Abstract
Classical tissue recombination studies demonstrated that initiation of tooth development depends on activation of odontogenic potential in the mesenchyme by signals from the presumptive dental epithelium. Although several members of the Wnt family of signaling molecules are expressed in the presumptive dental epithelium at the beginning of tooth initiation, whether Wnt signaling is directly involved in the activation of the odontogenic mesenchyme has not been characterized. In this report, we show that tissue-specific inactivation of β-catenin, a central component of the canonical Wnt signaling pathway, in the developing tooth mesenchyme caused tooth developmental arrest at the bud stage in mice. We show that mesenchymal β-catenin function is required for expression of Lef1 and Fgf3 in the developing tooth mesenchyme and for induction of primary enamel knot in the developing tooth epithelium. Expression of Msx1 and Pax9, two essential tooth mesenchyme transcription factors downstream of Bmp and Fgf signaling, respectively, were not altered in the absence of β-catenin in the tooth mesenchyme. Moreover, we found that constitutive stabilization of β-catenin in the developing palatal mesenchyme induced aberrant palatal epithelial invaginations that resembled early tooth buds both morphologically and in epithelial molecular marker expression, but without activating expression of Msx1 and Pax9 in the mesenchyme. Together, these results indicate that activation of the mesenchymal odontogenic program during early tooth development requires concerted actions of Bmp, Fgf and Wnt signaling from the presumptive dental epithelium to the mesenchyme.
Keywords: β-catenin, Cre/lox, epithelial-mesenchymal interaction, induction, odontogenic potential, signaling, tooth development, Wnt
Introduction
Similar to development of many other organs, development of the tooth, from its initiation through morphogenesis to cytodifferentiation, involves a series of sequential and reciprocal signaling interactions between the adjacent epithelium and mesenchyme (Thesleff et al., 1995; Pispa and Thesleff, 2003). In mice, tooth development begins as a thickening of the oral epithelium at around the 11th day of embryonic development (E11). The presumptive dental epithelium proliferates and buds into the underlying neural crest-derived mesenchyme at specific sites and induces the mesenchyme to condense around the epithelial buds from E12 to E13. At E13.5, cells at the tip of the epithelial tooth buds form the primary enamel knot, which secretes a number of growth factors to stimulate the proliferation of the surrounding dental epithelium. Subsequently, the dental epithelium folds and extends farther into the mesenchyme, wrapping itself around the condensing mesenchyme to form “cap” and then “bell”-shaped tooth germs. As development proceeds, the epithelial cells in contact with the dental mesenchyme differentiate into the enamel-producing ameloblasts and their adjacent mesenchymal cells differentiate into the dentin-producing odontoblasts (Thesleff and Hurmerinta, 1981). The ameloblasts and odontoblasts deposit enamel and dentin matrices, respectively back-to-back and subsequent mineralization of these matrices forms the hard tissues of the tooth (Lumsden, 1988).
Tissue recombination experiments have demonstrated that tooth inductive signals arise initially in the presumptive dental epithelium (Mina and Kollar, 1987; Lumsden, 1988; Ohazama et al., 2005). At the early tooth bud stage, however, the dental epithelium rapidly loses tooth inductive potential while the underlying mesenchyme acquires the capability to instruct tooth morphogenesis (Mina and Kollar, 1987; Lumsden, 1988). Whereas the exact molecular nature of the odontogenic potential has not been completely characterized, members of the bone morphogenetic protein (Bmp) and fibroblast growth factor (Fgf) families play critical roles in early tooth development (Reviewed Jernvall and Thesleff, 2000; Pispa and Thesleff, 2003). Bmp2, Bmp4, Fgf8, and Fgf9 are expressed in the presumptive dental epithelium at the beginning of tooth development (Wozney et al., 1988; Lyons et al., 1989; Vainio et al., 1993; Turecková et al., 1995; Neubuser et al., 1997; Dassule and McMahon, 1998). Bmp and Fgf signaling are necessary for activation of expression of the Msx1 and Pax9 transcription factors, respectively, in the presumptive tooth mesenchyme (Vainio et al., 1993; Neubuser et al., 1997; Tucker et al., 1998; Mandler and Neubuser, 2001). Mice lacking either Msx1 or Pax9 function exhibited tooth developmental arrest at the early bud stage (Satokata and Maas, 1994; Peters et al., 1998). Expression of Bmp4, which shifts from the presumptive dental epithelium to the developing tooth mesenchyme at about E12 during normal mouse tooth initiation, was significantly reduced in the developing tooth mesenchyme in either Msx1−/− or Pax9−/− mutant mice (Chen et al., 1996; Peters et al., 1998). Addition of recombinant Bmp4 protein rescued development of Msx1−/− mutant tooth germs to the late bell stage in explant cultures (Chen et al., 1996; Bei et al., 2000). In addition, Fgf8 induced Fgf3 expression in the dental mesenchyme in an Msx1-dependent manner (Bei and Maas, 1998). Although teeth developed normally in Fgf3−/−mutant mice (Mansour et al., 1994), mice homozygous for null mutations in both Fgf3 and Fgf10, which are both expressed in the developing tooth mesenchyme, had tooth developmental arrest at the bud stage (Wang et al., 2007). Moreover, mice with either tissue-specific inactivation in the oral epithelium of Bmpr1a, which encodes a type-1 receptor for Bmp signaling, or a deletion of Fgfr2b, which encodes the epithelial isoform of the type-2 Fgf receptor, exhibited tooth developmental arrest at the early bud stage (De Moerlooze et al., 2000; Andl et al., 2004; Liu et al., 2005). Thus, both Bmp and Fgf signaling are critical for the reciprocal interactions between the epithelium and mesenchyme during tooth initiation.
The Wnt signaling pathway also plays essential roles in early tooth development. The Wnt family of secreted proteins consists of 19 members in mammals and can trigger various cellular responses through several distinct pathways (Logan and Nusse, 2004). The canonical Wnt signaling pathway involves stabilization and nuclear accumulation of β-catenin. In the absence of Wnt signaling, cytoplasmic β-catenin is phosphorylated by the serine/threonine kinase GSK-3β, through interactions with the scaffolding proteins Axin and APC, and targeted for degradation by the ubiquitination-proteosome pathway. Activation of Wnt signaling inhibits β-catenin phosphorylation, leading to stabilization of β-catenin and its accumulation in the cellular nuclei where it interacts with and converts the TCF/Lef family DNA-binding proteins from transcriptional repressors to activators. Several Wnt genes, including Wnt4, Wnt6, Wnt10a, and Wnt10b, as well as Lef1, are strongly expressed in the presumptive dental epithelium at the tooth initiation stage in mice (Kratochwil et al., 1996; Dassule and McMahon, 1998; Sarkar and Sharpe, 1999). As the dental placode invaginated to form the tooth bud at E12, Lef1 mRNA expression shifted to the underlying presumptive dental mesenchyme (Kratochwil et al., 1996). At the same developmental stage, nuclear β-catenin was observed in both the dental epithelium and underlying mesenchyme (Liu et al., 2008). Application of Wnt-expressing cells specifically induced Lef1 mRNA expression in the mesenchyme of E11 mouse mandibular explants (Dassule and McMahon, 1998). These data indicate that the Wnt/β-catenin signaling pathway is activated in the dental mesenchyme during tooth initiation. However, whereas mice lacking Lef1 exhibited tooth developmental arrest at the bud stage (van Genderen et al., 1994; Kratochwil et al., 1996), tissue recombination experiments suggested that Lef1 function is required in the dental epithelium, but not in the dental mesenchyme, for tooth morphogenesis (Kratochwil et al., 1996; 2002). Consistent with an essential role of active Wnt/β-catenin signaling in the dental epithelium for early tooth development, epithelium-specific inactivation of β-catenin or epithelial expression of Dkk1, an inhibitor of canonical Wnt signaling, caused tooth developmental arrest at the early bud stage (Andl et al., 2002; Liu et al., 2008). However, whether Wnt/β-catenin signaling in the developing dental mesenchyme is required for early tooth morphogenesis is not known. Here we report that β-catenin function is required in the developing dental mesenchyme for the transition of tooth morphogenesis from the bud to cap stage. In addition, we show that constitutive stabilization of β-catenin in developing palatal mesenchyme induced de novo formation of palatal epithelial invaginations that resembled developing tooth buds. Together, these data indicate that Wnt/β-catenin signaling plays critical roles in the activation of the mesenchymal odontogenic potential during early tooth development.
Materials and Methods
Mouse strains, breeding and genotyping
Generation and characterization of Osr2-IresCre mice have been reported previously (Lan et al., 2007). Detailed analysis of Osr2-CreKI mice, in which a Cre cDNA replaced the coding region of the Osr2 gene, will be described elsewhere. The Catnbf/f mice, homozygous for a β-catenin allele with the DNA sequences from Exon-2 to Exon-6 flanked by two repeated loxP sites (Brault et al., 2001), and the R26R mice (Soriano, 1999) were purchased from the Jackson Laboratory (Bar Harbor, ME).
