Abstract
Several N-alkyl and N,N-dialkyl 5H-8,9-dimethoxy-5-(2-aminoethyl)-2,3-methylenedioxydibenzo[c,h][1,6]naphthyridin-6-ones have been identified as topoisomerase I-targeting agents with potent antitumor activity. In the present study, the impact on biological activity of substitution of a trifluoromethyl, cyano, aminocarbonyl, or ethynyl group on a N-methyl substituent of N,N-dimethyl-, N-methyl-N-ethyl-, and N-methyl-N-isopropyl 5H-8,9-dimethoxy-5-(2-aminoethyl)-2,3-methylenedioxydibenzo[c,h][1,6]-naphthyridin-6-ones was assessed.
Keywords: Topoisomerase I, Cytotoxicity, Antitumor, MDA-MB-435, Structure-activity, Non-camptothecin
1. Introduction
Topoisomerases are ubiquitous enzymes that participate in processes such as DNA replication, repair, transcription, and recombination as well as chromosome condensation and segregation.1,2 Topoisomerase I (TOP1) is the target of several antitumor agents based upon their ability to stabilize the enzyme–DNA cleavage complex, which results in DNA damage and ultimately cell death.3–5 Several 5-(2-aminoethyl)dibenzo[c,h][1,6]naphthyridin-6-ones have been identified as topoisomerase I-targeting agents with potent antitumor activity.6,7 One of the more extensively studied of these non-camptothecin TOP1-targeting agents is 5H-8,9-dimethoxy-5-(2-N,N-dimethylaminoethyl)-2,3-methylenedioxydibenzo[c,h][1,6]-naphthyridin-6-one (ARC-111), 1 (Fig. 1).8,9 Analogs of ARC-111 with various 5-[2-(N,N-dialkylaminoethyl)] substituents have exhibited potent activity.9 Compounds 2 and 3 (Fig. 1) are among the tertiary alkylamine analogs that exhibit similar TOP1-targeting activity and cytotoxic activity in RPMI8402 cells to 1 (IC50 values ranging from 2 to 6 nM).
Figure 1.
Structure of ARC-111 and related compounds.
The effect on biological activity of the addition of a trifluoromethyl, cyano, or ethynyl substituent on the N-methyl group of compounds related to ARC-111 was investigated. These studies were extended to examining the effect of an aminocarbonyl substituent on the N-methyl substituent of ARC-111 as well as the replacement of its 8,9-dimethoxy groups with 8,9-diethoxy substituents. These data provide further insight into the structure–activity associated with this family of potent non-camptothecin TOP1-targeting agents.
2. Results
2.1. Chemistry
The syntheses of compounds 4–6 were reported previously. The N-methyl-N-ethyl analog 2 of ARC-111 was prepared by reductive alkylation of 5. Compounds 4, 5, and 6 were treated with trifluoromethanesulfonic acid 2,2,2-trifluoroethyl ester and diisopropylethylamine (DIEA) to afford 7, 8, and 9, respectively (Scheme 1). Alkylation of 4, 5, and 6 using bromoacetonitrile provided compounds 10, 11, and 12, respectively (Scheme 1). Conversion of 4, 5, and 6 to compounds 13, 14, and 15 were carried out in DMF at 80 °C using 3-bromopropyne and anhydrous potassium carbonate (Scheme 1).
Scheme 1.
Preparation of 7–9, 10–12, and 13–15.
Compound 16 was synthesized from 4 by reaction with bromoacetamide and potassium carbonate as outlined in Scheme 2.
Scheme 2.
Preparation of 16.
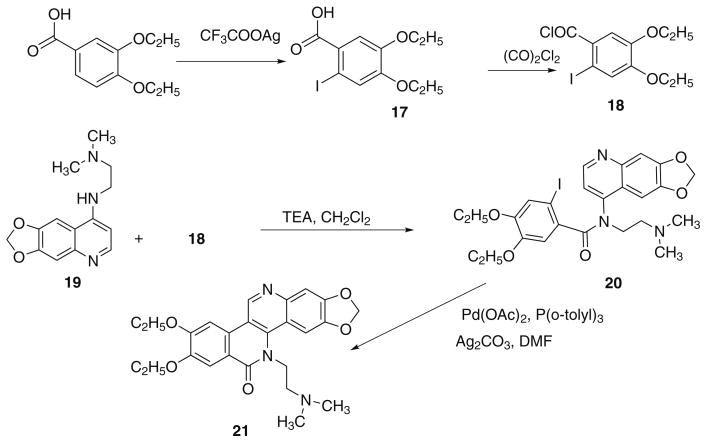
The preparation of the diethoxy analog of 1 was performed as outlined in Scheme 3. Using similar methodology as previously described,9 3,4-diethoxybenzoic acid was treated with iodine and silver trifluoroacetate in CHCl3 to yield 4,5-diethoxy-2-iodobenzoic acid 17 in 60% yield. Conversion of 17 to its acid chloride 18 was carried out in anhydrous CH2Cl2 with oxalyl chloride. Without further purification 18 was added directly to the solution of appropriate 4-amino-6,7-methylenedioxyquinoline7 and TEA in CH2Cl2. Intramolecular Heck cyclization of iodobenzamide 20 was performed in refluxing DMF for 2 h to afford 21 in 34% yield, using Pd(OAc)2, P(o-tolyl)3, and Ag2CO3.
Scheme 3.
Preparation of 21.
3. Results and discussion
The relative TOP1-targeting activities of the various N-substituted 5-[2-(N-alkylamino)ethyl]dibenzo[c,h][1,6]naphthyridines are listed in Table 1. The N-(2,2,2-trifluoroethyl) derivatives, 7–9, did not exhibit appreciable TOP1-targeting activity. The basis for this loss in activity cannot be explained on the basis of steric factors in light of the potent TOP1-targeting activity observed for 1–3.9 In contrast to these data, the cyano derivatives, 10–12, were highly active TOP1-targeting agents with similar potency to ARC-111 and camptothecin. The N-methyl-N-propargyl derivative, 13, and the N-methyl-N-acetamide derivative, 16, also retained potent TOP1-targeting activity relative to that observed for 1–3. The N-ethyl-N-propargyl derivative, 14, and the N-isopropyl-N-propargyl derivative, 15 were much less active as TOP1-targeting agents. The diethoxy analog of ARC-111, 21, exhibited a dramatic loss in TOP1-targeting activity.
