Abstract
Regulatory T cells (Tregs) and beta-galactoside-binding protein (βGBP), a regulatory protein often found expressed at sites of immunological privilege, have similar functions. Their presence affects the outcome of harmful autoimmunity and cancers, including experimental autoimmune encephalomyelitis and malignant gliomas. Here we report a novel pathway by which Tregs express and utilize βGBP to control CD8+ T cell responses partially activating TCR signaling but blocking PI3K activity. As a result, this leads to a loss of p21ras, ERK and Akt activities despite activation of TCR proximal signals, such as phosphorylation of CD3ζ, Zap70, Lat and PKCθ. Although non-processive TCR signaling often leads to cell anergy, Tregs/βGBP did not affect cell viability. Instead, βGBP/Tregs transiently prevented activation of CD8+ T cells with self-antigens, while keeping their responses to xenogeneic antigens unaffected.
Keywords: βGBP, galectin-1, regulatory T cells, TCR signaling, MAPK, ERK, anergy
Introduction
Regulatory T cells (Tregs) are a unique CD25+ subset of CD4+ cells that play an essential role in the regulation and control of peripheral tolerance to self- and allo-antigens (Sakaguchi et al., 1995;Sakaguchi, 2000). Infiltration of Tregs is associated with the progression of human metastatic brain tumors (Sugihara et al., 2009) and protection from CNS immune disease in murine models (Verhagen et al., 2009); and their dysfunction leads to the spontaneous onset of autoimmune disorders (Alyanakian et al., 2003), or inversely correlates with tumor immunosurveillance and positive disease outcome (Woo et al., 2002;Curiel et al., 2004;Viglietta et al., 2004). However, the nature of Tregs and the mechanism of their regulatory activity remain poorly understood. Due to the absence of definitive markers, Tregs are often difficult to segregate from activated CD4+ T cells, specifically Th2-polarized cells that can exert suppressive functions via production of immunosuppressive cytokines (Bonecchi et al., 1998). Although CD25− subset of CD4+ T cells (non-Tregs), particularly in greater numbers, were shown to be suppressive (Stephens and Mason, 2000;Annacker et al., 2001), their primary function is to suppress Th1-type polarization (presumably via production of immunomodulatory cytokines like IL-10) without affecting T cell proliferation (Baatar et al., 2007). In contrast, the majority of Tregs appear to use a contact-mediated regulatory process to suppress the proliferation of activated T cells and dendritic cells (Sakaguchi, 2000;Cavani et al., 2000;Zheng et al., 2004). Yet, the choice of a particular regulatory pathway may depend on the nature of cells or the strength/type of stimulation. For example, a different type of Tregs, such as so-called memory-type CD25+CD4+ Tregs (TREM) and Tr1, appear to primarily utilize soluble factors, such as IL-10 and TGFβ (Levings and Roncarolo, 2000;Jonuleit et al., 2001;Shevach, 2001), although Tr1 cells can utilize cell contact-dependent perforin/granzyme-mediated regulatory processes (Grossman et al., 2004;Gondek et al., 2005).
We have recently reported that granzymes are not expressed and not utilized by human peripheral blood Tregs (Baatar et al., 2007). These Tregs belong to the memory-type CCR4-expressing cells that are already primed and efficiently suppress the proliferation of CD8+ T cells. Recently, it was proposed that beta-galactoside-binding protein (βGBP) is expressed by Tregs (Garin et al., 2007), as well as activated T cells (Allione et al., 1998);(Blaser et al., 1998), to regulate T cells. βGBP is a 15 KDa monomeric form of homodimeric lectin-type protein Galectin-1 (Gal-1) which oligomerizes at concentrations greater than 7μM (Cho and Cummings, 1996). It is found often produced at sites of immunological privilege, including the CNS in hippocampus (Kajitani et al., 2009) and in the subventricular zone (Ishibashi et al., 2007). Its expression is detected in normal astrocytes and neural stem cells (Sakaguchi et al., 2006;Kajitani et al., 2009) and shown to be markedly up regulated in the area of neuronal death (Kajitani et al., 2009) and in brain tumors, such as gliomas and neuroblastomas (Le et al., 2009;Cimmino et al., 2009). TrkB-associated aggressiveness of neuroblastomas is mediated with βGBP/Gal-1 produced in response to brain-derived neutrophilic factor (Le et al., 2009;Cimmino et al., 2009). Although βGBP/Gal-1 can induce astrocyte differentiation (Sasaki et al., 2004) or promote proliferation of adult neural stem cells (Sakaguchi et al., 2006;Kajitani et al., 2009), it is mostly known for its immunosuppressive functions. It ameliorates harmful autoimmunity and prevents experimental autoimmune encephalomyelitis (Offner et al., 1990;Rabinovich et al., 1999) through the control of T cell responses, such as induction of cell-cycle arrest of activated T cells (Novelli et al., 1999;Ravatn et al., 2005), death of activated T cells (Allione et al., 1998), or promotion of pro-survival signals in naïve T cells (Endharti et al., 2005). The differential and opposing results appear to depend on the oligomerisation state of βGBP and the activation state of target cells. Unlike Gal-1 whose activity relates to the cross-linking of cell surface glycan complexes, βGBP monomers lack lectin properties and operate through high affinity (Kd 1.5×10−10M) to ~10×104 receptors/cell independently of saccharide determinants (Wells and Mallucci, 1991;Wells and Mallucci, 1992;Allione et al., 1998). As such it controls exit from G0 and transition from S phase to G2/M (Wells and Mallucci, 1991;Mallucci and Wells, 2005). Also it controls class IA and class IB phosphatidylinositol 3-OH kinase (PI3K) inhibiting its p110 catalytic subunit and resulting in suppression of Ras-GTP loading, consequent loss of ERK activation and block of somatic cell proliferation (Wells et al., 2007).
Here we report that βGBP is produced and utilized by Tregs to prevent self-antigen –induced activation of resting CD8+ T cells. To do this, βGBP controls PI3K signaling of CD8+ T cells blocking activation of ERK/MAPK and Akt signaling needed for promoting TCR –induced cell proliferation. Interestingly, this does not lead to inhibition of proximal TCR signaling events in CD8+ T cells, such as activation of CD3ζ, ZAP70 and Lat; and does not induce anergy. Instead the cells acquire significantly increased survival and respond stronger to xenogeneic antigens. Together, our data shed a new light on the mechanisms and unique biological role of βGBP in the control of peripheral tolerance to self-antigens.