For analysis of Cre activity in Osr2-IresCre mice, Osr2-IresCre mice were crossed to R26R mice and the embryos processed for X-gal staining. Mice lacking β-catenin in the dental mesenchymal cells was obtained by crossing Catnbf/+; Osr2-IresCre mice with Catnbf/f mice. For stabilization of β-catenin in the developing palatal mesenchyme, Catnblox(ex3) mice (Harada et al., 1999) were crossed to Osr2-CreKI mice. Timed mating was set up in the late afternoon and examined for the presence of vaginal plugs the next morning. The noon of plug day is assumed as 0.5 day of gestation (E0.5). Embryos were collected at various developmental stages as indicated in the Results section. For genotyping, genomic DNA from either yolk sac or tail tissues was extracted. Primers for genotyping Catnbf and R26R alleles have been described previously (Brault et al., 2001; Soriano, 1999). Other primers used for genotyping are: 1733, AGGGTACCTGAAGCTCAGCG and 1734, CAGTGGCTGACAGCAGCTTT for the Catnblox(ex3) allele; and Cre83, GTCCAATTTACTGACCGTACACC and Cre85, GTTATTCGGATCATCAGCTACACC for both Osr2-IresCre and Osr2-CreKI alleles.
X-Gal Staining
To detect β-galactosidase activity, embryos were fixed in 0.25% glutaraldehyde, passed through sucrose series, embedded in Negative-50 freezing medium (Richard Allan), and sectioned at 14 βm thickness using a cryostat microtome. X-gal staining of sections was performed as described previously (Hogan et al., 1994). Slides were counterstained with eosin.
Histology and immunohistochemical staining
For histological analysis, staged embryos were collected, fixed in Bouin's fixative or 4% paraformaldehyde (PFA), dehydrated through ethanol series, embedded in paraffin, and sectioned serially at 7 μm thickness. Slides were stained with hematoxylin and eosin, and mounted with Permount.
For immunostaining, frontal sections from 4% PFA-fixed embryos were treated with 3% hydrogen peroxide, boiled in a pressure cooker containing Trilogytm solution (Cell Marque, Rocklin, CA), and incubated with primary antibodies against phospho-Smad1/5/8 (polyclonal rabbit serum, Cell Signaling Technology; 1:200), β-catenin (mouse monoclonal clone 15B8, Sigma; 1:500), and active-caspase-3 (rabbit monoclonal antibody, BD Biosciences, 1:250). M.O.M kit (Vector Laboratories), in combination with streptavidin-conjugated Texas-Red (Vector Laboratories, 1:100) was used for detection of β-catenin. Histostain Plus Rabbit Primary (DAB) kit (Zymed Laboratories) was used for detection of phospho-Smad 1/5/8 and active-caspase-3, following manufacturer's instructions.
Section in situ hybridization
Embryos were fixed in 4% PFA, dehydrated through ethanol series, embedded in paraffin, and sectioned at 7 βm thickness. Frontal sections were hybridized with digoxigenin –labeled cRNA probes generated by in vitro transcription from linearized cDNA templates. Slides were subsequently incubated with an alkaline phosphatase-conjugated anti-DIG antibody (Roche). Hybridization signal was detected by BM purple substrate (Roche) as described previously (Zhang et al., 1999).
Kidney capsule culture of tooth germs
To investigate the effects of constitutive stabilization of β-catenin on tooth development and possible ectopic tooth initiation in the developing palate in the Catnblox(ex3)/+; Osr2-CreKI mice, molar tooth germs and palatal shelves were dissected from E13.5 embryos and grafted under the kidney capsule of adult CD-1 male mice according to standard procedure. Three weeks after transplantation, the recipient mice were sacrificed and the grafts processed for histological analysis. All animal procedures were carried out according to IACUC approved protocols.
Results
Deletion of β-catenin in early tooth mesenchyme caused tooth developmental arrest at the bud-to-cap transition
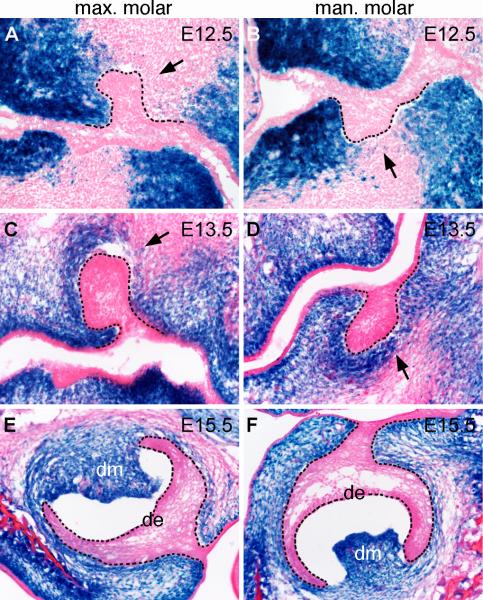
Since the tooth mesenchyme is derived from the cranial neural crest, the most straightforward approach for investigating the roles of canonical Wnt signaling in the developing tooth mesenchyme would be to specifically inactivate the β-catenin gene in the early neural crest cells. However, Cre/loxP-mediated tissue-specific inactivation of the β-catenin gene in the early neural crest cells in mice using the Wnt1-Cre transgenic mouse strain resulted in severe disruption of formation of the facial primordia prior to initiation of tooth development (Brault et al., 2001). We recently generated the Osr2-IresCre knockin mice, in which the IresCre bicistronic expression cassette was inserted in the 3’ region of the Osr2 gene, and showed that these mice exhibited Cre activity in the mesenchyme of the developing secondary palate and tooth germs (Lan et al., 2007). In contrast to the Wnt1-Cre transgenic mice, which express Cre in the premigratory neural crest cells by E8.5 (Danielian et al., 1998; Chai et al., 2000), Cre activity was not detected in the craniofacial tissues in the Osr2-IresCre mice until E10.5, after neural crest migration to the facial primordia was complete (Lan et al., 2007). To analyze the spatial and temporal pattern of Cre activity in Osr2-IresCre mice, we crossed Osr2-IresCre mice to the R26R reporter mice, in which LacZ expression is only activated by Cre-mediated recombination (Soriano, 1999). As shown previously, Cre-mediated activation of β-galactosidase expression occurred throughout the developing palatal mesenchyme as well as in the developing molar and incisor tooth mesenchyme by E14.5 in Osr2-IresCre;R26R mice (Lan et al., 2007). We further examined in detail Cre-mediated activation of β-galactosidase expression during molar tooth development. At E12.5, as the molar tooth epithelium invaginated into the mesenchyme, β-galactosidase activity was detected in mesenchymal cells lingual to the molar tooth buds, while mesenchymal cells buccal to the molar tooth buds showed very little β-galactosidase activity (Fig. 1A, B). The lingual bias of Cre activity in the developing tooth mesenchyme corresponds to preferential expression of the Osr2 gene in the lingual side of the developing tooth mesenchyme (Gao et al., 2009; Zhang et al., 2009). At E13.5, β-galactosidase activity in the upper molar mesenchyme still exhibited lingual bias (Fig. 1C), while it was detected in the mesenchymal cells in both lingual and buccal side of lower molar tooth buds (Fig. 1D). By E15.5, β-galactosidase was expressed robustly in the dental papilla in both upper molar and lower molar tooth germs (Fig. 1E, F). Importantly, no β-galactosidase expression was detected in the palatal and tooth epithelial cells throughout these stages.
Fig. 1.
Cre-mediated activation of LacZ expression during molar tooth development in the Osr2-IresCre; R26R embryos. (A-F) Frontal sections from Osr2-IresCre; R26R embryos at E12.5 (A, B), E13.5 (C, D), and E15.5 (E, F) were assayed by X-gal staining. Note that X-gal staining was only detected in dental mesenchymal cells, but not in dental epithelia. Black dashed lines mark the boundary between the developing dental epithelium and mesenchyme. Arrows in A-D point to the mesenchyme lingual to the developing tooth buds. de, dental epithelium; dm, dental mesenchyme; max. molar, maxillary first molar tooth germs; man. molar, mandibular first molar tooth germs.