Table 1.
TOP1-targeting activity and cytotoxicity in RPMI8402 and P388 cells and their camptothecin-resistant variants
| Compound | TOP1-mediated cleavagea | Cytotoxicity IC50 (μM) | |||
|---|---|---|---|---|---|
| RPMI8402 | CPT-K5 | P388 | P388/CPT 45 | ||
| 1 | 0.3 | 0.002 | 0.90 | 0.001 | 0.23 |
| 7 | >10 | 0.25 | >10 | 0.25 | >10 |
| 8 | >10 | 0.7 | >10 | 0.61 | >10 |
| 9 | >10 | 1.9 | >10 | 1.1 | >10 |
| 10 | <0.2 | 0.004 | 0.98 | 0.003 | 0.22 |
| 11 | <0.2 | 0.001 | 0.67 | 0.001 | 0.13 |
| 12 | <0.2 | 0.002 | 1.3 | 0.002 | 0.06 |
| 13 | 0.1 | 0.008 | 4.5 | 0.02 | 0.4 |
| 14 | >10 | 0.017 | >10 | 0.035 | 2.3 |
| 15 | >10 | 0.065 | >10 | 0.08 | 3.7 |
| 16 | 0.3 | 0.003 | 3.5 | 0.009 | 0.39 |
| 21 | >10 | 0.15 | 0.14 | 0.015 | 0.013 |
| CPT | 0.2 | 0.006 | >10 | 0.014 | >10 |
| Topotecan | 1.0 | 0.021 | >10 | 0.045 | >10 |
Topoisomerase I cleavage values are reported as REC, relative effective concentration. These are concentrations relative to topotecan, whose value is arbitrarily assumed as 1, that are able to produce 10% cleavage of the plasmid DNA in the presence of human topoisomerase I.
Those derivatives with potent TOP1-targeting activity also exhibited the more pronounced cytotoxicity. The cytotoxicity of compounds 10–12 did not vary dramatically from one another with IC50 values that ranged from 3 to 7 nM and 2 to 4 nM in RPMI8402 and P388, respectively. Compound 13 was less cytotoxic with IC50 values that ranged from 2-to 20-fold higher than 10–12 in these cell lines. Compounds 14 and 15 had similar cytotoxicity in RPMI8402 to each other, but were 2- to 6-fold less cytotoxic than 13. The aminocarbonyl derivative, 16, exhibited similar cytotoxicity to 10–12.
CPT-K5 cell line, a variant of RPMI8402 cells, and P388/CPT45 a variant of P388 have been used to investigate the role of TOP1 in the mechanism of cytotoxicity of suspect TOP1-targeting agents.10,11 Resistance to CPT-K5 cells is associated with its mutant form of the enzyme TOP1 as well as its ability to expresses the efflux transporter BCRP.12 Resistance to P388/CPT45, which has minimal expression of TOP1, is consistent with TOP1-targeting as the primary mechanism associated with cytotoxic activity. With the notable exception of 21, these results suggest that TOP1-targeting is associated with cytotoxicity, even in the case of those derivatives with comparatively weak TOP1-targeting activity.
Data are provided in Table 2 on the relative cytotoxicity in the parent cell line, KB3-1, KBV-1, a variant that overexpresses the efflux transporter MDR1 (ABCB1/P-glycoprotein/PGY1/CD243), and KBH5.0, a variant that overexpresses BCRP (ABCG2/MXR/ABCP1). Differences in relative cytotoxicity between these variant cell lines and the parent cell line, KB3-1, may be indicative of a compound that is a substrate for an efflux transporter.13,14 Topotecan has been shown to be substrates for the MDR1 efflux transporter.13 Topotecan, as well as the active metabolite of irinotecan, SN-38, are resistant to tumor cells that express BCRP.15,16 In light of their 7-fold difference in IC50 values between KB3-1 cells and KBV-1 cells, the data suggest that 10–12 and 16 are substrates for MDR1. While not among the more potent cytotoxic agents, 13 does not appear to be a substrate for either MDR1 or BCRP. It is of interest to note that, of the compounds evaluated; only 16 appears to be a substrate for BCRP.
Table 2.
Cytotoxicity of N-substituted 5-[2-(N-alkylamino)ethyl]dibenzo[c,h][1,6]naphthyridines in KB3-1 cells and its variant cell lines, KBV-1 and KBH5.0
| Compound | Cytotoxicity IC50 (μM) | ||
|---|---|---|---|
| KB3-1 | KBV-1 | KBH5.0 | |
| 1 | 0.005 | 0.005 | 0.006 |
| 7 | 0.41 | 1.0 | 0.63 |
| 8 | 0.65 | 2.9 | 0.75 |
| 9 | 1.0 | 4.4 | 1.1 |
| 10 | 0.005 | 0.043 | 0.027 |
| 11 | 0.004 | 0.034 | 0.007 |
| 12 | 0.006 | 0.043 | 0.009 |
| 13 | 0.02 | 0.07 | 0.034 |
| 14 | 0.07 | 0.1 | 0.11 |
| 15 | 0.10 | 0.43 | 0.07 |
| 16 | 0.01 | 0.38 | 0.32 |
| 21 | 0.07 | 0.19 | 0.1 |
| Topotecan | 0.04 | 0.44 | 0.44 |
Compounds 8 and 10–16 were evaluated for antitumor activity in vivo in athymic nude mice with MDA-MB-435 human tumor xenografts. The results of this bioassay are outlined in Table 3 and are illustrated in Figures 2–4.
Table 3.