Materials and Methods
Chemicals and reagents
Reagents were purchased from Sigma (St. Louis, MO), unless specified otherwise. Human βGBP and neutralizing βGBP Ab was reported elsewhere (Wells and Mallucci, 1991;Allione et al., 1998). Anti-human Gal-1 (AF1152 and BAF1152), phycoerythrin (PE)-conjugated anti-CD8 Abs, FITC-conjugated anti-CCR4 Ab, and T cell enrichment columns were from R&D Systems Inc. (Minneapolis, MN). RPMI 1640 medium, fetal bovine serum, human AB serum, Dynabeads® CD8, Dynabeads® CD25, Dynabeads® CD4, DETACHaBEAD® reagent, carboxyfluorescein diacetate succinimidyl ester (CFSE), Alexa-conjugated secondary antibodies were purchased from Invitrogen Corp. (Carlsbad, CA). Fix Buffer I, Perm Buffer III, anti-CD3ε Ab (NA/LE, UCHT1 clone), anti-CD3ζpY142-PE and anti-phospho ERK 1/2-PE Abs were from BD Biosciences (San Jose, CA). Anti-phospho-ERK1/2 (Thr202/Tyr204), phospho-ZAP-70 (Tyr319)/Syk(Tyr352), ZAP70, phospho-LAT (Tyr171), LAT, phopsho-PKCθ (Thr538), PKCθ, phosphor-Akt (Thr308 and Ser473), and β-tubilin Abs were from Cell Signaling Technology Inc. (Danvers, MA). RIPA lysis buffer and anti-ERK2 Abs were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). RDI-pHRP20-SA and TMB-S for ELISA were from RDI Division of Fitzgerald Industries Intl. (Concord MA). Jurkat T cell line (Clone E6-1), human acute T-lymphoblastic leukemia cell lines CCRF-CEM (CEM, CCL-119), and MOLT-4 (CRL-1582) were purchased from ATCC (Manassas, VA).
Isolation of T cells
Human peripheral blood was taken from healthy donors with written informed consent in accordance with Human Subject Protocol #2003054 of the Health Apheresis Unit and the Clinical Core Laboratory of the National Institute on Aging. T cells were isolated from Ficoll-Paque (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) density gradient–separated PBMCs using human CD3+ T cell enrichment columns. CD8+ T cells were selected from T cells using Dynabeads® CD8. CD25+ T cells (Tregs) were selected from CD8+-depleted T cells using Dynabeads® CD25. The CD25-depleted fraction of CD4+T cells was used as a control for non-Tregs. Beads were removed from cells using DETACHaBEAD reagent. Monocyte/macrophage-enriched PBMCs (antigen presenting cells, APCs) were isolated by plastic adherence and irradiated with 4500 rad before use in cell proliferation assays.
Flow cytometry analysis
βGBP expression was detected by staining cells with goat anti-Gal-1 Ab or non-immune goat IgG and Alexa 555-conjugated anti-goat IgG. For some experiments Tregs were double stained with FITC-conjugated anti-CCR4 Ab and anti-Gal-1 Ab and βGBP expression was evaluated separately on CCR4-positive and CCR4-negative subsets of Tregs. For detection of phosphorylated CD3ζ and ERK1/2 in CD8+Tcells, cells were first fixed and permeabilized using Fix Buffer I and Perm Buffer III, respectively and, thereafter, were stained with anti-CD3ζpY142 and anti-phospho-ERK1/2-PE Abs, respectively. To co-localize βGBP with FoxP3 expressing population of cells, freshly isolated human CD4 T cells were first fixed (Fix/Perm sol) and permeabilized (Perm sol), then blocked in 10% donkey goat serum and stained with goat anti-Gal-1 Ab (R&D) or control IgG and Alexa 488-conjugated anti-goat IgG (Invitrogen) and PE-conjugated FoxP3 (Clone 259D, Biolegend). Flow cytometry analysis was performed using a FACScan flow cytometer and Cell Quest Pro software (BD Biosciences).
Confocal microscopy
CD8+ T cells (5×104) were labeled with CFSE and were mixed with Tregs or non-Tregs (ratio 4:1). Cells were stained with anti-GAL-1 Ab in the same way as described for flow cytometry analysis. The cells were spun onto glass slides using a Shandon Cytospin® 3 Cytocentrifuge (Thermo Fisher Scientific, Inc., Waltham, MA), and were treated with 3.7% formaldehyde and ProLong anti-fade reagent (Invitrogen Corp.). Confocal images were acquired with a 40 × objective on a Zeiss LSM 510 confocal system (Carl Zeiss, Heidelberg, Germany), and were then processed using Zeiss LSM Image Browser (Carl Zeiss).
Suppression Assay
To test Treg-mediated suppression, titrated numbers of Tregs or non-Tregs were mixed with the CFSE-labeled CD8+ T cells (5×104 each). The cells were stimulated with soluble CD3 Ab (1 μg/ml) in the presence of autologous APCs (5×104) in V-bottom 96-well plates for 4 days. To test a direct role of βGBP, cells were cultured in the presence or absence of βGBP neutralizing Ab or control non-immune mouse IgG. To evaluate effect of pre-incubation with βGBP, CFSE-labeled CD8+ T cells were first cultured in the presence or absence of 1 μg/ml βGBP for 3 hours; then cells were washed and cultured in a fresh cRPMI for additional 24 hours (pre-incubation) and were stimulated with soluble CD3 Ab (1 μg/ml for proliferation and 10 μg/ml for ERK activation). Some cells were treated with βGBP at the time of activation with CD3 Ab (co-incubation). Cells were either lysed for western blotting or allowed to proliferate for 4 days in the presence of autologous APCs. The propidium iodide (PI) staining was used to exclude/evaluate dead cells. Proportion of CD8+ T cells that underwent divisions and had decreased CFSE expression was evaluated (proliferation rate).