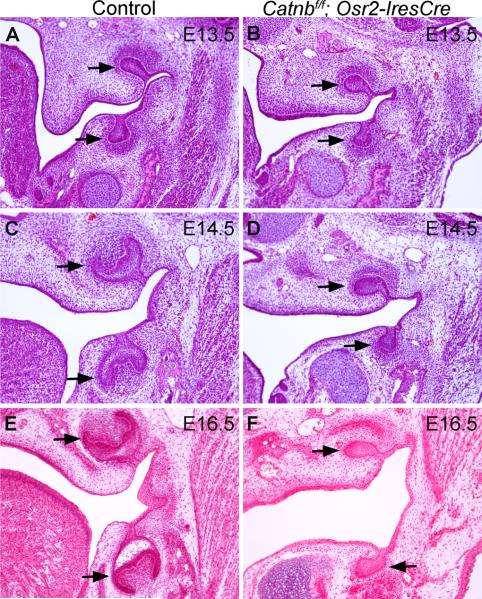
To investigate whether β-catenin function in the developing tooth mesenchyme is required for tooth morphogenesis, we crossed Osr2-IresCre mice to mice carrying a targeted conditional β-catenin allele, Catnbf/f (Brault et al., 2001). Catnbf/f;Osr2-IresCre pups were born with cleft palate and died shortly after birth. To examine tooth development in Catnbf/f;Osr2-IresCre mice, we carried out histological analyses of embryos harvested at E12.5 to E16.5. At E12.5, molar tooth development had initiated and tooth epithelium invaginated into the underlying mesenchyme in both control and mutant embryos (data not shown). At E13.5, molar tooth germs had reached the bud stage in both control and mutant embryos (Fig. 2A, B). By E14.5, tooth development in the control embryos had reached the cap stage (Fig. 2C), while molar tooth germs in the Catnbf/f; Osr2-IresCre mutants remained at the bud stage (Fig. 2D). At E16.5, molar tooth germs in the control embryos continued to develop toward the bell stage (Fig. 2E), but the molar tooth germs in the Catnbf/f; Osr2-IresCre mutants remained arrested at the bud stage (Fig. 2F). Moreover, examination of sections through the developing incisor regions revealed that development of both upper and lower incisors were arrested at the late bud to early cap stage in the Catnbf/f; Osr2-IresCre mutant mice (Fig. 3). These data indicate that β-catenin function is required in the developing dental mesenchyme for tooth morphogenesis beyond the bud stage.
Fig. 2.
Molar tooth development arrested at the bud stage in the Catnbf/f; Osr2-IresCre mutant mice. (A-F) Histology of frontal sections through the developing molar tooth germs in control (A, C, E) and Catnbf/f; Osr2-IresCre mutant (B, D, F) embryos at E13.5 (A, B), E14.5 (C, D) and E15.5 (E, F). Arrows point to the molar tooth germs.
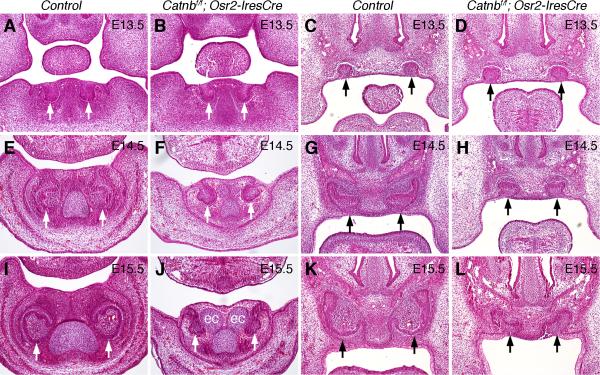
Fig. 3.
Incisor tooth development arrested at the late bud to early cap stages in the Catnbf/f; Osr2-IresCre mutant mice. White arrows point to mandibular tooth germs, and black arrows point to maxillary tooth germs. (A-D) At E13.5, both maxillary and mandibular incisors developed to the bud stage in control (A, C) and Catnbf/f; Osr2-IresCre mutant (B, D) embryos. (E-H) At E14.5, the incisor tooth germs have developed to the cap stage in the control embryos (E, G) but incisor tooth germs in the Catnbf/f; Osr2-IresCre mutant littermates (F, H) were still at the bud stage. (I-L) At E15.5, the incisor tooth germs developed to bell stage in the control embryos (I, K) but the mandibular incisors arrested at the bud stage whereas the maxillary incisor tooth germs arrested at the early cap stage in the Catnbf/f; Osr2-IresCre mutant embryos. Ectopic cartilage (ec) developed next to the incisor tooth germs in the Catnbf/f; Osr2-IresCre mutant embryos by E15.5 (J).
β-catenin function is required in the developing tooth mesenchyme for induction of primary enamel knot
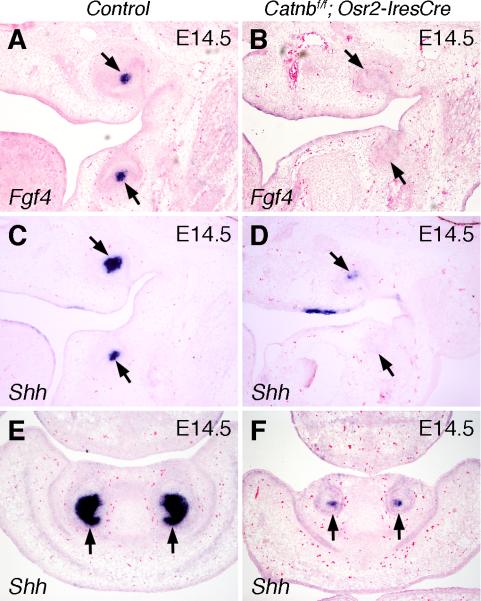
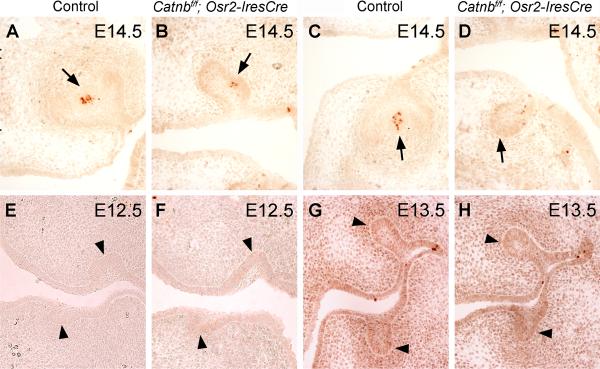
As the developing tooth germ transitions from the bud to the cap stage, mesenchymal signals induce the cells at the tip of the epithelial tooth bud to form the primary enamel knot, an epithelial signaling center that expresses multiple signaling molecules and transcriptional factors. The enamel knot is an important regulator of tooth shape, and its induction is a prerequisite for the tooth germ to develop into the cap stage (reviewed by Jernvall and Thesleff, 2000). To investigate whether induction of enamel knot had taken place in Catnbf/f; Osr2-IresCre mutant embryos, we analyzed expression of Fgf4 and Shh, two well established molecular markers of the primary enamel knot, in the developing tooth germs. At E14.5, the first molar tooth germs in control embryos exhibited strong localized expression of Fgf4 and Shh mRNAs at the center of the cap-shaped tooth epithelium (Fig. 4A, C). In contrast, examination of serial sections throughout the developing first molar tooth germs in the Catnbf/f; Osr2-IresCre mutant littermates failed to detect any Fgf4 expression in the developing tooth epithelium while only a few cells at the tip of the maxillary, but not mandibular, first molar tooth buds showed weak Shh expression (Fig. 4B, D). Similarly, in the developing incisor tooth germs at E14.5, only a few cells at the tip of the Catnbf/f; Osr2-IresCre mutant tooth buds showed weak Shh expression, in contrast to the robust Shh expression in the distal incisor epithelium in the control littermates (Fig. 4E, F).
Fig. 4.
β-catenin function is required in the developing tooth mesenchyme for induction of primary enamel knot. (A) At E14.5, Fgf4 mRNA was strongly expressed in the primary enamel knot cells (arrows) in the developing first molar tooth germs in the control embryo. (B) Fgf4 expression was not detected in the developing first molar tooth germs in the E14.5 Catnbf/f; Osr2-IresCre mutant embryos. Arrows point to the developing tooth germs. (C) At E14.5, Shh mRNA was strongly expressed in the primary enamel knot cells in the developing first molar tooth germs in the control embryo. (D) Shh mRNA expression was detected in a few cells in the maxillary first molar tooth germ but not in the mandibular molar tooth germ in the E14.5 Catnbf/f; Osr2-IresCre mutant embryo. (E, F) At E14.5, Shh mRNA was strongly expressed in the inner enamel epithelium in the developing incisors in control embryo (E) but was only weakly expressed in a few cells in the developing incisor epithelium in the Catnbf/f; Osr2-IresCre mutant littermate (F).
While the primary enamel knot acts as a signaling center for stimulating tooth morphogenesis from the bud to the cap stage, the enamel knot cells themselves undergo programmed cell death (reviewed by Jernvall and Thesleff, 2000). Consistent with the Shh expression data shown above, we found activated Caspase3 activity in a few cells in the center of the maxillary first molar tooth buds (Fig. 5B), but not in the mandibular first molar tooth buds (Fig. 5D), in the E14.5 Catnbf/f; Osr2-IresCre mutant embryos, compared with strong and localized activated Caspase3 activity in the enamel knot cells in both the maxillary and mandibular first molar tooth germs in the control littermates (Fig. 5A, C). Importantly, no increase in activation of Caspase3 activity was detected in the developing tooth epithelium and mesenchyme in the mutant embryos from E12.5 through E14.5, compared with their control littermates (Fig. 5A-H). We further carried out TUNEL assays and did not find any increase in cell death in the developing tooth epithelium and mesenchyme in the mutant embryos (data not shown). These data indicate that the tooth developmental arrest in the Catnbf/f; Osr2-IresCre mutant embryos was not associated with increased cell death.