Antitumox activity in athymic nude mice with the human tumor xenograft MDA-MB-435
| Compound | Route | Average tumor volume (mm3) | Total dose (mg/kg)/mouse | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 | Day 49 | |||
| 8 | I.P.a | 127 | 176 | 268 | 310 | 435 | 543 | 718 | 111 |
| 10 | I.P.a | 149 | 222 | 370 | 392 | 528 | 669 | 919 | 111 |
| 11 | I.P.a | 100 | 156 | 178 | 202 | 293 | 361 | 435 | 117 |
| 12 | I.P.b | 142 | 175 | 213 | 220 | 250 | 286 | 314 | 168 |
| 13 | I.P.c | 144 | 167 | 218 | 284 | 317 | 358 | 433 | 223 |
| 14 | I.P.a | 115 | 106 | 170 | 204 | 331 | 349 | 488 | 117 |
| 15 | I.P.a | 99 | 112 | 165 | 170 | 234 | 310 | 357 | 117 |
| 16 | I.P.d | 147 | 178 | 230 | 319 | 344 | 434 | 527 | 301 |
| Vehicle | I.P.e | 126 | 181 | 332 | 408 | 573 | 747 | 966 | |
| 1 | I.P.f | 120 | 123 | 145 | 158 | 175 | 189 | 242 | 37.5 |
Initial dose was 2.0 mg/kg qd × 5/week for 2 weeks and was gradually increased to 6.0 mg/kg × 5/week.
Initial dose was 5.0 mg/kg qd × 5/week and was increased to 6.0 mg/kg qd × 5/week for 2 weeks, then adjusted to 6.0 mg/kg qd × 3/week for one week. This dose was then again modified to the initial dose of 5.0 mg/kg qd × 5/week.
Initial dose was 5.0 mg/kg qd × 5/week for 1 week and was gradually increased to 7.0 mg/kg × 5/week.
Initial dose was 5.0 mg/kg qd × 5/week for one and a half weeks and was increased to 6.0 mg/kg qd × 5/week for two and a half weeks. Administration was increased to 14.0 mg/kg qd × 5/week for one and a half weeks then adjusted back to 10.0 mg/kg qd × 5/week for 1 week and finally adjusted to 12.0 mg/kg qd × 5/week.
Vehicle consisted of 0.1% citrate in H2O and was administered qd × 5/week.
Initial dose was 1.5 mg/kg, which was administered qd × 3/5 days.
Figure 2.
Antitumor activity of compounds 10, 13, 16, vehicle, and ARC-111.
Figure 4.
Antitumor activity of compounds 12 and 15, vehicle, and ARC-111.
Figure 2 clearly illustrates that the N-methyl-N-cyanomethyl analog 10 is less active than the N-methyl-N-propargyl 13 or N-methyl-N-acetamide 16 at their maximally tolerated doses. The N-methyl-N-2,2,2-trifluoroethyl analog 8 was also less efficacious than the N-ethyl-N-cyanomethyl analog 11 or the N-ethyl-N-propargyl derivative 14 as illustrated in Figure 3. While in vitro studies did indicate that 14 and 15 were less cytotoxic than their structurally related cyano derivatives, 11 and 12, this did result in notable differences in their antitumor activity in vivo. Both 11 and 14 had similar antitumor activity, but were less potent and effective than ARC-111 in this bioassay. The N-isopropyl-N-cyanomethyl and the N-isopropyl-N-propargyl derivatives, 12 and 15, respectively, also had similar antitumor activity (Fig. 4). Both of these derivatives, however, appear from these in vivo studies to be less potent and less efficacious than ARC-111 (Table 3).
Figure 3.
Antitumor activity of compounds 8, 11, 14, vehicle, and ARC-111.
These comparative studies did not result in the identification of an analog of ARC-111 with comparable in vivo potency and efficacy. The presence of electron-withdrawing substituents on the N-methyl substituents of these various analogs of compounds 1–3 did negatively affect the relative ease with which they could be formulated for injection. The decreased basicity of these derivatives lessened the solubility of their citrate salts and may have also impacted their absorption and distribution. Further studies are in process to assess the influence of other more polar substituents on the amino group of the 5-(2-aminoethyl) substituent of ARC-111 and related compounds.
4. Experimental
Melting points were determined with either a Thomas-Hoover Unimelt or Meltemp capillary melting point apparatus. Column chromatography refers to flash chromatography conducted on Sil-iTech 32–63 μm, (ICN Biomedicals, Eschwege, Germany) using the solvent systems indicated. Proton (1H NMR) and carbon (13C NMR) nuclear magnetic resonance were recorded on a Varian Gemini-2000 Fourier Transform spectrometer. Infrared spectral data were obtained using a Thermo-Nicolet Avatar 360 Fourier transform spectrophotometer and are reported in cm−1. NMR spectra (200 MHz 1H and 50 MHz 13C) were recorded in the deuterated solvent indicated with chemical shifts reported in δ units downfield from tetramethylsilane (TMS). Coupling constants are reported in hertz (Hz). Mass spectra were obtained from Washington University Resource for Biomedical and Bio-organic Mass Spectrometry within the Department of Chemistry at Washington University, St. Louis, MO, USA. All starting materials and reagents were purchased from Aldrich. Solvents were purchased from Fisher Scientific, and were A.C.S. grade or HPLC grade. Methylene chloride was freshly distilled from calcium hydride. All other solvents were used as provided without further purification. Compounds 5–7 were prepared as previously described.9 Methods for the preparation of intermediate 19 have also been previously reported.7 Trifluoromethanesulfonic acid 2,2,2-trifluoroethyl ester was purchased from TCI America (Portland, OR).