ELISA and Western blot assays
βGBP was measured in culture media by ELISA using anti-Gal-1 Ab (1 μg/mL; AF1152) for capture and biotin-conjugated anti-Gal-1 (200 ng/mL, BAF1152) for detection. CD8+ T cell TCR signaling was assessed in western blotting after Tregs or non-Tregs were removed with Dynabeads® CD4. For Jurkat E6.1 cells, ERK phosphorylation was tested after serum starvation for 16 hours. To test the role of PKC, CD8+ T cells were also treated with 10 nM phorbol myristate acetate for 20 minutes at 37°C. Blots were first probed with antibodies specific to phosphorylated form of proteins. Then, the blots were stripped and re-probed with Abs specific for corresponding proteins. To confirm equal loading, the blots were also re-probed with anti-β-tubulin Ab. Active p21Ras levels were measured as described previously (Wells and Mallucci, 1991) in Jurkat cell lysates containing 100mM NaCl, 50mM Tris, pH 7.5, 1% Triton X-100, 1mM EDTA, 10mM MgCl2, 20mM sodium fluoride, 1mM sodium vanadate, 10ug/ml aprotinin, 10ug/ml leupeptin (Sigma, UK). The lysates were incubated with the Ras binding domain of Raf-1- coupled to GST-agarose beads to precipitate active p21Ras and western blots of active p21Ras were probed with anti-pan-Ras Ab (BD Transduction Laboratories, Franklin Lakes, NJ). Total p21Ras was assessed using the same anti-pan-Ras Ab in Western blots of total cell lysates. PI3K activity was measured as described previously (Wells and Mallucci, 1991) by immunoprecipitating it from cell lysates using an anti-PI3K p85 Ab (Upstate Biotechnology, Lake Placid, NY) and protein A- agarose. The immunoprecipitated PI3K was incubated in a kinase reaction for 3 h with 40 pmol PI(4,5)P2 substrate and the PI(3,4,5)P3 generated was assayed in a competitive ELISA (Echelon Biosciences, Salt Lake City, UT). Absorbance of the samples was measured at 450nm and the PI(3,4,5)P3 was quantified by comparison with a PI(3,4,5)P3 standard curve carried out in parallel with the experimental samples and plotted on a log scale. Quantitative evaluation of intensity of Western blot bands was performed using ImageJ 1.37V software (NIH, USA). Data are presented as the ratio of band intensity of phosphorylated protein to that of total protein.
Effects of bGBP on antigen-specific cell proliferation
T cell responses to self and xeno peptides were tested using mouse gp10025-33 (EGSRNQDWL) and human gp10025–33 (KVPRNQDWL) peptides that are specifically recognized by H-2Db-restricted TCR transgenic CD8+ T cells (Vα1Vβ13) from Pmel-1 mice (Overwijk et al., 2003). Mice were bred and housed at the National Institute of Aging animal facility in Baltimore, MD. Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication no. 86-23, 1985). Pmel mice splenocytes were harvested and cultured in complete RPMI 1640 medium containing 10% FBS, 20 IU/ml recombinant human IL-2, and 1 μg/ml corresponding peptide (mouse gp100 and human gp100, respectively) in the presence of 1 μg/ml of βGBP for 4 days. Proliferation of splenocytes was measured by BrdU incorporation using Cell Proliferation ELISA, BrdU kit (Roche Applied Science, Indianapolis, IN).
Statistical Analysis
All data are representative of at least 2 experiments. For all graphs the data represent the mean ± SD. Differences were tested using Student's t test and p-value less than 0.05 was considered statistically significant.
RESULTS
Human peripheral blood-derived natural Tregs use βGBP to regulate T cell proliferation
Besides activated T cells (Allione et al., 1998);(Blaser et al., 1998), βGBP is reported to be also produced by Tregs (Garin et al., 2007). However, compared with non-Tregs and CD8+ T cells, TCR stimulation induced significantly higher βGBP production from Tregs (Fig.1a). It was also found expressed on the surface of freshly isolated Tregs (Fig.1b,c), but not non-Tregs or CD8+ T cells (Fig.1b,c). Tregs were indeed the major source of βGBP, as almost every FoxP3+ cells expressed βGBP (Fig.1d). Recently, we have reported the existence of two discrete Treg subsets in human peripheral blood, CCR4+ and CCR4− Tregs, where former was already primed to suppress proliferation of CD8+ T cells (Baatar et al., 2007). βGBP was primarily expressed on the surface of freshly isolated CCR4+ Tregs, but not CCR4− Tregs (Fig.2a), indicating its potential role in the regulation of CD8+ T cell proliferation. Indeed, the presence of βGBP neutralizing, but not isotype-matched, Ab significantly reversed the Treg-mediated suppression of CD8+ T cell proliferation in a dose-dependent manner (closed circles, Fig.2b). This is truly a Treg-mediated suppression, as CD8+ T cell proliferation was not affected by co-culture with non-Treg CD4+ cells (triangles, Fig.2b), regardless of the presence or absence of Ab. As we previously reported (Baatar et al., 2007), Tregs did not induce cell death of target CD8+ T cells (data not shown). Similarly, nM amounts of human βGBP also blocked proliferation CD8+ T cells (Fig.2c) without induction of cell death (Fig.2d) after CD3 Ab stimulation.
Figure 1. βGBP is expressed on the surface of Tregs and secreted upon activation.
(A) ELISA results of βGBP in conditioned media of T cells (Tregs, non-Tregs and CD8+ T cells T cells, each 5×105) after 2 days of activation with CD3Ab. *P<0.05 versus CD8+ cells. (B) βGBP expression (red) on non-activated freshly isolated human peripheral blood Tregs or non-Tregs mixed with CFSE-labeled CD8+ T cells (green). Cells were stained with anti-Gal-1 Ab followed by Alexa 555-conjugated secondary Ab and analyzed by confocal microscopy. (C) Tregs or non Tregs were stained for βGBP as for Figure 1B after 2 days of activation with plate-bound CD3Ab and were analyzed by flow cytometry. (D) FoxP3-expressing subsets, Tregs, mostly express βGBP. Data are from intracellular staining of freshly isolated Tregs with antibodies for βGBP and FoxP3. βGBP expression is shown by thick line (C and D). Thin line represents staining with control Ab (Cnt Ab). All results were independently reproduced at least three times using different donor blood from normal humans.
Figure 2.