Fig. 5.
Tooth developmental arrest in the Catnbf/f; Osr2-IresCre mutant mice was not accompanied by increased cell apoptosis. (A, C) At E14.5, the primary enamel knot cells at the center of the developing inner enamel epithelium in the maxillary (A) and mandibular (C) molar tooth germs in the control embryo exhibited active caspase-3 activity. (B, D) A rudimentary primary enamel knot, as shown by a few clustered cells expressing active caspase-3, formed in the developing maxillary molar tooth germ (B), but not in the mandibular molar tooth germ (D), in the E14.5 Catnbf/f; Osr2-IresCre mutant embryos. Arrows in A – D point to the corresponding primary enamel knot region of the developing molar tooth germs. No active caspase-3 activity was detected in the developing tooth mesenchyme in either the control (A, C) or Catnbf/f; Osr2-IresCre mutant (B, D) embryos. (E-H) Detection of active caspase-3 activity in the developing molar tooth germs in E12.5 (E, F) and E13.5 (G, H) control (E, G) and Catnbf/f; Osr2-IresCre mutant (F, H) embryos. Arrowheads in E – H point to the developing tooth buds. No differences in cell apoptosis were detected in the control and mutant tooth germs.
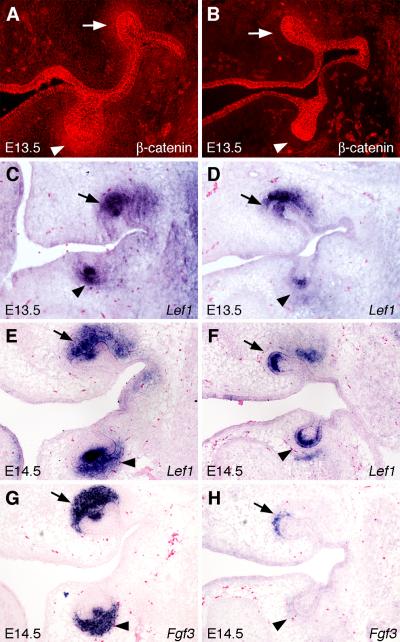
The subtle differences between the progression of maxillary and mandibular first molar development may be due to the differences in the domains of Cre-mediated β-catenin inactivation in the developing tooth mesenchyme. We compared the expression of β-catenin protein in the developing tooth germs in the control and Catnbf/f; Osr2-IresCre embryos by immunohistochemical staining. While β-catenin was most strongly expressed in the epithelium, including the oral and dental epithelium, significant amount of β-catenin protein was detected in the developing tooth mesenchyme (Fig. 6A). By comparison, β-catenin protein levels were dramatically reduced throughout the developing tooth mesenchyme surrounding the mandibular first molar tooth buds by E13.5 in the Catnbf/f; Osr2-IresCre embryos (Fig. 6B). Consistent with the lingual bias of Cre activity, in particular around the developing maxillary molar tooth germs, β-catenin was dramatically reduced in the developing tooth mesenchyme lingual and distal to, but not in the buccal side of, the maxillary first molar tooth buds in the E13.5 Catnbf/f; Osr2-IresCre embryos (Fig. 6B). Importantly, the levels of β-catenin protein in the developing tooth epithelium was not altered in the Catnbf/f; Osr2-IresCre embryos, in comparison with the control littermates (Fig. 6A, B). Thus, whereas previous studies showed that β-catenin function is required in the developing tooth epithelium for early tooth morphogenesis (Liu et al., 2008), these data indicate that β-catenin function is also required in the developing tooth mesenchyme for the induction of primary enamel knot formation.
Fig. 6.
Alterations in gene expression during tooth development in the Catnbf/f; Osr2-IresCre mutant embryos. (A, B) Immunofluorescent detection of β-catenin protein expression in the developing molar tooth germs in E13.5 control (A) and Catnbf/f; Osr2-IresCre mutant (B) littermates. Arrows point to developing maxillary molar tooth mesenchyme and arrowheads point to the developing mandibular molar tooth mesenchyme. (C-F) Lef1 mRNA expression in E13.5 (C, D) and E14.5 (E, F) control (C, E) and Catnbf/f; Osr2-IresCre mutant (D, F) molar tooth germs, respectively. (G, H) Fgf3 mRNA expression was dramatically reduced in the E14.5 Catnbf/f; Osr2-IresCre mutant molar tooth germs (H), in comparison with that in the control littermate (G).
Cell autonomous requirement of β-catenin for maintenance of expression of Lef1 and Fgf3 in the developing tooth mesenchyme
Induction of the primary enamel knot and transition of tooth development from the bud to the cap stage depends on adequate Bmp4 expression in the developing tooth mesenchyme (Chen et al., 1996; Bei et al., 2000; Zhao et al., 2000). Mice lacking either Msx1 or Pax9, two transcription factors expressed in the early developing tooth mesenchyme, exhibited tooth developmental arrest at the bud stage with loss of mesenchymal Bmp4 expression (Chen et al., 1996; Peters et al., 1998). Addition of exogenous Bmp4 to tooth germ explant cultures or transgenic expression of Bmp4 in the tooth mesenchyme rescued Msx1−/− mutant molar tooth development to the cap stage (Bei et al., 2000; Zhao et al., 2000). We thus compared the expression of Bmp4, Msx1, and Pax9 during tooth development in the Catnbf/f; Osr2-IresCre mutant and control embryos. We found that expression of these genes in the developing tooth mesenchyme was similar in the Catnbf/f; Osr2-IresCre mutant and control littermates (Fig. S1 in the supplementary material).
Mice lacking the transcription factor Lef1 exhibited tooth developmental arrest at late bud stage without disrupting mesenchymal Bmp4 expression (Kratochwil et al., 1996), similar to the Catnbf/f; Osr2-IresCre mutant mice. We compared the expression of Lef1 mRNA during tooth development in the Catnbf/f; Osr2-IresCre mutant and control littermates. At E13.5, Lef1 mRNA was highly expressed in the distal tooth bud epithelium and mesenchyme in control embryos (Fig. 6C). In comparison, Lef1 mRNA expression was dramatically reduced in the developing tooth mesenchyme in the Catnbf/f; Osr2-IresCre mutant littermates (Fig. 6D). In particular, the domains of Lef1 downregulation correlated well with the domains of loss of β-catenin in the tooth mesenchyme such that Lef1 mRNA expression was reduced throughout the mandibular molar tooth mesenchyme but persisted on the buccal side of the maxillary molar tooth mesenchyme (compare Fig. 6D with Fig. 6B). By E14.5, Lef1 mRNA was strongly expressed in the enamel knot and the underlying dental mesenchyme in control embryos (Fig. 6E). In contrast, little Lef1 mRNA expression was detected in the developing tooth mesenchyme while strong Lef1 mRNA expression was present in the distal tooth epithelium in Catnbf/f; Osr2-IresCre mutant littermates (Fig. 6F). These data indicate that β-catenin function is required cell-autonomously in the developing tooth mesenchyme for maintenance of Lef1 mRNA expression.
Lef1−/− mutant embryos lacked Fgf3 and Fgf4 expression in the developing tooth mesenchyme and epithelium, respectively (Kratochwil et al., 2002). We found that Fgf4 expression was absent in the developing tooth epithelium of Catnbf/f; Osr2-IresCre mutant embryos although strong Lef1 expression persisted in the mutant tooth epithelium (Fig. 4B and Fig. 6F). We further examined expression of Fgf3 expression during tooth development in the Catnbf/f; Osr2-IresCre mutant embryos and found that expression of Fgf3 was significantly reduced in the developing tooth mesenchyme in Catnbf/f; Osr2-IresCre mutant embryos compared with the control littermates (Fig. 6G, H).
Constitutive stabilization of β-catenin in the palatal mesenchyme induced tooth bud-like structures from the palatal epithelium
To further investigate the role of β-catenin in the activation of mesenchymal odontogenic potential, we crossed the Catnblox(ex3) mice, in which Exon-3 of the β-catenin gene was flanked by loxP sequences (Harada et al., 1999), to Osr2-CreKI mice. Deletion of Exon-3 from the β-catenin gene results in generation of a constitutively more stable β-catenin protein product due to lack of phosporylation sites for GSK3β, thus mimicking activation of canonical Wnt signaling (Harada et al., 1999; Jarvinen et al., 2006; Liu et al., 2008). Similar to Osr2-IresCre mice, Osr2-CreKI mice exhibit highly specific Cre expression in the Osr2-expressing cells, including the developing palatal and tooth mesenchyme. Whereas a small percentage of Osr2-IresCre mice exhibited ectopic Cre activity (Lan et al., 2007), none of over 200 Osr2-CreKI mouse embryos displayed ectopic Cre activity outside of Osr2-expressing tissues (Lan et al., unpublished data).