4.1. 2,3-Methylenedioxy-8,9-dimethoxy-5-[N-ethyl-N-methyl aminoethyl]dibenzo[c,h][1,6]naphthyridin-6-one (2)
A mixture of 5 (150 mg, 0.37 mmol), acetaldehyde (0.2 mL, 3.38 mmol), and sodium cyanoborohydride (78 mg, 1.1 mmol) in ethanol (15 mL) was heated to 75 °C in a sealed tube for 6 h. The mixture was filtered and the filtrate was evaporated. The residue was partitioned between chloroform (50 mL) and 10% NaOH (30 mL), and the aqueous phase was extracted with chloroform (2× 50 mL). The combined organic phases were evaporated and chromatographed through a short column of silica eluting with 97:3 chloroform/methanol, to provide 89 mg (55% yield); mp 251–253 °C; IR (neat) 1650; 1H NMR (CDCl3): δ 1.01 (t, J = 7.4, 3H), 2.27 (s, 3H), 2.46 (q, J = 7.4, 2H), 3.01 (t, J = 7, 2H), 4.04 (s, 3H), 4.10 (s, 3H), 4.60 (t, J = 7, 2H), 6.15 (s, 2H); 7.44 (s, 1H), 7.65 (s, 1H), 7.87 (s, 1H), 7.92 (s, 1H), 9.34 (s, 1H), 13C NMR (CDCl3 + CD3OD) δ 11.1, 40.9, 47.8, 50.8, 53.9, 55.1, 55.3, 100.0, 101.0, 101.3, 105.2, 107.6, 110.0, 113.7, 118.1, 126.5, 139.9, 142.1, 145.6, 146.8, 149.1, 149.3, 153.3, 163.2; HRMS (M++H) Calcd for C24H25N3O5H: 436.1872; found: 436.1874.
4.2. General procedure for the preparation of 2,3-methylenedioxy-8,9-dimethoxy-5-[N-alkyl-N-(2,2,2-trifluoro ethyl)aminoethyl]dibenzo[c,h][1,6]naphthyridin-6-ones (7–9)
To a mixture of 2,3-methylenedioxy-8,9-dimethoxy-5-[2-alkylaminoethyl]dibenzo[c,h]-[1,6]naphthyridin-6-one (1.0 mmol equiv) in DMF (8 mL per mmol equiv) was added DIEA (20.0 mmol equiv) and 2,2,2-trifluoroethyl trifluoromethanesulfonate (5.0 mmol equiv) at room temperature. The resulting reaction mixture was heated to 80 °C with stirring. The reaction mixture was allowed to cool to room temperature, and then diluted with CHCl3. The CHCl3 solution was washed with water, then brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was chromatographed on silica gel using dichloromethane:methanol.
4.2.1. 2,3-Methylenedioxy-8,9-dimethoxy-5-[N-methyl-N-(2,2,2-trifluoroethyl)aminoethyl]dibenzo[c,h][1,6] naphthyridin-6-one (7)
Prepared from 4 (50 mg, 0.12 mmol); (43% yield); reaction time 4 h; mp 237–239 °C; IR (neat): 1647; 1H NMR (CDCl3) δ 2.43 (s, 3H), 3.04 (q, 2H, J = 9.4), 3.22 (t, 2H, J = 6.8), 4.04 (s, 3H), 4.11 (s, 3H), 4.62 (t, 2H, J = 6.8), 6.16 (s, 2H), 7.44 (s, 1H), 7.64 (s, 1H), 7.70 (s, 1H), 7.84 (s, 1H), 9.33 (s, 1H); 13C NMR (CDCl3) δ 42.2, 47.9, 55.2, 55.4, 55.4, 57.3 (q, J = 30.8), 99.8, 101.0, 101.3, 106.2, 107.7, 110.9, 113.8, 118.3, 124.6 (q, J = 279.6), 126.7, 140.0, 142.5, 146.2, 146.8, 149.0, 149.3, 153.2, 163.3; HRMS m/z Calcd for C25H27N3O5H: 490.1590; found: 490.1567.
4.2.2. 2,3-Methylenedioxy-8,9-dimethoxy-5-[N-ethyl-N-(2,2,2-trifluoroethyl)aminoethyl]dibenzo[c,h][1,6]naphthyridin-6-one (8)
Prepared from 5 (100 mg, 0.20 mmol); (31% yield); reaction time 7 h; mp 246–248 °C; IR (neat): 1644; 1H NMR (CDCl3) δ 0.98 (t, 3H, J = 7.0), 2.72 (q, 2H, J = 7.0), 3.11 (q, 2H, J = 9.6), 3.26 (t, 2H, J = 7.4), 4.05 (s, 3H), 4.12 (s, 3H), 4.60 (t, 2H, J = 7.4), 6.17 (s, 2H), 7.44 (s, 1H), 7.64 (s, 1H), 7.70 (s, 1H), 7.85 (s, 1H), 9.34 (s, 1H); 13C NMR (CDCl3) δ 11.1, 48.1, 48.2, 52.1, 53.8 (q, J = 30.4), 55.4, 55.4, 99.8, 100.9, 101.4, 106.2, 107.7, 110.8, 113.7, 118.3, 124.7 (q, J = 279.4), 126.7, 139.8, 142.5, 146.3, 146.8, 149.0, 149.3, 153.2, 163.3; HRMS m/z Calcd for C25H24F3N3O5H: 504.1746; found: 504.1748.
4.2.3. 2,3-Methylenedioxy-8,9-dimethoxy-5-[N-isopropyl-N-(2,2,2-trifluoroethyl)aminoethyl]dibenzo[c,h][1,6] naphthyridin-6-one (9)
Prepared from 6 (50 mg, 0.12 mmol); (52% yield); reaction time 7 h; mp 258–261 °C; IR (neat): 1650; 1H NMR (CDCl3) δ 1.01 (d, 6H, J = 6.6), 3.0 (m, 1H), 3.07 (q, 2H, J = 9.6), 3.24 (t, 2H, J = 7.6), 4.05 (s, 3H), 4.12 (s, 3H), 4.58 (t, 2H, J = 7.6), 6.17 (s, 2H), 7.46 (s, 1H), 7.67 (s, 1H), 7.73 (s, 1H), 7.87 (s, 1H), 9.37 (s, 1H); 13C NMR (CDCl3) δ 17.4, 48.9, 49.2, 51.3 (q, J = 31.9), 51.8, 55.4, 55.4, 99.8, 100.9, 101.3, 106.2, 107.7, 110.8, 113.8, 118.3, 124.6 (q, J = 279.6), 126.8, 139.9, 142.5, 146.2, 146.8, 149.0, 149.3, 153.2, 163.3; HRMS m/z Calcd for C26H26F3N3O5H: 518.1903; found: 518.1903.