(A) βGBP is only expressed on the surface of freshly-isolated CCR4+ Tregs, but not CCR4− Tregs. For comparison, mean fluorescence intensity (MFI) of non-Tregs is shown. “Cnt Ab” represents staining with control isotype-matched Ab. (B) Tregs utilize βGBP to mediate regulation of CD8+ T cells. CFSE-labeled CD8+ T cells (5×104) were incubated with Tregs or non-Tregs (ratio 4:1) in the presence of indicated amounts of βGBP-neutralizing Ab (βGBP Ab) or control mouse IgG (Cnt Ab). Cells were stimulated with CD3 Ab (1 μg/ml) in the presence of autologous APCs for 4 days. Proportion of proliferated CD8+CFSElow cells was evaluated by flow cytometry analysis. Results are presented in percentage of the proliferation rate of CD8+ T cells cultured alone. *P<0.05 is for comparisons with corresponding doses of Cnt Ab. (C) Free βGBP also inhibits CD8+ T cell proliferation. Cells were cultured in the presence of titrated amounts of βGBP and stimulated as for Figure 2A. Results are expressed as percentage of the proliferation rate of untreated (0) cells. (D) Suppressive doses of βGBP are not cytotoxic for CD8+T cells. CFSE-labeled CD8+ T cells were stimulated as for Figure 2A and were cultured in the presence of titrated amounts of βGBP for 2 days. Cells were stained with propidium iodide (PI) and analyzed by flow cytometry. Data have been independently reproduced at least three times using different donor blood from normal humans, and mean ± SD of triplicates shown. *P<0.05 is for comparisons with untreated cells (0).
Tregs inhibit ERK phosphorylation without affecting proximal TCR signaling of target T cells
Next, since T cell proliferation requires activation of TCR, we have hypothesized that by releasing βGBP Tregs may interfere with TCR signaling in CD8+ T cells. Thus, we tested activation of TCR ζ-chain, an early event induced by the binding of a cognate antigen. Surprisingly, CD3 Ab–induced phosphorylation of ζ-chain of CD8+ T cells was not blocked, but rather slightly enhanced by co-culture with Tregs, but not non-Tregs (Fig.3a). Similarly, Tregs also enhanced the activation of its downstream signaling molecule Zap70 in CD8+ T cells instead of inhibiting it (Fig.3b), indicating that Tregs do not suppress proximal TCR signaling. In contrast, Tregs significantly inhibited CD3 Ab-induced phosphorylation of ERK in CD8+ T cells (Fig.3c,d). To do this Tregs utilized βGBP, as its inhibitory activity was neutralized by the presence of neutralizing βGBP Ab, but not control Ab (Fig.3c,d). Neither antibodies affected ERK phosphorylation of CD8+ T cells cultured with non-Treg CD4+ T cells (Fig.3c,d). Furthermore, CD3 Ab-induced ERK phosphorylation in CD8+ T cells was also inhibited by treatment with nM amounts of βGBP alone (Fig.3e). Together, these results presumably explain the anti-proliferative activity of Tregs/βGBP, as ERK is a major signaling molecule that controls cell proliferation in response to TCR stimulation. However, this process required TCR signaling through Zap70, as βGBP failed to inhibit ERK phosphorylation in human transformed T cells with non-functional Zap70 (p116 Jurkat cells (Williams et al., 1999;Shan et al., 2001)) (Fig.4a). In contrast and importantly, this inability was reversed by reintroduction of the wild-type Zap70 gene into P116 cells (P116.cl39) inducing significant inhibition of ERK phosphorylation (Fig.4a) at the same extent as in control parental Jurkat E6.1 cells (Fig.4a). Interestingly, TCR signaling was also required for cytotoxicity of high doses of βGBP (Gal-1, ≥ 50 μg/ml, Fig.4b), as unlike parental Jurkat E6.1 cells, it did not affect viability of the T cell lines that were deficient in ZAP70 (P116) or TCR (CEM cells).
Figure 3.
(A) Tregs do not suppress CD3 Ab-induced CD3ζ phosphorylation. CFSE-labeled CD8+ T cells were co-cultured with Tregs or non Tregs (ratio 4:1) for 6 hours and then were activated with CD3 Ab for 5 min, fixed, permeabilized and stained with anti-CD3ζpY142 Ab and were analyzed by flow cytometry. Histogram shows expression of CD3ζ phosphorylated at tyrosine 142 in CD8+ T cells (CFSE-gated cells). (B) Tregs do not suppress CD3 Ab-induced ZAP70 phosphorylation in CD8+ T cells. Cells were cultured as for Figure 3A and then, Tregs and non- Tregs were removed from the mixtures using Dynabeads® CD4, and the remaining CD8+ T cells were either lysed immediately (non-activated, NA) or activated as above (Act) and then, lysed to test for phosphorylated form of Zap70 (pZap70). (C and D) Tregs utilize βGBP to suppress CD3 Ab-induced ERK phosphorylation of CD8+ T cells. Flow cytometry specifically gated on CD8+ T cells (C) or western blotting on lysates from purified CD8+ T cells (D) that were cultured with either Tregs or non-Tregs (ratio 4:1) in the presence of βGBP-neutralizing Ab (βGBP Ab) or control isotype-matched IgG (Cnt Ab) for 6 hours prior to activation with soluble CD3 Ab (5 min). For Figure 3D Tregs and non-Tregs were removed from cell mixtures prior to activation as described in Materials and Methods. *P<0.05 is for comparison with βGBP Ab in CD8+Treg group (D). Lower panel in Figure 3D are data after quantitative densitometry. (E) Western blotting of lysates from CD8+ T cells incubated with titrated amounts of βGBP (μg/ml) for 3 hours prior to stimulation with CD3 Ab. The results have been independently reproduced at least three times using different donor blood from normal humans, and mean ± SD of the individual experiments in duplicates.
Figure 4.
(A) The suppression is ZAP70-dependent, βGBP did not affect ERK activity in ZAP70-deficient Jurkat cells (p116). However, the ability of βGBP to suppress ERK activity was restored in p116 that were retransfected with Zap70 (P116cl.39), which was as good as in w.t. Jurkat cells (E6.1). The cells were treated with 1 μg/ml βGBP for 3 hours. (B) βGBP at high doses (50 μg/ml) are cytotoxic for Jurkat cells, but not for TCR-deficient CEM or Zap70-deficient p116 cells. The viability was evaluated by Anexin V/PI staining. *P<0.05 is for comparisons with untreated cells.