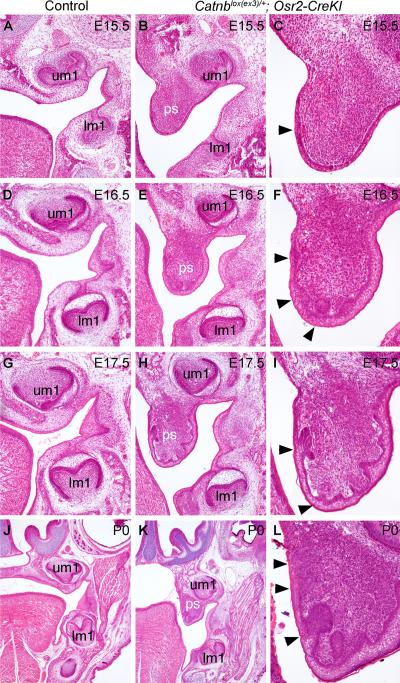
Catnblox(ex3)/+;Osr2-CreKI mice died shortly after birth and displayed cleft secondary palate (Fig. S2 in the supplementary material). Histological analyses revealed that development of molar teeth in Catnblox(ex3)/+;Osr2-CreKI mutant embryos (Fig. 7B, E, H, K) progressed morphologically similarly to their control littermates (Fig. 7A, D, G, J). However, multiple epithelial invaginations were detected on the nasal side of the developing palatal shelves by E16.5 in the Catnblox(ex3)/+;Osr2-CreKI mutants (Fig.7F). From E17.5 to P0, the ectopic epithelial invaginations at the nasal side of the palatal shelves in the Catnblox(ex3)/+;Osr2-CreKI mutants formed morphologically distinct epithelial buds (Fig. 7I, L).
Fig. 7.
Constitutive stabilization of β-catenin in the developing palatal mesenchyme resulted in ectopic tooth-like epithelial buds in the palate. (A, B) At E15.5, the Catnblox(ex3)/+;Osr2-CreKI mutant embryo (B) exhibited comparable molar tooth development with that in the control littermate (A), but the mutant palatal shelves were still vertically oriented (B) while palatal shelves had elevated to the horizontal position above the tongue in the control littermates. (C) High magnification view of the Catnblox(ex3)/+;Osr2-CreKI mutant palatal shelf at E15.5. Arrowhead points to thickened palatal epithelium. (D - F) At E16.5, while the developing molar tooth germs exhibited similar morphology in control (D) and the Catnblox(ex3)/+;Osr2-CreKI mutant (E) littermates, the mutant palatal shelves (F) exhibited epithelial invaginations (arrowheads in F). (G-I) At E17.5, the molar tooth germs appear smaller in the Catnblox(ex3)/+;Osr2-CreKI mutant (H) than those in the control embryo (G). Some of the epithelial invaginations in the mutant palate (I) appeared to form early “cap”-like structures (arrowheads in I). (J-L) At P0, the molar tooth germs in the Catnblox(ex3)/+;Osr2-CreKI mutant (K) appeared retarded, in comparison with those in control littermate (J). Multiple epithelial invaginations (arrowheads in L) were detected in the mutant palatal shelves and the palatal mesenchyme appeared to condense under the epithelial invaginations.
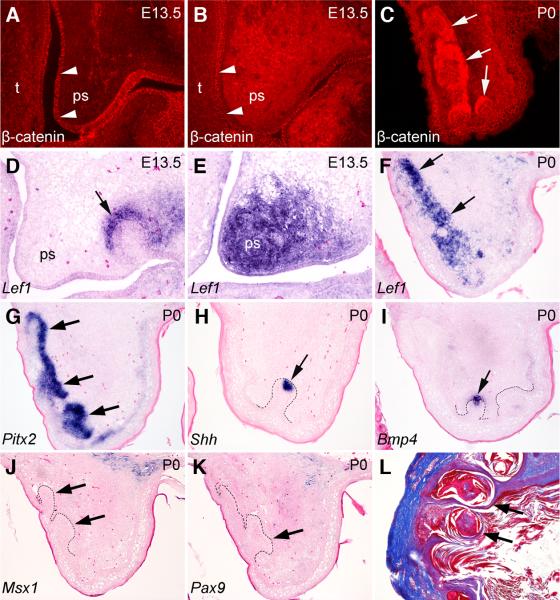
To ascertain that the palatal epithelial invaginations resulted from stabilization of β-catenin in the palatal mesenchyme, we examined β-catenin protein expression in the control and Catnblox(ex3)/+;Osr2-CreKI mutant embryos. At E13.5, there is little β-catenin protein detected in the palatal mesenchyme in the control embryos (Fig. 8A). In contrast, strong β-catenin staining was detected throughout the palatal mesenchyme in the Catnblox(ex3)/+;Osr2-CreKI mutant littermates (Fig. 8B) while β-catenin staining in the palatal epithelium was comparable in the control and mutant embryos (Fig. 8A, B). Interestingly, following the formation of the palatal epithelial invaginations in the Catnblox(ex3)/+;Osr2-CreKI mutant embryos, intense β-catenin staining localized to the mesenchyme cells immediately underlying the invaginated palatal epithelium (Fig. 8C). Consistent with the changes in the pattern of β-catenin protein distribution, Lef1 mRNA expression was ectopically activated throughout the developing palatal mesenchyme in the Catnblox(ex3)/+;Osr2-CreKI mutant embryos at E13.5 (Fig. 8E), while no Lef1 expression was detected in the palatal mesenchyme in the control littermates (Fig. 8D). In the Catnblox(ex3)/+;Osr2-CreKI mutants at P0, strong Lef1 mRNA expression was localized in the palatal mesenchyme immediately adjacent to the invaginated palatal epithelium (Fig. 8F). These data suggest, following the initial overall accumulation of stabilized β-catenin and activation of the canonical Wnt signaling pathway resulting from Cre-mediated deletion of Exon-3 of the β-catenin gene in the palatal mesenchyme in the Catnblox(ex3)/+;Osr2-CreKI mutant embryos, that subsequent β-catenin expression and accumulation in the palatal mesenchyme underlying the invaginated palatal epithelium was regulated by epithelial-mesenchymal interactions.
Fig. 8.
Constitutive stabilization of β-catenin in the palatal mesenchyme induced tooth bud-like structures from the palatal epithelium. (A-C) Immunofluorescent detection of β-catenin protein in the palate in E13.5 (A, B) and P0 (C) control (A) and Catnblox(ex3)/+;Osr2-CreKI mutant (B, C) mice. Much more β-catenin protein (red color) accumulated in the developing palate mesenchyme in E13.5 Catnblox(ex3)/+;Osr2-CreKI mutant (B) than in the control littermate (A). By P0, β-catenin protein was preferentially accumulated in the mesenchymal cells underlying the epithelial invaginations in the mutant palate (C). Arrowheads in A and B point to the palatal epithelium, whereas arrows in C point to the β-catenin positive palatal mesenchyme underlying the invaginated palatal epithelium. (D-F) In situ hybridization detection of Lef1 mRNA expression (blue color) in the palate in E13.5 (D, E) and P0 (F) control (D) and Catnblox(ex3)/+;Osr2-CreKI mutant (E, F) mice. Arrow in D points to Lef1-positive tooth mesenchyme, whereas arrows in F point to Lef1 mRNA expression in the palatal mesenchyme underlying the invaginated palatal epithelium in the Catnblox(ex3)/+;Osr2-CreKI mutant mouse. (G-I) Expression of Pitx2 (G), Shh (H), and Bmp4 (I) mRNAs indicate that the invaginated epithelial structures in the palate in the Catnblox(ex3)/+;Osr2-CreKI mutant mice resemble developing tooth germs. Arrows in G point to Pitx2 mRNA expression in the invaginated palatal epithelium. Arrows in H and I point to the Shh- and Bmp4-positive domain in the distal region of the invaginated palatal epithelium, which resemble primary enamel knot in gene expression pattern. (J, K) The mesenchyme underlying palatal epithelial invaginations (arrows) in Catnblox(ex3)/+;Osr2-CreKI mutant mice did not show detectable expression of Msx1 (J) and Pax9 mRNA (K). Black dashed line in each panel mark the boundary between the invaginated epithelium and mesenchyme. (L) Kidney capsule graft of E13.5 Catnblox(ex3)/+;Osr2-CreKI mutant palatal shelf had encapsulated cysts (arrows) but no organized tooth structures.