4.3. General procedure for the preparation of 2,3-methylenedioxy-8,9-dimethoxy-5-[N-alkyl-N-cyanomethyl aminoethyl]dibenzo[c,h][1,6]naphthyridin-6-ones (10–12)
To a solution of 2,3-methylenedioxy-8,9-dimethoxy-5-[2-alkylaminoethyl]dibenzo[c,h][1,6]naph-thyridin-6-one (1.0 mmol equiv) in DMF (8 mL per mmol equiv) was added potassium carbonate (20.0 mmol equiv) and bromoacetonitrile (3.0 mmol equiv) at room temperature. The resulting reaction mixture was heated at 75 °C with stirring. The reaction mixture was allowed to cool to room temperature, and then diluted with CHCl3. The CHCl3 solution was washed with water, and then brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography using dichloromethane: methanol.
4.3.1. 2,3-Methylenedioxy-8,9-dimethoxy-5-[N-cyanomethyl-N-methylaminoethyl]dibenzo[c,h][1,6]naphthyridin-6-one (10)
Prepared from 4 (50 mg, 0.12 mmol); (64% yield); reaction time 35 min; mp 235–236 °C; IR (neat): 2239, 1639; 1H NMR (CDCl3): δ 2.32 (s, 3H), 3.07 (t, 2H, J = 6.6 Hz), 3.43 (s, 2H), 4.08 (s, 3H), 4.14 (s, 3H), 4.70 (t, 2H, J = 6.6 Hz), 6.20 (s, 2H), 7.50 (s, 1H), 7.66 (s, 1H), 7.69 (s, 1H), 7.89 (s, 1H), 9.39 (s, 1H); 13C NMR (CDCl3 + CD3OD): δ 35.9, 40.8, 44.7, 53.0, 55.4, 99.4, 101.1, 105.3, 107.7, 111.1, 113.7, 116.1, 118.1, 126.3, 140.0, 142.2, 145.5, 147.0, 149.2, 149.4, 153.2, 163.5; HRMS m/z Calcd for C24H22N4O5H 447.1668; found: 447.1660.
4.3.2. 2,3-Methylenedioxy-8,9-dimethoxy-5-[N-cyanomethyl-N-ethylaminoethyl]dibenzo[c,h][1,6]naphthyridin-6-one (11)
Prepared from 5 (100 mg, 0.24 mmol); (80% yield); reaction time 40 min; mp 226–227 °C; IR (neat): 2285, 1634; 1H NMR (CDCl3): δ 0.83 (t, 3H, J = 7.2), 2.49 (q, 2H, J = 7.2), 3.05 (t, 2H, J = 6.6), 3.42 (s, 2H), 4.08 (s, 3H), 4.14 (s, 3H), 4.72 (t, 2H, J = 6.6), 6.20 (s, 2H), 7.51 (s, 1H), 7.67 (s, 1H), 7.68 (s, 1H), 7.89 (s, 1H), 9.38 (s, 1H); 13C NMR (CDCl3 + CD3OD): δ 11.4, 40.8, 47.2, 47.3, 50.9, 55.2, 55.4, 99.4, 100.0, 101.6, 104.9, 107.6, 111.2, 113.7, 114.0, 118.0, 126.2, 140.1, 141.7, 144.8, 147.0, 149.4, 153.3, 163.3; HRMS m/z Calcd for C25H24N4O5H 461.1825; found: 461.1826.
4.3.3. 2,3-Methylenedioxy-8,9-dimethoxy-5-[N-cyanomethyl-N-isopropyl-aminoethyl]dibenzo[c,h][1,6]naphthyridin-6-one (12)
Prepared from 6 (100 mg, 0.23 mmol); (49% yield); reaction time 90 min; mp 219–220 °C; IR (neat): 2287, 1644; 1H NMR (CDCl3): δ 0.90 (d, 6H, J = 6.6), 2.78 (m, 1H), 3.12 (t, 2H, J = 6.6), 3.41 (s, 2H), 4.07 (s, 3H), 4.14 (s, 3H), 4.68 (t, 2H, J = 6.6), 6.19 (s, 2H), 7.48 (s, 1H), 7.66 (s, 2H), 7.88 (s, 1H), 9.36 (s, 1H); 13C NMR (CDCl3): δ 18.3, 37.7, 47.3, 47.6, 52.4, 55.4, 99.4, 100.9, 101.4, 105.7, 107.9, 111.1, 113.7, 116.1, 118.3, 126.3, 140.0, 142.0, 145.5, 146.9, 149.2, 149.4, 153.2, 163.3; HRMS m/z Calcd for C26H26N4O5H 475.1981; found: 475.1982.
4.4. General procedure for the preparation of 2,3-methylenedioxy-8,9-dimethoxy-5-[N-alkyl-N-(prop-2-ynyl)aminoethyl]dibenzo[c,h][1,6]naphthyridin-6-ones (13–15)
To a mixture of 2,3-methylenedioxy-8,9-dimethoxy-5-[2-alkylaminoethyl]dibenzo[c,h][1,6]naph-thyridin-6-one (1.0 mmol equiv) in DMF (8 mL per mmol equiv) was added potassium carbonate (20.0 mmol equiv) and 3-bromopropyne (3.0 mmol equiv) at room temperature. The resulting reaction mixture was heated up to 80 °C with stirring. The reaction mixture was allowed to cool to room temperature, and then diluted with CHCl3. The CHCl3 solution was washed with water, then brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was chromatographed on silica gel using dichloromethane:methanol.
4.4.1. 2,3-Methylenedioxy-8,9-dimethoxy-5-[N-methyl-N-(prop-2-ynyl)aminoethyl]dibenzo[c,h][1,6]naphthyridin-6-one (13)
Prepared from 4 (100 mg, 0.25 mmol); (65% yield); reaction time 30 min; mp 239–241 °C; IR (neat): 1648; 1H NMR (CDCl3) δ 2.22 (t, 1H, J = 2.2), 2.34 (s, 1H), 3.09 (t, 2H, J = 6.6), 3.30 (d, 2H, J = 2.2), 4.04 (s, 3H), 4.11 (s, 3H), 4.61 (t, 2H, J = 6.6), 6.16 (s, 2H), 7.42 (s, 1H), 7.65 (s, 1H), 7.77 (s, 1H), 7.85 (s, 1H), 9.31 (s, 1H); 13C NMR (CDCl3) δ 42.0, 46.4, 48.9, 54.0, 56.3, 56.3, 73.4, 78.6, 101.0, 102.0, 102.2, 107.1, 108.8, 111.7, 114.8, 119.3, 127.6, 140.9, 143.4, 147.2, 147.7, 149.9, 150.2, 154.1, 164.1; HRMS m/z Calcd for C25H23N3O5H: 446.1716; found: 446.1716.