βGBP activates non-processive TCR signaling that is blocked at PI3K-Ras step
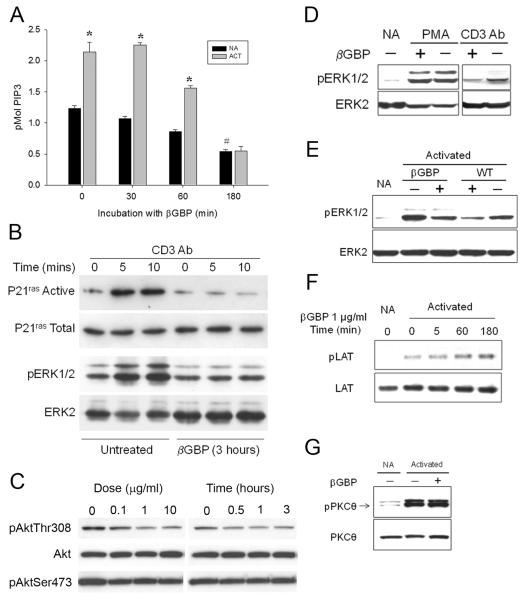
TCR-mediated T cell proliferation requires ERK activation that is controlled by sequential activation of Ras, Raf, and MEK, and PKC (Downward et al., 1990;Mitin et al., 2005). We have recently reported that in somatic cells βGBP inhibits growth factor-induced activation of the Ras-ERK signaling cascade by targeting the p110 catalytic subunit of PI3K (Wells et al., 2007). Thus, βGBP may also similarly act in T cells. Indeed, Figure 5a (grey bars) shows that the response to CD3 stimulation in terms of phosphatidylinositol(3,4,5)P3 (PIP3) generated, the product of PI3K catalyic activity, was reduced below basal levels within a period of 3 hours (180 minutes) from the addition of βGBP. Notably, such a response did not relate to CD3 Ab activation as βGBP could bring down basal PIP3 levels to similar values and within a similar time scale in the absence of CD3 stimulation (Fig.5a, dark bars), furthermore, no Ras-GTP loading did occur once PI3K activity had been brought down by 50% below basal levels (Fig.5b). This was also associated with suppression of its other downstream signaling events such as Akt phosphorylation at Thr308 position (Fig. 5c). Demonstration that prevention of ERK phosphorylation related to the abrogation of Ras activation rather than to an effect downstream of Ras was obtained bypassing Ras by the addition of 20 nM 12-o-tetradecanol phorbol-13-acetate (PMA), a phorbol ester that activates ERK signaling via PKC to MEK (Schonwasser et al., 1998). Figure 5d shows that βGBP-mediated inhibition of ERK phosphorylation was abrogated by co-incubation with PMA, but not otherwise. These results copy the results previously obtained in somatic cells. To validate the functional link between inhibition of PI3K activity by βGBP and blockage of ERK activation in cells ex vivo, we carried out parallel experiments in CD8+ T cells using βGBP and wortmannin, a pharmacological inhibitor of the p110 catalytic subunit of PI3K. Figure 5e shows that βGBP and wortmannin similarly inhibited CD3 Ab induced ERK phosphorylation.
Figure 5. Mechanism of antiproliferative effects of βGBP.
(A) βGBP suppresses basal and CD3 Ab-induced PI3K activity in T cells. Serum-starved Jurkat T cells were treated with βGBP for 3 hours and were activated with CD3 Ab for 5 min. Black and grey bars stand for non-activated (NA) and activated (Act) with CD3 Ab cells, respectively. *P<0.05 is for comparisons with NA at the corresponding time point. #P<0.05 is for comparison with NA at 0 min. βGBP suppresses CD3 Ab-induced Ras activation (B) and phosphorylation of Akt at Thr308 (C). Serum-starved Jurkat T cells were treated with βGBP for 3 hours and thereafter were activated with CD3 Ab for either 5 or 10 min and levels of active P21ras and Akt. Note that Akt at Ser473 is not affected by βGBP due to its constitutively phosphorylated state in Jurkat cells. (D) βGBP does not suppress PMA- induced ERK phosphorylation. CD8+ T cells were pretreated with 1 μg/ml βGBP for 3 hours and were activated with PMA (10 nM) or soluble CD3 Ab (10 μg/ml). NA: non-activated cells. (E) Wortmannin suppresses CD3 Ab-induced ERK phosphorylation. CD8+ T cells were pretreated with 100 nM wortmannin (WT) for 30 minutes or with βGBP for 3 hours and then, were activated with CD3 Ab for 5 min prior to lysis and western blotting for pERK1/2 and ERK2. (F) βGBP does not suppress CD3 Ab-induced LAT phosphorylation. CD8+ T cells were incubated with βGBP (1 μg/ml) for the indicated time periods prior to activation with CD3 Ab for 5 min (Activated). Cells were lysed and analyzed for pLAT and LAT expression by western blotting. NA: non-activated cells. (G) βGBP does not suppress CD3 Ab-induced PKCθ phosphorylation. CD8+ T cells were incubated with 1 μg/ml βGBP for 3 hours and activated with CD3 Ab for 5 minutes prior to lysis and western blotting.
βGBP transiently suppresses T cell responses to self, but not xeno, antigens
TCR/ZAP70 activation that does not lead to T cell proliferation is usually associated with so-called “non-processive” TCR signaling (Chung et al., 2000) that hypophosphorylates the linker for activation of T cells (LAT). However, βGBP did not suppress but rather enhanced CD3 Ab-induced LAT phosphorylation in CD8+ T cells (Fig.5f), indicating that the ability of LAT to recruit various signaling proteins was not necessarily affected. Similarly, βGBP did not inhibit PKCθ activation after CD3 Ab treatment (Fig.5g). Together, these data are in concordance with our previous observation that Treg/βGBP only suppress proliferation of resting CD8+ T cells without induction of cell death (Fig.2d and see (Baatar et al., 2007)). However, the Tregs/βGBP-induced regulation was transient, as CD8+ T cells readily responded to TCR stimulations by proliferating (Pre-incubation, Fig.6a) and activating ERK (Fig.6b, right panel) after removal of βGBP/Tregs. The CD8+ T cells could proliferate even after several days of βGBP pretreatment (data not shown). In addition, Tregs/βGBP only inhibited ERK activity and proliferation of CD8+ T cells stimulated with soluble CD3 Ab, a treatment considered to be sub-optimal. In concordance with our previous report (Baatar et al., 2007), Tregs/βGBP poorly inhibited proliferation and ERK activity of CD8+ T cells that were stimulated with strong activators, such as plate-bound CD3 Ab (Fig.6b, left panels). Thus, Tregs might utilize βGBP mainly to regulate CD8+ T cell responses to low affinity signals such as self-antigens. Indeed, while βGBP readily inhibited proliferation of murine TCR transgenic CD8+ T cells stimulated with a cognate peptide from mouse gp100, it did not have any effects on cells that were stimulated with high-affinity peptide from human gp100 (Fig.6c). Together, Tregs prevent anti-self hyperactivity of resting CD8+ T cells using βGBP that induces transient and reversible blockage of TCR signaling at the PI3K-induced Ras/MAPK activation step.
Figure 6.