To investigate the identity of the invaginated palatal epithelial structures in the Catnblox(ex3)/+;Osr2-CreKI mutants, we performed section in situ hybridization with probes for genes important for tooth development. Pitx2 is a marker for developing tooth epithelium and is not expressed in other embryonic epithelial appendages (Mucchielli et al., 1997; Liu et al., 2008). Pitx2 expression was detected throughout the invaginated palatal epithelium in the Catnblox(ex3)/+;Osr2-CreKI mutants (Fig. 8G). Shh is normally expressed in the enamel knot cells in the developing tooth germs (Dassule et al., 2000). Shh expression was observed specifically at the tip of some of the palatal epithelial buds in the Catnblox(ex3)/+;Osr2-CreKI mutants (Fig. 8H). Similarly, Bmp4 expression was detected at the tip of some of the palatal epithelial buds and in the underlying mesenchymal cells in the Catnblox(ex3)/+;Osr2-CreKI mutants (Fig. 8I), which closely resemble Bmp4 expression in the enamel knot and mesenchyme of the late bud stage tooth germs. These data suggest that the palatal epithelial invaginations in the Catnblox(ex3)/+;Osr2-CreKI mutant mice resemble developing tooth buds both morphologically and in molecular marker expression.
As mentioned above, both the Msx1 and Pax9 transcription factors are required in the developing tooth mesenchyme for tooth development beyond the bud stage. However, we found that neither Msx1 nor Pax9 was activated in the mesenchyme underlying the palatal epithelial invaginations in the Catnblox(ex3)/+;Osr2-CreKI mutant mice. To further investigate whether the palatal epithelial invagiations in the Catnblox(ex3)/+;Osr2-CreKI mutant mice could undergo tooth-like morphogenesis beyond the bud stage, we grafted either E13.5 or E17.5 palatal explants from the Catnblox(ex3)/+;Osr2-CreKI mutant embryos under kidney capsules of adult male mice for three weeks. As control, we grafted E13.5 molar tooth germs or palatal explants of wildtype mouse embryos. No teeth formed from the palatal explants from either wildtype or Catnblox(ex3)/+;Osr2-CreKI mutant embryos (Fig. 8L, and data not shown), whereas the E13.5 mouse molar tooth germs gave rise to well-differentiated tooth structures (Fig. 9K, and data not shown). Taken together, these data indicate that β-catenin function is required in the developing tooth mesenchyme, together with Msx1 and Pax9, for the complete activation of mesenchymal odontogenic potential.
Fig. 9.
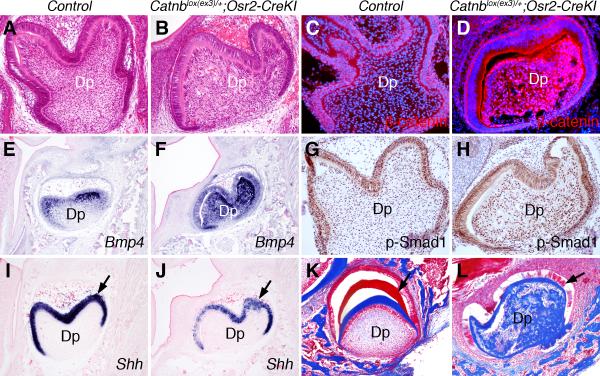
Persistent stabilization of β-catenin in the tooth mesenchyme disrupts cytodifferentiation during later tooth morphogenesis in the Catnblox(ex3)/+;Osr2-CreKI mutant mice. (A, B) Frontal sections through the mandibular first molar tooth germs of control (A) and Catnblox(ex3)/+;Osr2-CreKI mutant mice at P0 stained with hematoxylin and eosin. Note the uniform distribution of dental mesenchymal cells in the dental pulp in the control mouse versus the disorganized dental pulp compartment in the mutant mouse. (C, D) Immunostaining showing β-catenin distribution (red color) in the control (C) and Catnblox(ex3)/+;Osr2-CreKI mutant (D) molar tooth germs at P0. Cellular nuclei were counterstained with 4’-6-diamidino-2-phenylindole (DAPI) and shown in blue color. Note the much higher level of β-catenin protein in the dental mesenchyme of the mutant mouse (D) than that in the control littermate (C). (E, F) In situ hybridization detection of Bmp4 mRNA (shown in blue color) in the frontal sections of molar tooth germs in control (E) and Catnblox(ex3)/+;Osr2-CreKI mutant (F) mice. (G, H) Immunohistochemical staining (detected in brown color) of the frontal sections of molar tooth germs in control (G) and Catnblox(ex3)/+;Osr2-CreKI mutant (H) mice with an antibody against phosporylated-Smad1/5/8. The sections were counterstained with hematoxylin (blue). (I, J) In situ hybridization detection of Shh mRNA expression in the molar tooth germs in control (I) and Catnblox(ex3)/+;Osr2-CreKI mutant (J) mice. (K, L) Kidney capsule grafts of the molar tooth germs from E13.5 control (K) and Catnblox(ex3)/+;Osr2-CreKI mutant (L) embryos assayed by trichrome staining. The enamel matrix (arrows) is stained red and the dentin matrix is stained blue. Dp, dental pulp.
Persistent stabilization of β-catenin in the dental mesenchyme disrupts differentiation of odontoblasts and ameloblasts
Although molar tooth germs in Catnblox(ex3)/+;Osr2-CreKI mutant mice progressed to the bell stage by birth, careful examination of histological sections of newborn pups indicated that the dental mesenchyme was aberrant (Fig. 9A, B). By Immunofluorescent staining, we detected high levels of β-catenin protein in the dental mesenchyme cells in Catnblox(ex3)/+;Osr2-CreKI mutant pups, in comparison with their control littermates (Fig. 9C, D). Expression of Bmp4 mRNA was strongly upregualted throughout the dental mesenchyme in Catnblox(ex3)/+;Osr2-CreKI mutant mice, in comparison to the restricted expression of Bmp4 mRNA in differentiating odontoblasts in close contact with the dental epithelial cells in the control mice (Fig. 9E, F). Consistent with the Bmp4 expression pattern, Bmp signaling, as revealed by immunostaining with an antibody against phosphorylated-Smad1/5/8, was strongly elevated throughout the dental mesenchyme in Catnblox(ex3)/+;Osr2-CreKI mutant mice, in comparison with the control littermates (Fig. 9G, H). In contrast, expression of Shh in the differentiating ameloblasts was significantly reduced in Catnblox(ex3)/+;Osr2-CreKI mutant mice in comparison with the control littermates (Fig. 9I, J), suggesting that ameloblast differentiation was impaired in the mutant mice. To investigate further the defects in cell differentiation in the tooth germs in Catnblox(ex3)/+;Osr2-CreKI mutant mice, we transplanted E13.5 control and Catnblox(ex3)/+;Osr2-CreKI mutant molar tooth germs under kidney capsules of adult mice for three weeks and then analyzed the tooth morphology by histological analysis. As shown in Fig. 9K, the control tooth germs had well-organized enamel and dentin layers in between the ameloblast and odontoblast cell layers. In contrast, very little enamel matrix was deposited in the Catnblox(ex3)/+;Osr2-CreKI mutant tooth germ (Fig. 9L). In addition, whereas the control dental pulp exhibited uniform distribution of undifferentiated dental mesenchyme cells (Fig. 9K), the Catnblox(ex3)/+;Osr2-CreKI mutant dental pulp cells prematurely differentiated and produced large amounts of dentin-like matrix throughout the dental pulp compartment (Fig. 9L). These data indicate that persistent stabilization of β-catenin in the dental mesenchyme cells disrupted differentiation of both the dental epithelium and mesenchyme. Thus, whereas β-catenin signaling in the early tooth mesenchyme is required for tooth development from the bud to the cap stage, its activity in the developing dental mesenchyme is spatiotemporally controlled for proper differentiation of the ameloblasts and odontoblasts during later stages of tooth morphogenesis.
Discussion
At the beginning of tooth development, the presumptive dental epithelium provides the tooth initiation signals to activate odontogenic potential in the developing tooth mesenchyme (Mina and Kollar, 1987; Lumsden, 1988; Ohazama et al., 2005). Several signaling molecules, including Bmp2, Bmp4, Fgf8, Fgf9, Shh, Wnt4, Wnt6, Wnt10a, and Wnt10b, are expressed in the presumptive dental epithelium at the beginning of tooth development (Wozney et al., 1988; Lyons et al., 1989; Neubuser et al., 1997; Dassule and McMahon, 1998; Sarkar and Sharpe, 1999). Previous studies demonstrated that both Bmp and Fgf signaling play essential roles in the induction of odontogenic potential in the developing tooth mesenchyme by activating expression of the Msx1 and Pax9 transcription factors, respectively (Neubuser et al., 1997; Mandler and Neubuser, 2001). In contrast, Shh signaling appears not directly involved in the activation of odontogenic potential in the developing tooth mesenchyme because mice lacking Smoothened, the obligate transducer of Shh signaling, throughout cranial neural crest derived tissues, developed molars and upper incisors (Jeong et al., 2004). In this report, we provide the first experimental evidence that β-catenin mediated canonical Wnt signaling is also required for the activation of odontogenic potential in the developing tooth mesenchyme for tooth development beyond the bud stage.