4.4.2. 2,3-Methylenedioxy-8,9-dimethoxy-5-[N-ethyl-N-(prop-2-ynyl)aminoethyl]dibenzo[c,h][1,6]naphthyridin-6-one (14)
Prepared from 5 (100 mg, 0.20 mmol); (64% yield); reaction time 60 min; mp 227–229 °C; IR (neat): 1648; 1H NMR (CDCl3) δ 0.95 (t, 3H, J = 7.4), 2.18 (t, 1H, J = 2.2), 2.56 (q, 2H, J = 7.4), 3.12 (t, 2H, J = 7.0), 3.33 (d, 2H, J = 2.2), 4.04 (s, 3H), 4.10 (s, 3H), 4.56 (t, 2H, J = 7.0), 6.15 (s, 2H), 7.41 (s, 1H), 7.59 (s, 1H), 7.78 (s, 1H), 7.84 (s, 1H), 9.28 (s, 1H); 13C NMR (CDCl3) δ 12.7, 42.1, 47.9, 49.1, 51.6, 56.3, 56.3, 73.0, 78.7, 101.1, 101.9, 102.2, 107.0, 108.8, 111.6, 114.7, 119.3, 127.4, 140.8, 143.3, 147.1, 147.6, 149.8, 150.2, 154.0, 164.0; HRMS m/z Calcd for C26H25N3O5H: 460.1872; found: 460.1890.
4.4.3. 2,3-Methylenedioxy-8,9-dimethoxy-5-[N-isopropyl-N-(prop-2-ynyl)aminoethyl]dibenzo-[c,h][1,6]naphthyridin-6-one (15)
Prepared from 6 (100 mg, 0.23 mmol); (58% yield); reaction time 60 min; mp 217–219 °C; IR (neat): 1649; 1H NMR (CDCl3) δ 0.98 (t, 6H, J = 6.6), 2.13 (t, 1H, J = 1.8), 2.93 (m, 1H,), 3.16 (t, 2H, J = 7.0), 3.33 (d, 2H, J = 1.8), 4.04 (s, 3H), 4.11 (s, 3H), 4.58 (t, 2H, J = 7.0), 6.15 (s, 2H), 7.41 (s, 1H), 7.60 (s, 1H), 7.80 (s, 1H), 7.85 (s, 1H), 9.29 (s, 1H); 13C NMR (CDCl3) δ 19.5, 39.6, 48.1, 49.7, 52.4, 56.3, 56.3, 72.4, 80.8, 101.2, 101.9, 102.2, 107.0, 108.8, 111.7, 114.8, 119.4, 127.6, 141.0, 143.4, 147.1, 147.6, 149.8, 150.2, 154.1, 164.1; HRMS m/z Calcd for C27H28N3O5H: 474.2029; found: 474.2031.
4.5. 2,3-Methylenedioxy-8,9-dimethoxy-5-[N-(2-amino-2-oxoethyl)-N-methylaminoethyl]dibenzo[c,h][1,6]naphthyridin-6-ones (16)
Prepared from 4 (150 mg, 0.37 mmol) and bromoacetamide (56 mg, 0.41 mmol); reaction time 40 min; (47% yield); mp 228–229 °C; IR (neat): 3423, 1671, 1647; 1H NMR (CDCl3 + CD3OD): δ 2.17 (s, 3H), 2.93 (s, 2H), 3.00 (t, 2H, J = 6.6 Hz), 4.00 (s, 3H), 4.08 (s, 3H), 4.14 (s, 3H), 4.63 (t, 2H, J = 6.6 Hz), 6.14 (s, 2H), 7.39 (s, 1H), 7.63 (s, 1H), 7.65 (s, 1H), 7.80 (s, 1H), 9.30 (s, 1H); 13C NMR (CDCl3 + CD3OD): δ 41.9, 47.2, 47.6, 55.2, 60.2, 99.4, 101.1, 101.5, 105.2, 107.6, 111.5, 113.7, 116.0, 118.0, 126.3, 141.0, 142.0, 144.8, 147.0, 149.5, 153.4, 163.3, 171.4; HRMS m/z Calcd for C24H24N4O6H 465.1774; found: 465.1791.
4.6. 4,5-Diethoxy-2-iodobenzoic acid (17)
To a suspension of 3,4-diethoxybenzoic acid (1.8 g, 8.6 mmol) and silver trifluoroacetate (2.0 g, 9.02 mmol) in 15 mL CHCl3 was added I2 (2.29 g, 9.0 mmol) portionwise at 0 °C. The resulting reaction mixture was warmed up to room temperature with stirring overnight. The reaction mixture was quenched with 10 mL water. The organic layer was washed with satd Na2S2O3 solution (2× 10 mL), brine (1 × 10 mL), dried over Na2SO4, and concentrated under reduced pressure. The residue was chromatographed on silica gel using ethyl acetate/hexanes (1:1), yielding 1.4g in 50% yield as a light brown solid; mp 195–197 °C; IR (neat): 1694; 1H NMR (CDCl3) δ 1.49 (m, 6H), 4.14 (m, 4H), 7.44 (s, 1H), 7.63 (s, 1H), 9.2 (s, 1H); 13C NMR (CDCl3) δ 13.7, 13.7, 63.8, 64.0, 84.7, 115.4, 124.4, 124.4, 147.2, 151.6, 170.5; HRMS m/z Calcd for C11H13IO4H: 334.9775; found: 334.9784.