(A and B) The suppressive effect of βGBP is reversible. Cells were either incubated with βGBP for 3 hours, washed and cultured in a fresh medium overnight at 37°C prior to stimulation (pre-incubation) or were treated with βGBP for 3 hours immediately prior to stimulation (co-incubation). Cells were activated with either soluble CD3 Ab (soluble CD3) or plate-bound CD3 Ab (plate CD3). Legend indicates titrated amounts of βGBP (μg/ml). (A) Proportion of proliferated CD8+CFSElow cells was evaluated after 4 days of culture by flow cytometry analysis. Results are presented in percentage of the proliferation rate of untreated CD8+ T cells (0).*P<0.05 is for comparisons with untreated cells (0) within the group. (B) CD8+ T cells treated with 1 μg/ml βGBP for 3 hours and then were stimulated with either soluble or plate-bound CD3 Ab for 5 min, lysed and were analyzed by western blotting for pERK and ERK levels. *Cells were treated with βGBP for 3 hours one day prior to stimulation. (C) βGBP suppresses proliferation of TCR transgenic CD8+ T cells from splenocytes of pmel mice induced by mouse, but not human, gp100 peptide. Pmel mice splenocytes were cultured in the presence or absence (Cnt) of 1 μg/ml βGBP and indicated peptides (both at 1 μg/ml) for 4 days. No peptide was added to control cells (unstimulated). Proliferation was evaluated by BrdU incorporation and ELISA. Data represent mean ± SD of OD values at 450 nm. The peptide stimulation yielded more than 95% CD8+ cells at the time of assay (data not shown). *P<0.05 is for comparison with the mgp100 Cnt group.
DISCUSSION
Despite the wealth of information associating both Tregs and βGBP with the induction of tolerance, amelioration of harmful autoimmunity, or poor prognosis of cancers, very little is known about the molecular mechanisms of their regulatory activity. Here we demonstrate that CCR4+Tregs, express βGBP to prevent activation of resting CD8+ T cells with self-antigens. The molecular mechanism involved lies in the ability of βGBP to block TCR signaling at the PI3K-induced Ras/MAPK activation step that results in the inability to phosphorylate ERK and Akt. Thus, it appears that βGBP regulates TCR signaling in the same way as we have recently reported in somatic cells, i.e. inhibition of the p110 catalytical subunit of PI3K leading to suppression of Ras-GTP loading and loss of ERK activation (Wells et al., 2007). On the other hand, Tregs/βGBP did not affect proximal TCR signaling in CD8+ T cells, such as phosphorylation of TCRζ and Zap70. Similarly, we did not detect any inhibitory effects on LAT and PKCθ, indicating that the ability of LAT to recruit various signaling proteins was likely not affected. This is in concordance with our recent report that βGBP does not affect Grb2 recruitment during the inhibition of growth factor-induced activated Ras in somatic cells (Wells et al., 2007). Non-processive TCR signaling such as phosphorylation of TCRζ was also observed by others in murine hybridoma cells after treatment with 10 μM βGBP (Chung et al., 2000). However, unlike our finding, this did not lead to LAT phosphorylation. It is reasonable to assume that such a discrepancy is explainable by the differential use of βGBP, as it has a biphasic “cytokine = βGBP” and as a lectin (Gal-1) functions depending on its oligomerization state (Vas et al., 2005;Barrionuevo et al., 2007) (see also (Camby et al., 2006)). βGBP at higher than 7 μM concentrations forms Gal-1 dimers (Cho and Cummings, 1996) acquiring lectin and cytotoxic activities (Perillo et al., 1995). In contrast, βGBP as a monomer (nM amounts) may operate as an effector molecule via receptor mediated interaction to control PI3K function and consequently to affect mitogenic and survival signaling (Wells and Mallucci, 1991;Wells and Mallucci, 1992;Mallucci and Wells, 2005;Wells et al., 2007).
Tregs (such as Tr1) have been reported to utilize perforin- and Granzyme (Gz) A and B -dependent processes in regulation of T cells (Grossman et al., 2004), indicating that they may be used to induce cell death. On the other hand, human natural Tregs presumably function to prevent activation of naïve T cells without killing them. We have recently reported that human peripheral blood contains two functionally different types of Tregs that did not express GZ-A and GZ-B, the so-called “memory-type” CCR4+Tregs and the “naïve-type” CCR4−Tregs (Baatar et al., 2007). Naïve-type CCR4−Tregs required TCR pre-stimulation in the secondary lymphoid organs in order to acquire a regulatory activity, while CCR4+Tregs represented already primed cells that readily exerted a cell contact-mediated regulation of naïve CD8+ T cells (Baatar et al., 2007). However, as shown here, CCR4+Tregs also expressed βGBP to reversibly abrogate TCR signaling of CD8+ T cells, as the cells were able to be fully activated once βGBP/Tregs was removed. In this respect, this process appears to be quite similar to the activation-induced non-responsiveness (AINR) of CD8+ T cells, a transient process that, unlike “classical” anergy, occurs despite the cells receiving both signal 1 and signal 2 (Tham and Mescher, 2001). Similarly with AINR, Treg-induced suppression of CD8+ T cells required the blockage of Ras -dependent ERK activity. In both cases, cell proliferation was restored when the cells were stimulated with a PKC-inducer, PMA, that activates Ras by suppressing the activity of the GTPase (Downward et al., 1990;Liu and Heckman, 1998). Similarly, the strong TCR-activating signals (e.g. immobilized CD3 Ab, model of xeno- and foreign antigens) overcame the suppressive effects of βGBP (or Tregs), indicating that the regulation is effective only for weak TCR signals. This is in concordance with our previous report that human natural Tregs could not efficiently regulate CD8+ T cells activated with strong TCR stimuli (Baatar et al., 2007). Consistent with this notion, βGBP only inhibited activation of murine TCR transgenic CD8+ T cells with the cognate murine gp100 peptide (a normal melanocyte differentiation antigen often expressed in melanomas), while proliferation with higher affinity xenogeneic human gp100 peptide was even slightly increased in the presence of βGBP. Thus, it is tempting to conclude that the key biological feature of βGBP/Tregs is presumably to prevent the activation of naïve CD8+ T cells to self-antigen and sub-optimal TCR stimulation, an important feature of self tolerance. As others reported that βGBP protects survival of naïve T cells without promoting cell proliferation (Endharti et al., 2005), Tregs/βGBP induced neither apoptosis nor anergy of non-activated CD8+ T cells even after several days of treatment (unpublished data). Instead, this could be a mechanism to sensitize CD8+ T cells to foreign antigens, as the βGBP -pretreated CD8+ T cells were not only protected but also acquired significantly increased potency to respond to subsequent TCR stimulation.