Wnt/β-catenin signaling plays a direct and essential role in activation of odontogenic mesenchyme
Several Wnt genes are expressed specifically in the developing dental epithelium during tooth bud formation and exogenous Wnt1, as well as Wnt10b, induced Lef1 mRNA expression in the E11.5 mouse mandibular mesenchyme (Dassule and McMahon, 1998; Sarkar and Sharpe, 1999), suggesting that canonical Wnt signaling may be involved in the epithelial-mesenchymal interactions during tooth initiation. However, expression of TOPGAL, a lacZ transgenic reporter gene under the control of a synthetic Wnt/β-catenin-responsive promoter containing three repeats of LEF1/TCF-binding sequences (DasGupta and Fuchs, 1999), has only been detected in the tooth epithelium, but not in the tooth mesenchyme, during the bud and cap stages of tooth development (Liu et al., 2008). Since Bmp2 and Bmp4 could also induce Lef1 mRNA expression in mouse mandibular mesenchyme (Chen et al., 1996; Kratochwil et al., 1996; Dassule and McMahon, 1998), the lack of TOPGAL expression in the early tooth mesenchyme raises questions about whether Lef1 is a direct target of canonical Wnt signaling in the tooth mesenchyme and whether Wnt signaling plays a direct role in the activation of the tooth mesenchyme. In this report, we show that tissue-specific inactivation of β-catenin in the developing tooth mesenchyme resulted in cell-autonomous loss of Lef1 mRNA expression, without affecting Bmp4 expression, in the developing tooth mesenchyme in the Catnbf/f; Osr2-IresCre mutant embryos. Thus, in the absence of β-catenin mediated canonical Wnt signaling, Bmp4 was insufficient to maintain Lef1 expression in the developing tooth mesenchyme. These data suggest that Lef1 mRNA expression is directly regulated by Wnt/β-catenin signaling in the developing tooth mesenchyme. With regards to lack of TOPGAL expression in the early tooth mesenchyme, several reports have indicated that the Wnt-responsive transgenic reporters, including TOPGAL and BAT-gal (Maretto et al., 2003), do not label all sites of known Wnt/β-catenin signaling (Dessimoz et al., 2005; Fathke et al., 2006; reviewed by Barolo, 2006). Our results suggest that Wnt/β-catenin signaling is capable of directly regulating the expression of Lef1 and other target genes in the developing tooth mesenchyme without activating TOPGAL expression.
The tooth developmental arrest phenotype of the Catnbf/f; Osr2-IresCre mutant mice is remarkably similar to that of the Lef1−/− mutant mice. Both mutant strains exhibited tooth developmental arrest at the late bud stage with losses of Fgf4 and Shh expression from the tooth epithelium and of Fgf3 expression in the tooth mesenchyme (Kratochwil et al., 1996). However, tissue recombination experiments indicated that Lef1 function was required in the tooth epithelium, but not in the tooth mesenchyme, for tooth morphogenesis in explant cultures (Kratochwil et al., 1996). Further studies demonstrated that Wnt signaling was able to directly activate reporter gene expression driven by the Fgf4 gene promoter and that exogenous Fgf4 rescued development of the Lef1−/− mutant tooth germs (Kratochwil et al., 2002). These studies led to the hypothesis that Lef1-mediated canonical Wnt signaling acts as a relay mechanism within the developing tooth epithelium through regulation of Fgf4 expression, which in turn signals to the mesenchyme to activate Fgf3 and other mesenchymal odontogenic factors to induce tooth development from the bud to the cap stage (Kratochwil et al., 2002). In the Catnbf/f; Osr2-IresCre mutant mice, however, β-catenin was inactivated in the developing tooth mesenchyme but not in the tooth epithelium. Lef1 expression was specifically down-regulated in the developing tooth mesenchyme but not in the tooth epithelium in these mutants. The lack of Fgf4 expression in the developing tooth epithelium in Catnbf/f; Osr2-IresCre mutant embryos indicates that intraepithelial Wnt/Lef1 signaling is insufficient to activate Fgf4 expression in the developing tooth epithelium in the absence of signals downstream of Wnt/β-catenin signaling in the developing tooth mesenchyme. The difference between our finding that Wnt/β-catenin signaling in the developing tooth mesenchyme is required for tooth development in vivo and the finding of Kratochwil et al. (1996) that Lef1 function was not required in the developing tooth mesenchyme for tooth morphogenesis in recombinant explants is probably due to functional redundancy between Lef1 and other Tcf family members. Indeed, Tcf1 is co-expressed with Lef1 in many embryonic tissues, including the developing tooth mesenchyme (Oosterwegel et al., 1993; Galceran et al., 1999). Moreover, Lef1 appears to function partially redundantly with Tcf1 and Tcf4, respectively, during limb and midfacial development (Galceran et al., 1999; Brugmann et al., 2007). Thus, by specifically inactivating β-catenin in the developing tooth mesenchyme, we have revealed an essential role for Wnt/β-catenin signaling in the activation of the odontogenic mesenchyme during early tooth development.
Wnt/β-catenin signaling and ectopic tooth initiation
Our finding that constitutive stabilization of β-catenin in the developing palatal mesenchyme caused initiation of tooth bud-like structures from the palatal epithelium is very intriguing. Two laboratories recently independently demonstrated that constitutively stabilizing β-catenin through epithelium-specific deletion of Exon-3 of the β-catenin gene using the K14-Cre transgenic mice resulted in continuous sequential tooth production from the embryonic molar tooth germs (Jarvinen et al., 2006; Liu et al., 2008). In addition, K14-Cre mediated epithelium-specific deletion of the Apc gene resulted in extra tooth formation next to the molar and incisor tooth germs in the K14-Cre;Apccko/cko mutant mice (Kuraguchi et al., 2006; Wang et al., 2009). These results indicate that constitutive activation of Wnt/β-catenin signaling in the oral epithelium can trigger ectopic initiation of tooth development. Interestingly, although many epithelial invaginations formed in the oral epithelium in the β-catex3K14/+ mice, extra teeth only developed from the dental epithelium (Jarvinen et al., 2006; Tummers and Thesleff, 2009), indicating that ectopic intraepithelial Wnt/β-catenin signaling was insufficient to activate the odontogenic program outside of the tooth developmental field. In Catnblox(ex3)/+;Osr2-CreKI mutant mice, tooth bud-like structures formed from the palatal epithelium but not from other regions, including the molar tooth epithelium, that are associated with Cre-expressing mesenchyme. One possible explanation of the restricted tissue response is the presence of other factors that antagonize the odontogenic program. We recently reported that a normal in vivo function of the Osr2 transcription factor is to restrict mesenchymal odontogenic activity within the tooth morphogenetic field (Zhang et al., 2009). Interestingly, although Osr2 is initially expressed throughout the palatal mesenchyme at E12.5, it is down-regulated from the medial side of the developing palatal shelves during palatal outgrowth such that Osr2 mRNA expression exhibits a lateral-medial gradient in the palatal mesenchyme by E13.5 (Lan et al., 2001; Lan et al., 2004). The fact that we only observed tooth bud-like epithelial invaginations from the medial side of the palatal shelves in the Catnblox(ex3)/+;Osr2-CreKI mutant mice suggest that the endogenous Osr2 transcription factor may antagonize Wnt/β-catenin-mediated activation of mesenchymal odontogenic potential in other regions where β-catenin is stabilized.
Although the tooth bud-like epithelial invaginations from the palatal epithelium in Catnblox(ex3)/+;Osr2-CreKI mutant mice expressed several tooth epithelial markers, transplantation of the palatal shelves from the Catnblox(ex3)/+;Osr2-CreKI mutant mice into adult mouse kidney capsules did not result in complete tooth morphogenesis. Further examination of expression of tooth developmental genes showed that the palatal epithelial invaginations in the Catnblox(ex3)/+;Osr2-CreKI mutant mice did not have expression of either Msx1 or Pax9 in the underlying mesenchyme. Both Msx1 and Pax9 are required for the normal tooth development beyond the bud stage (Satokata and Maas, 1994; Chen et al., 1996; Peters et al., 1998), which is probably at least part of the reason why those tooth bud-like structures could not complete tooth morphogenesis in the kidney capsule cultures. The lack of expression of Msx1 and of Pax9 in the mesenchyme underlying the palatal epithelial invaginations in the Catnblox(ex3)/+;Osr2-CreKI mutant mice, together with the finding that expression of Msx1 and of Pax9 were not altered in the tooth mesenchyme in the Catnbf/f; Osr2-IresCre mutant embryos, indicate that both the Wnt/β-catenin and the Pax9-Msx1 pathways are required and act in parallel to activate mesenchymal odontogenic potential during early tooth development. Further investigation of how Wnt/β-catenin signaling interacts with the Pax9-Msx1 and other pathways to activate the mesenchymal odontogenic potential will guide future research efforts in biological tooth regeneration for replacement therapy.