4.7. N-([1,3]Dioxolo[4,5-g]quinolin-8-yl)-N-(2-(dimethylamino)ethyl)-4,5-diethoxy-2-iodobenzamide (20)
Oxalyl chloride (1.3 mL, 11.3 mmol) was added to a mixture of 2-iodo-4,5-diethoxybenzoic acid (1.95 g, 5.7 mmol) in methylene chloride (40 mL), and the mixture was heated to reflux under nitrogen with stirring for 4 h. The mixture was concentrated to dryness under vacuum to provide crude 18. The acid chloride was used without purification and redissolved in 40 mL of methylene chloride, and a solution of 197 (1.24 g, 4.78 mmol) added, then triethylamine (2.0 mL, 27 mmol) was added. The mixture was heated to reflux with stirring for 16 h and was then cooled to room temperature. The mixture was washed with saturated NaHCO3 (3× 100 mL) and extracted into dilute aqueous HCl (3× 100 mL). The combined aqueous phases were washed with chloroform (2× 100 mL), basified (30% NaOH), and extracted with chloroform (3 × 100 mL). The combined organic layers were washed with brine (100 mL), dried (Mg2SO4), and evaporated, yielding 2.6 g in 94% yield as a light brown sticky glue; IR (neat): 1654. 1H NMR (CDCl3) δ 1.00 (t, 3H, J = 7.0), 1.28 (t, 3H, J = 7.0), 2.19 (s, 6H), 2.55 (m, 2H), 3.25 (m, 1H), 3.52 (q, 2H, J = 7.0), 3.85 (q, 2H, J = 7.0), 4.44 (m, 1H), 6.09 (m, 2H), 6.31 (s, 1H), 6.97 (s, 1H), 7.25 (d, 1H, J = 4.6), 7.29 (s, 1H), 7.35 (s, 1H), 7.25 (d, 1H, J = 4.6); 13C NMR (CDCl3) δ 14.3, 14.6, 45.6, 46.9, 56.6, 64.3, 64.6, 82.8, 98.5, 102.2, 106.7, 112.3, 120.2, 123.0, 123.2, 133.6, 146.0, 147.5, 148.3, 148.4, 149.0, 149.6, 151.0, 170.0; HRMS m/z Calcd for C25H28IO5N3H: 578.1146; 2029; found: 578.1149.
4.8. 2,3-Methylenedioxy-8,9-diethoxy-5-(2-N,N-dimethylaminoethyl)dibenzo[c,h][1,6]naphthyridin-6-one (21)
A mixture of 19 (1.5 g, 2.6 mmol), Pd(OAc)2 (117 mg, 0.52 mmol), P(o-tolyl)3 (390 mg, 1.3 mmol), and Ag2CO3 (1.43 g, 5.2 mmol) in dimethylformamide (DMF) (35 mL) was heated to reflux with stirring for 30 min. The reaction mixture was cooled, diluted with chloroform, and filtered through Celite, and the solvent was removed under vacuum. The crude residue was chromatographed in 99:1 chloroform/methanol to provide 500 mg (34%) of the cyclized product as a white solid; mp 229–232 °C; IR (neat): 1649; 1H NMR (CDCl3) δ 1.58 (m, 6H), 2.38 (s, 6H), 2.99 (t, 2H, J = 6.6), 4.30 (m, 4H), 4.58 (t, 2H, J = 6.6), 6.16 (s, 2H), 7.43 (s, 1H), 7.63 (s, 1H), 7.85 (s, 1H), 9.31 (s, 1H); 13C NMR (CDCl3) δ 14.7, 14.7, 45.8, 49.1, 57.9, 64.7, 64.9, 101.2, 102.2, 103.2, 107.0, 109.8, 111.7, 114.8, 119.1, 127.3, 140.8, 143.5, 147.2, 147.6, 149.8, 149.9, 153.8, 164.1; HRMS m/z Calcd for C29H27O5N3H: 450.2029; found: 450.2030.
4.9. Topoisomerase-mediated DNA cleavage assays
Human topoisomerase I was expressed in Escherichia coli and isolated as a recombinant fusion protein using a T7 expression system as described previously.17 Plasmid YepG was also purified by the alkali lysis method followed by phenol deproteination and CsCl/ethidium isopycnic centrifugation method as described.18 The 3′ endlabeling of the plasmid was accomplished by digestion with a restriction enzyme followed by end filling with Klenow polymerase as previously described.19 The cleavage assays were performed as previously reported.20,21 The drug and the DNA in presence of topoisomerase I was incubated for 30 min at room temperature. The reactions were terminated by the addition of 5 μL of 5% SDS and 1 mg/mL protein kinase K with an additional 1 h of incubation at 37 °C. Samples were then alkali denatured by the addition of NaOH, EDTA, sucrose, and bromophenol blue to final concentrations of 75 mM, 2.5%, and 0.05 mg/mL, respectively, prior to loading onto a neutral agarose gel. After development of the gels, typically 24-h exposure was used to obtain autoradiograms outlining the extent of DNA fragmentation. Topoisomerase I-mediated DNA cleavage values are reported as relative effective concentration (REC), that is, concentrations relative to camptothecin, whose value is arbitrarily assumed as 0.2, that are able to produce the same 10% cleavage on the plasmid DNA in the presence of human topoisomerase I.
4.10. Cytotoxicity assays
The cytotoxicity was determined using the MTT-microtiter plate tetrazolinium cytotoxicity assay (MTA). The human lymphoblast RPMI 8402 and its camptothecin-resistant variant cell line, CPT-K5 was provided by Dr. Toshiwo Andoh (Aichi Cancer Center Research Institute, Nagoya, Japan).10 The P388 mouse leukemia cell line and its CPT-resistant TOP1-deficient variant P388/CPT4511 were obtained from Michael R. Mattern and Randal K. Johnson (GlaxoSmithKline, King of Prussia, PA). The KB3-1 cell line and its multidrug-resistant variant KBV-113 were obtained from K.V. Chin (The Cancer Institute of New Jersey, New Brunswick, NJ). The KBH5.0 cell line as noted previously8 was derived from KB3-1 by stepwise selection against Hoechst 33342. The cytotoxicity assay was performed using 96-well microtiter plates. Cells were grown in suspension at 37 °C in 5% CO2 and maintained by regular passage in RPMI medium supplemented with 10% heat inactivated fetal bovine serum, l-glutamine (2 mM), penicillin (100 U/mL), and streptomycin (0.1 mg/mL). For determination of IC50, cells were exposed continuously for four days to varying concentrations of drug, and MTT assays were performed at the end of the fourth day. Each assay was performed with a control that did not contain any drug. All assays were performed at least twice in six replicate wells.