Our data also raises an interesting possibility that the basis of the βGBP-induced T cell survival may be in the inhibition of Ras/ERK pathway that abrogates the MAPK-catalyzed negative feedback loop via Thr155 phosphorylation of LAT and attenuation of subsequent downstream events (Matsuda et al., 2004). It remains to be tested whether βGBP/Tregs did not affect the proximal TCR signaling (such as LAT/PKC) is to allow NF-κB- mediated survival events to occur in CD8+ T cells. Taken together, our data indicate that Tregs regulate resting CD8+ T cell responses to self-antigenic stimuli. To do this, they employ βGBP to induce non-cytotoxic and non-processive TCR signaling that blocks the PI3K-induced Ras/MAPK activation step and prevents self-antigen –induced ERK activation and cell proliferation. Although a predominant proportion of CCR4+Tregs we used in this study expressed skin homing marker CLA (Baatar et al., 2007), they can also infiltrate other peripheral sites including metastatic brain (Sugihara et al., 2009). It is tempting to speculate that Tregs support brain metastasis the same way as in case of breast cancer lung metastasis. We have recently demonstrated that Tregs were required in lung metastasis to specifically eliminate protective NK cells in the lungs utilizing βGBP (Olkhanud et al., 2009). Thus, in addition to the regulation of the activity of resting T cells to self-antigens, the role Tregs in harmful autoimmunity and tumors of CNS (Offner et al., 1990;Rabinovich et al., 1999) could be attributed to βGBP-mediated elimination of activated T cells (Allione et al., 1998) and NK cells.
Acknowledgements
We are grateful to Karen Madara (Apheresis Unit and the Clinical Core Laboratory, NIA) for providing human blood samples; Gary Collins (NIA) and Dr. Giovanni Almanzar (NIA), for help with processing human blood samples; Brittany Frank (NIA) for help with confocal microscopy imaging; Dr. Dan L. Longo, Dr. Paritosh Gosh, and Ana Lustig (NIA) for critical reading of the manuscript and helpful comments and suggestions. This research was supported by the Intramural Research Program of the National Institute on Aging, NIH.
References Cited
- Allione A, Wells V, Forni G, Mallucci L, Novelli F. Beta-galactoside-binding protein (beta GBP) alters the cell cycle, up-regulates expression of the alpha- and beta-chains of the IFN-gamma receptor, and triggers IFN-gamma-mediated apoptosis of activated human T lymphocytes. J.Immunol. 1998;161(5):2114–2119. [PubMed] [Google Scholar]
- Alyanakian MA, You S, Damotte D, Gouarin C, Esling A, Garcia C, Havouis S, Chatenoud L, Bach JF. Diversity of regulatory CD4+T cells controlling distinct organ-specific autoimmune diseases. Proc.Natl.Acad.Sci.U.S.A. 2003;100(26):15806–15811. doi: 10.1073/pnas.2636971100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J.Immunol. 2001;166(5):3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- Baatar D, Olkhanud P, Sumitomo K, Taub D, Gress R, Biragyn A. Human Peripheral Blood T Regulatory Cells (Tregs), Functionally Primed CCR4+ Tregs and Unprimed CCR4− Tregs, Regulate Effector T Cells Using FasL. J.Immunol. 2007;178(8):4891–4900. doi: 10.4049/jimmunol.178.8.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo P, Beigier-Bompadre M, Ilarregui JM, Toscano MA, Bianco GA, Isturiz MA, Rabinovich GA. A novel function for galectin-1 at the crossroad of innate and adaptive immunity: galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J.Immunol. 2007;178(1):436–445. doi: 10.4049/jimmunol.178.1.436. [DOI] [PubMed] [Google Scholar]
- Blaser C, Kaufmann M, Muller C, Zimmermann C, Wells V, Mallucci L, Pircher H. Beta-galactoside-binding protein secreted by activated T cells inhibits antigen-induced proliferation of T cells. Eur.J.Immunol. 1998;28(8):2311–2319. doi: 10.1002/(SICI)1521-4141(199808)28:08<2311::AID-IMMU2311>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J.Exp.Med. 1998;187(1):129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camby I, Le MM, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16(11):137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- Cavani A, Nasorri F, Prezzi C, Sebastiani S, Albanesi C, Girolomoni G. Human CD4+ T lymphocytes with remarkable regulatory functions on dendritic cells and nickel-specific Th1 immune responses. J.Invest Dermatol. 2000;114(2):295–302. doi: 10.1046/j.1523-1747.2000.00881.x. [DOI] [PubMed] [Google Scholar]
- Cho M, Cummings RD. Characterization of monomeric forms of galectin-1 generated by site-directed mutagenesis. Biochemistry. 1996;35(40):13081–13088. doi: 10.1021/bi961181d. [DOI] [PubMed] [Google Scholar]
- Chung CD, Patel VP, Moran M, Lewis LA, Miceli MC. Galectin-1 induces partial TCR zeta-chain phosphorylation and antagonizes processive TCR signal transduction. J.Immunol. 2000;165(7):3722–3729. doi: 10.4049/jimmunol.165.7.3722. [DOI] [PubMed] [Google Scholar]
- Cimmino F, Schulte JH, Zollo M, Koster J, Versteeg R, Iolascon A, Eggert A, Schramm A. Galectin-1 is a major effector of TrkB-mediated neuroblastoma aggressiveness. Oncogene. 2009;28(19):2015–2023. doi: 10.1038/onc.2009.70. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat.Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Downward J, Graves JD, Warne PH, Rayter S, Cantrell DA. Stimulation of p21ras upon T-cell activation. Nature. 1990;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Endharti AT, Zhou YW, Nakashima I, Suzuki H. Galectin-1 supports survival of naive T cells without promoting cell proliferation. Eur.J.Immunol. 2005;35(1):86–97. doi: 10.1002/eji.200425340. [DOI] [PubMed] [Google Scholar]
- Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109(5):2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J.Immunol. 2005;174(4):1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21(4):589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Kuroiwa T, Sakaguchi M, Sun L, Kadoya T, Okano H, Mizusawa H. Galectin-1 regulates neurogenesis in the subventricular zone and promotes functional recovery after stroke. Exp.Neurol. 2007;207(2):302–313. doi: 10.1016/j.expneurol.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J.Exp.Med. 2001;193(11):1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani K, et al. Galectin-1 promotes basal and kainate-induced proliferation of neural progenitors in the dentate gyrus of adult mouse hippocampus. Cell Death.Differ. 2009;16(3):417–427. doi: 10.1038/cdd.2008.162. [DOI] [PubMed] [Google Scholar]