Supplementary Material
Acknowledgements
We thank Di Chen for the Catnblox(ex3)/+ mice, Zunyi Zhang for technical advice on kidney capsule transplantation procedures, and Kathleen Maltby for technical assistance. This work was supported by NIH/NIDCR grant R01DE013681 to R.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev. Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw EB, III, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Barolo S. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene. 2006;25:7505–7511. doi: 10.1038/sj.onc.1210057. [DOI] [PubMed] [Google Scholar]
- Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development. 1998;125:4325–4333. doi: 10.1242/dev.125.21.4325. [DOI] [PubMed] [Google Scholar]
- Bei M, Kratochwil K, Maas RL. BMP4 rescues a non-cell-autonomous function of Msx1 in tooth development. Development. 2000;127:4711–4718. doi: 10.1242/dev.127.21.4711. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Goodnough LH, Gregorieff A, Leucht P, ten Berge D, Fuerer C, Clevers H, Nusse R, Helms JA. Wnt signaling mediates regional specification in the vertebrate face. Development. 2007;134:3283–3295. doi: 10.1242/dev.005132. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr., Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–3044. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Dassule HR, McMahon AP. Analysis of epithelial.mesenchymal interactions in the initial morphogenesis of the mammailan tooth. Dev. Biol. 1998;202:215–227. doi: 10.1006/dbio.1998.8992. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Dessimoz J, Bonnard C, Huelsken J, Grapin-Botton A. Pancreas-specific deletion of β-catenin reveals Wnt-dependent and Wnt-independent functions during development. Curr. Biol. 2005;15:1677–1683. doi: 10.1016/j.cub.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Fathke C, Wilson L, Shah K, Kim B, Hocking A, Moon R, et al. Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol. 2006;7:4. doi: 10.1186/1471-2121-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Lan Y, Ovitt CE, Jiang R. Functional equivalence of the zinc finger transcription factors Osr1 and Osr2 in mouse development. Dev. Biol. 2009 doi: 10.1016/j.ydbio.2009.01.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: A laboratory manual. 2nd ed Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1994. [Google Scholar]
- Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18627–18632. doi: 10.1073/pnas.0607289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Dull M, Farinas I, Galceran J, Grosschedl R. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–1394. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(−/−) mice. Genes Dev. 2002;16:3173–3185. doi: 10.1101/gad.1035602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraguchi M, Wang XP, Bronson RT, Rothenberg R, Ohene-Baah NY, Lund JJ, Kucherlapati M, Maas RL, Kucherlapati R. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PloS. Genet. 2006;2:e146. doi: 10.1371/journal.pgen.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Kingsley PD, Cho ES, Jiang R. Osr2, a new mouse gene related to Drosophila odd-skipped exhibits dynamic expression patterns during craniofacial, limb, and kidney development. Mech. Dev. 2001;107:175–179. doi: 10.1016/s0925-4773(01)00457-9. [DOI] [PubMed] [Google Scholar]
- Lan Y, Ovitt CE, Cho E-S, Maltby KM, Wang Q, Jiang R. Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development. 2004;131:3207–3216. doi: 10.1242/dev.01175. [DOI] [PubMed] [Google Scholar]
- Lan Y, Wang Q, Ovitt CE, Jiang R. A unique mouse strain expressing Cre recombinase for tissue-specific analysis of gene function in palate and kidney development. Genesis. 2007;45:618–624. doi: 10.1002/dvg.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, Taketo MM, Morrisey EE, Atit R, Dlugosz AA, Millar SE. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol. 2008;313:210–224. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Sun X, Braut A, Mishina Y, Behringer RR, Mina M, Martin JF. Distinct functions for Bmp signaling in lip and palate fusion in mice. Development. 2005;132:1453–1461. doi: 10.1242/dev.01676. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lumsden AG. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development. 1988;103:155–169. doi: 10.1242/dev.103.Supplement.155. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development. Genes Dev. 1989;3:1657–1668. doi: 10.1101/gad.3.11.1657. [DOI] [PubMed] [Google Scholar]
- Mandler M, Neubuser A. FGF signaling is necessary for the specification of the odontogenic mesenchyme. Dev. Biol. 2001;240:548–559. doi: 10.1006/dbio.2001.0490. [DOI] [PubMed] [Google Scholar]
- Mansour SL. Targeted disruption of int-2 (fgf-3) causes developmental defects in the tail and inner ear. Mol. Reprod. Dev. 1994;39:62–68. doi: 10.1002/mrd.1080390111. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, et al. Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina M, Kollar EJ. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch. Oral Biol. 1987;32:123–127. doi: 10.1016/0003-9969(87)90055-0. [DOI] [PubMed] [Google Scholar]
- Mucchielli ML, Mitsiadis TA, Raffo S, Brunet JF, Proust JP, Goridis C. Mouse Otlx2/RIEG expression in the odontogenic epithelium precedes tooth initiation and requires mesenchyme-derived signals for its maintenance. Dev. Biol. 1997;189:275–284. doi: 10.1006/dbio.1997.8672. [DOI] [PubMed] [Google Scholar]
- Neubuser A, Peters H, Balling R, Martin GR. Antagonistic interactions between FGF and BMP signalling pathways: a mechanism for positioning the sites of tooth formation. Cell. 1997;90:247–255. doi: 10.1016/s0092-8674(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Tucker A, Sharpe PT. Organized tooth-specific cellular differentiation stimulated by BMP4. J. Dent. Res. 2005;84:603–606. doi: 10.1177/154405910508400704. [DOI] [PubMed] [Google Scholar]
- Oosterwegel M, van de Wetering M, Timmerman J, Kruisbeek A, Destree O, Meijlink F, Clevers H. Differential expression of the HMG box factors TCF-1 and LEF-1 during murine embryogenesis. Development. 1993;118:439–448. doi: 10.1242/dev.118.2.439. [DOI] [PubMed] [Google Scholar]
- Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pispa J, Thesleff I. Mechanisms of ectodermal organogenesis. Dev. Biol. 2003;262:195–205. doi: 10.1016/s0012-1606(03)00325-7. [DOI] [PubMed] [Google Scholar]
- Sarkar L, Sharpe PT. Expression of Wnt signalling pathways genes during tooth development. Mech. Dev. 1999;85:197–200. doi: 10.1016/s0925-4773(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Satokata I, Maas R. Msx-1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat. Genet. 1994;6:348–356. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Hurmerinta K. Tissue interactions in tooth development. Differentiation. 1981;18:75–88. doi: 10.1111/j.1432-0436.1981.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Vaahtokari A, Kettunen P, Aberg T. Epithelial-mesenchymal signaling during tooth development. Connect. Tissue Res. 1995;32:9–15. doi: 10.3109/03008209509013700. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Matthews KL, Sharpe PT. Transformation of tooth type induced by inhibition of BMP signaling. Science. 1998;282:1136–1138. doi: 10.1126/science.282.5391.1136. [DOI] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. The importance of signal pathway modulation in all aspects of tooth development. J. Exp. Zoolog. B. Mol. Dev. Evol. 2009 doi: 10.1002/jez.b.21280. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Tureckova J, Sahlberg C, Åberg T, Ruch JV, Thesleff I, Peterkova R. Comparison of expression of the msx-1, msx-2, BMP-2 and BMP-4 genes in the mouse upper diastemal and molar tooth primordia. Int. J. Dev. Biol. 1995;39:459–468. [PubMed] [Google Scholar]
- Vainio S, Karavanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- Van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, Maas RL, Chuong CM, Schimmang T, Thesleff I. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 2007;5:e159. doi: 10.1371/journal.pbio.0050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-P, O'Connell DJ, Lund JJ, Saadi I, Kuraguchi M, et al. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development. 2009;136:1939–1949. doi: 10.1242/dev.033803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Zhang YD, Zhao X, Hu Y, St. Amand TR, Ramamurthy R, Qiu MS, Chen Y. Msx1 is required for the induction of Patched by Sonic hedgehog in the mammalian tooth germ. Dev. Dyn. 1999;215:45–53. doi: 10.1002/(SICI)1097-0177(199905)215:1<45::AID-DVDY5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lan Y, Chai Y, Jiang R. Antagonistic actions of Msx1 and Osr2 pattern mammlian teeth into a single row. Science. 2009;323:1232–1234. doi: 10.1126/science.1167418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhang Z, Song Y, Zhang X, Zhang Y, Hu Y, Fromm SH, Chen Y. Transgenically ectopic expression of Bmp4 to the Msx1 mutant dental mesenchyme restores downstream gene expression but represses Shh and Bmp2 in the enamel knot of wild type tooth germ. Mech. Dev. 2000;99:29–38. doi: 10.1016/s0925-4773(00)00467-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.