–93. doi: 10.1128/iai.66.11.5485-5493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowden RA, Cloeckaert A, Zygmunt MS, Bernard S, Dubray G. Surface exposure of outer membrane protein and lipopolysaccharide epitopes in Brucella species studied by enzyme-linked immunosorbent assay and flow cytometry. Infect Immun. 1995;63(10):3945–52. doi: 10.1128/iai.63.10.3945-3952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lerouge I, Vanderleyden J. O-antigen structural variation: mechanisms and possible roles in animal/plant-microbe interactions. FEMS Microbiol Rev. 2002;26(1):17–47. doi: 10.1111/j.1574-6976.2002.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 35.Jimenez De Bagues MP, Terraza A, Gross A, Dornand J. Different Responses of Macrophages to Smooth and Rough Brucella spp.: Relationship to Virulence. Infect Immun. 2004;72(4):2429–33. doi: 10.1128/IAI.72.4.2429-2433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishibashi Y, Claus S, Relman DA. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18) J Exp Med. 1994;180(4):1225–33. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yates RM, Russell DG. Phagosome maturation proceeds independently of stimulation of toll-like receptors 2 and 4. Immunity. 2005;23(4):409–17. doi: 10.1016/j.immuni.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115(Pt 12):2603–11. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]