- Le MM, Fortin S, Mathieu V, Kiss R, Lefranc F. Galectins and Gliomas. Brain Pathol. 2009 doi: 10.1111/j.1750-3639.2009.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings MK, Roncarolo MG. T-regulatory 1 cells: a novel subset of CD4 T cells with immunoregulatory properties. J.Allergy Clin.Immunol. 2000;106(1 Pt 2):S109–S112. doi: 10.1067/mai.2000.106635. [DOI] [PubMed] [Google Scholar]
- Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10(8):529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- Mallucci L, Wells V. Potential role of the antiproliferative cytokine beta-galactoside binding protein in cancer therapy. Curr.Opin.Investig.Drugs. 2005;6(12):1228–1233. [PubMed] [Google Scholar]
- Matsuda S, Miwa Y, Hirata Y, Minowa A, Tanaka J, Nishida E, Koyasu S. Negative feedback loop in T-cell activation through MAPK-catalyzed threonine phosphorylation of LAT. EMBO J. 2004;23(13):2577–2585. doi: 10.1038/sj.emboj.7600268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitin N, Rossman KL, Der CJ. Signaling interplay in Ras superfamily function. Curr.Biol. 2005;15(14):R563–R574. doi: 10.1016/j.cub.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Novelli F, Allione A, Wells V, Forni G, Mallucci L. Negative cell cycle control of human T cells by beta-galactoside binding protein (beta GBP): induction of programmed cell death in leukaemic cells. J.Cell Physiol. 1999;178(1):102–108. doi: 10.1002/(SICI)1097-4652(199901)178:1<102::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Offner H, Celnik B, Bringman TS, Casentini-Borocz D, Nedwin GE, Vandenbark AA. Recombinant human beta-galactoside binding lectin suppresses clinical and histological signs of experimental autoimmune encephalomyelitis. J.Neuroimmunol. 1990;28(2):177–184. doi: 10.1016/0165-5728(90)90032-i. [DOI] [PubMed] [Google Scholar]
- Olkhanud PB, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and T regulatory cells. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-08-4619. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J.Exp.Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378(6558):736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, Chernajovsky Y. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J.Exp.Med. 1999;190(3):385–398. doi: 10.1084/jem.190.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravatn R, Wells V, Nelson L, Vettori D, Mallucci L, Chin KV. Circumventing multidrug resistance in cancer by beta-galactoside binding protein, an antiproliferative cytokine. Cancer Res. 2005;65(5):1631–1634. doi: 10.1158/0008-5472.CAN-04-1970. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M, et al. A carbohydrate-binding protein, Galectin-1, promotes proliferation of adult neural stem cells. Proc.Natl.Acad.Sci.U.S.A. 2006;103(18):7112–7117. doi: 10.1073/pnas.0508793103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J.Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- Sasaki T, Hirabayashi J, Manya H, Kasai K, Endo T. Galectin-1 induces astrocyte differentiation, which leads to production of brain-derived neurotrophic factor. Glycobiology. 2004;14(4):357–363. doi: 10.1093/glycob/cwh043. [DOI] [PubMed] [Google Scholar]
- Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol.Cell Biol. 1998;18(2):790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Balakir R, Criado G, Wood JS, Seminario MC, Madrenas J, Wange RL. Zap-70-independent Ca(2+) mobilization and Erk activation in Jurkat T cells in response to T-cell antigen receptor ligation. Mol.Cell Biol. 2001;21(21):7137–7149. doi: 10.1128/MCB.21.21.7137-7149.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J.Exp.Med. 2001;193(11):F41–F46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J.Immunol. 2000;165(6):3105–3110. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- Sugihara AQ, Rolle CE, Lesniak MS. Regulatory T cells actively infiltrate metastatic brain tumors. Int.J.Oncol. 2009;34(6):1533–1540. doi: 10.3892/ijo_00000282. [DOI] [PubMed] [Google Scholar]
- Tham EL, Mescher MF. Signaling alterations in activation-induced nonresponsive CD8 T cells. J.Immunol. 2001;167(4):2040–2048. doi: 10.4049/jimmunol.167.4.2040. [DOI] [PubMed] [Google Scholar]
- Vas V, Fajka-Boja R, Ion G, Dudics V, Monostori E, Uher F. Biphasic effect of recombinant galectin-1 on the growth and death of early hematopoietic cells. Stem Cells. 2005;23(2):279–287. doi: 10.1634/stemcells.2004-0084. [DOI] [PubMed] [Google Scholar]
- Verhagen J, Gabrysova L, Minaee S, Sabatos CA, Anderson G, Sharpe AH, Wraith DC. Enhanced selection of FoxP3+ T-regulatory cells protects CTLA-4-deficient mice from CNS autoimmune disease. Proc.Natl.Acad.Sci.U.S.A. 2009;106(9):3306–3311. doi: 10.1073/pnas.0803186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J.Exp.Med. 2004;199(7):971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells V, Downward J, Mallucci L. Functional inhibition of PI3K by the betaGBP molecule suppresses Ras-MAPK signalling to block cell proliferation. Oncogene. 2007 doi: 10.1038/sj.onc.1210580. [DOI] [PubMed] [Google Scholar]
- Wells V, Mallucci L. Identification of an autocrine negative growth factor: mouse beta-galactoside-binding protein is a cytostatic factor and cell growth regulator. Cell. 1991;64(1):91–97. doi: 10.1016/0092-8674(91)90211-g. [DOI] [PubMed] [Google Scholar]
- Wells V, Mallucci L. Molecular expression of the negative growth factor murine beta-galactoside binding protein (mGBP) Biochim.Biophys.Acta. 1992;1121(3):239–244. doi: 10.1016/0167-4838(92)90152-4. [DOI] [PubMed] [Google Scholar]
- Williams BL, Irvin BJ, Sutor SL, Chini CC, Yacyshyn E, Bubeck WJ, Dalton M, Chan AC, Abraham RT. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-gamma1 and Ras activation. EMBO J. 1999;18(7):1832–1844. doi: 10.1093/emboj/18.7.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J.Immunol. 2002;168(9):4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J.Immunol. 2004;172(5):2778–2784. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]