4.11. Human tumor xenograft
Bioassays were performed using female NCR/NU NU mice of approximately 9 weeks of age as obtained from Taconic Farms, Inc. (Germantown, NY, USA). Mice were housed 4 per cage in laminar flow HEPA filtered microisolator caging (Allentown Caging Equipment Co., Allentown, NJ, USA) with two cages used per experimental group. Mice were fed Purina autoclavable breeder chow #5021 and given drinking water, purified by reverse-osmosis, ad libitum. Five days after arrival within the animal facility, the mice were inoculated on the right flank with 1.5 × 106 MDA-MB-435 tumor cells in 0.1 mL of RPMI 1640 Media by sc injection (25 gauge needle × 5/8″). The MDA-MB-435 cells were grown in 75 cm2 flasks using RPMI 1640 Media and 10% fetal bovine serum. Tumors were of sufficient size at 19–20 days after inoculation. Tumor-bearing mice were evenly matched in each experimental group based on tumor volume. Tumor volume was calculated by measuring the tumor with a microcaliper. The length (l) is the maximum two dimensional distance of the tumor and the width (w) is the maximum distance perpendicular to this length measured in mm. Tumor volume was calculated using the formula (l*w2)/2. Every mouse in this study was weighed individually on a daily basis. Dose adjustments for each experimental group, as indicated in Table 3, were made throughout the study based upon the effect or lack of an effect of treatment on average body weights. Tumor volume was determined for each individual mouse every other day.
Acknowledgments
This study was supported by National Cancer Institute Grants CA098127 (E.J.L) and CA39662 (L.F.L.) and Genzyme Corporation.
References and notes
- 1.Champoux JJ. Annu Rev Biochem. 2001;70:369. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 2.Wang JC. Nat Rev Mol Cell Biol. 2002;3:430. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 3.Chen AY, Liu LF. Annu Rev Pharmacol Toxicol. 1994;34:191. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- 4.Li TK, Liu LF. Annu Rev Pharmacol Toxicol. 2001;41:53. doi: 10.1146/annurev.pharmtox.41.1.53. [DOI] [PubMed] [Google Scholar]
- 5.Pommier Y. Nat Rev Cancer. 2006;6:789. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 6.Ruchelman AL, Singh SK, Wu XH, Ray A, Yang JM, Li TK, Liu A, Liu LF, LaVoie EJ. Bioorg Med Chem Lett. 2002;12:3333. doi: 10.1016/s0960-894x(02)00737-0. [DOI] [PubMed] [Google Scholar]
- 7.Ruchelman AL, Singh SK, Ray A, Wu X, Yang JM, Li TK, Liu A, Liu LF, LaVoie EJ. Bioorg Med Chem. 2003;11:2061. doi: 10.1016/s0968-0896(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 8.Li TK, Houghton PJ, Desai SD, Daroui P, Liu AA, Hars ES, Ruchelman AL, LaVoie EJ, Liu LF. Cancer Res. 2003;63:8400. [PubMed] [Google Scholar]
- 9.Ruchelman AL, Zhou N, Liu A, Liu LF, LaVoie EJ. J Med Chem. 2005;48:792. doi: 10.1021/jm049447z. [DOI] [PubMed] [Google Scholar]
- 10.Andoh T, Ishii K, Suzuki Y, Ikegami Y, Kusunoki Y, Takemoto Y, Okada K. Proc Natl Acad Sci. 1987;84:5565. doi: 10.1073/pnas.84.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woessner RD, Eng WK, Hofmann GA, Rieman DJ, McCabe FL, Hertzberg RP, Mattern MR, Tan KB, Johnson RK. Oncol Res. 1992;4:481. [PubMed] [Google Scholar]
- 12.Rajendra R, Gounder MK, Saleem A, Schellens JHM, Ross DD, Bates SE, Sinko P, Rubin EH. Cancer Res. 2003;63:3228. [PubMed] [Google Scholar]
- 13.Gervasoni JE, Jr, Fields SZ, Krishna S, Baker MA, Rosado M, Thuraisamy K, Hindenburg AA, Taub RN. Cancer Res. 1991;51:4955. [PubMed] [Google Scholar]
- 14.Chen AY, Yu C, Potmesil M, Wall ME, Wani MC, Liu LF. Cancer Res. 1991;51:6039. [PubMed] [Google Scholar]
- 15.Kawabata S, Oka M, Shiozawa K, Tsukamoto K, Nakatomi K, Soda H, Fududa M, Ikegami Y, Sugahara K, Yamada Y, Kamihira S, Doyle LA, Ross DD, Kohno S. Biochem Biophys Res Commun. 2001;280:1216. doi: 10.1006/bbrc.2001.4267. [DOI] [PubMed] [Google Scholar]
- 16.Yang CH, Schneider E, Kuo ML, Volk EL, Roccchi E, Chen YC. Biochem Pharmacol. 2000;60:831. doi: 10.1016/s0006-2952(00)00396-8. [DOI] [PubMed] [Google Scholar]
- 17.Makhey D, Yu C, Liu A, Liu LF, LaVoie EJ. Bioorg Med Chem. 2000;8:1171. doi: 10.1016/s0968-0896(00)00048-1. [DOI] [PubMed] [Google Scholar]
- 18.Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning, A Laboratory Manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NJ: 1982. pp. 149–185. [Google Scholar]
- 19.Tewey KM, Rowe TC, Yang L, Hallogan BC, Liu LF. Science. 1984;226:466. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- 20.Gatto B, Sanders MM, Yu C, Wu HY, Makhey D, LaVoie EJ, Liu LF. Cancer Res. 1996;56:2795. [PubMed] [Google Scholar]
- 21.Wang H, Mao Y, Chen A, Zhou N, LaVoie EJ, Liu LF. Biochemistry. 2001;40:3316. doi: 10.1021/bi002786j. [DOI] [PubMed] [Google Scholar]